The prevalence of asthma has been on the rise in recent decades, including in children and adolescents( Reference Asher, Montefort and Björkstén 1 ). It is estimated that about 300 million people suffer from asthma and that this number will have increased by 100 million by 2025( Reference Masoli, Fabian and Holt 2 ). It is also estimated that over 6 million children struggle with asthma in the USA alone( 3 ). The rise in asthma prevalence represents a serious public health concern, in consideration of the economic cost of asthma and the impaired quality of life of asthmatic individuals( Reference Masoli, Fabian and Holt 2 ). Several hypotheses have been put forth to explain the upsurge in the prevalence of asthma, including the ‘hygiene hypothesis’( Reference Yazdanbakhsh, Kremsner and van Ree 4 ), the rising obesity prevalence( Reference Ford 5 ) alongside insufficient physical activity( Reference Ford, Heath and Mannino 6 ), and less exposure to indoor allergens( Reference Shi, Dal Grande and Taylor 7 ). However, there is accumulating evidence suggesting a link between dietary intake and asthma aetiology( Reference McKeever and Britton 8 – Reference Kim, Ellwood and Asher 11 ).
Sugar-sweetened beverage (SSB) consumption is also on the rise in both children( Reference French, Lin and Guthrie 12 ) and adults( Reference Bleich, Wang and Wang 13 ). One study found that the prevalence of soft drink consumption in youth increased from 37 % in 1977/1978 to 56 % in 1994/1998, and that the average daily soft drink intake increased from 148 ml (5 fl oz) to 355 ml (12 fl oz)( Reference French, Lin and Guthrie 12 ). More recently, surveillance data have indicated that among European adolescents, beverages provide approximately 1609 kJ/d (385 kcal/d), of which 30 % comes from SSB( Reference Duffey, Huybrechts and Mouratidou 14 ). SSB consumption is associated with poor health, including conditions such as obesity( Reference Malik, Popkin and Bray 15 ), CVD( Reference Fung, Malik and Rexrode 16 ), type 2 diabetes( Reference Malik, Popkin and Bray 15 ) and metabolic syndrome( Reference Malik, Popkin and Bray 15 ). Interestingly, recent data also link SSB with airway health, specifically asthma( Reference Shi, Dal Grande and Taylor 7 , Reference Stensson, Wendt and Koch 17 – Reference Park, Blanck and Sherry 19 ). One recent cross-sectional study found that SSB consumption was positively associated with asthma in a sample of 16 907 Australian adults aged 16 years and older( Reference Shi, Dal Grande and Taylor 7 ). Research has also shown that asthmatic children consume more SSB than children without asthma( Reference Stensson, Wendt and Koch 17 ). The likely mechanism by which SSB intake promotes the development of asthma is oxidative stress and inflammation consequent of the added sugars present in SSB( Reference Shi, Dal Grande and Taylor 7 ).
While previous studies have investigated SSB intake and asthma in children and adults, no extant studies have examined the relationship between SSB consumption and airway health longitudinally, across pubertal growth. Puberty is an important time course to examine, as previous research indicates that asthma prevalence can vary from pre- to post-puberty( Reference De Marco, Locatelli and Sunyer 20 , Reference Guerra, Wright and Morgan 21 ). Asthma is one of the most prevalent childhood chronic diseases( Reference Van Cleave, Gortmaker and Perrin 22 , Reference Winer, Qin and Harrington 23 ) and while it is widely believed that asthma primarily remits during puberty, it has been reported that about 60 % of children present unremitting asthma from pre- to post-puberty( Reference Guerra, Wright and Morgan 21 ). Thus, public health solutions are needed to ameliorate poor airway health outcomes, namely asthma, during this important period of development.
Narrowing of the airways during and/or following exercise (post-exercise airway narrowing) is a predictor of future asthma development in non-asthmatic individuals and useful in diagnoses of airway health( Reference Hopp, Townley and Biven 24 ). Thus, the purpose of the present longitudinal study was to investigate whether SSB consumption is associated with post-exercise airway narrowing across puberty. We hypothesized that SSB intake would be positively correlated with post-exercise airway narrowing pre-puberty, post-puberty and across puberty.
Methods
We recruited twenty healthy participants (ten boys, ten girls) from forty subjects who were previously tested in our laboratory approximately 5 years ago( Reference Rosenkranz, Swain and Rosenkranz 25 ). One male subject was tested at both time points but failed to complete dietary questionnaires and one female subject reported a diagnosis of asthma and was therefore excluded. The remaining nineteen were unable to be contacted. All participants were non-asthmatic and free of pulmonary disease at both time points. All research components were reviewed and approved by the Institutional Review Board of Human Subjects at Kansas State University.
Maximal aerobic capacity and pulmonary function
Our detailed study protocol is outlined elsewhere( Reference Emerson, Kurti and Rosenkranz 26 ). In brief, participants reported to the lab on four occasions: twice at baseline (pre-puberty) and twice approximately 5 years later for follow-up (post-puberty). On the first visit, participants’ height and weight were measured using a calibrated eye-level physical scale with height-rod (Detecto, Webb City, MO, USA). Participants also completed medical history and nutrition and physical activity questionnaires. Tanner stage of maturation was assessed pre-puberty by the parent and post-puberty via self-report. Next, standard pulmonary function tests were performed, followed by an incremental maximal exercise test to exhaustion on a cycle ergometer (VO2max), and then post-exercise pulmonary function tests. Post-exercise airway narrowing was assessed by determining the percentage change in forced expiratory volume in 1 s from pre- to post-exercise (ΔFEV1). On the second visit, participants underwent a dual-energy X-ray absorptiometry scan to determine body composition.
Questionnaires
To determine nutrition intake, the BS-FJV-FFQ( Reference Cullen, Baranowski and Baranowski 27 ) was used at both time points. The BS-FJV-FFQ is a 7 d recall that comprises four 100 % fruit juice options, fifteen fruit categories, thirty-two vegetable categories and thirty-four drink categories. Beverage or food consumption for a specific beverage or food category (e.g. regular soft drinks) was determined by number of servings in the past week. There were seven choices for each food or beverage item, ranging from ‘none’ to ‘15 or more servings last week’. Regular soft drinks, fruit drinks, punches, and popular commercial fruit drinks and sport drinks were included in the determination of total SSB intake. To measure physical activity status, we administered the BS-BAQ( Reference Cullen, Baranowski and Baranowski 27 ), which is an age-appropriate physical activity questionnaire consisting of thirty-seven activities (e.g. running, swimming, basketball, soccer, etc.). Previous-day physical activity in each category was determined using three possible choices: ‘none’, ‘less than 15 minutes’ or ‘15 minutes or more’. All of the activities in which the participant reported to participate for 15 min or more were used to determine his/her physical activity level. The BS-FJV-FFQ and BS-BAQ are previously validated questionnaires from Baranowski’s work with Boy Scouts and detailed explanation of the questionnaires’ composition and use is provided elsewhere( Reference Rosenkranz, Swain and Rosenkranz 25 ).
Statistical analyses
Pearson correlations were used to assess the relationship between post-exercise airway narrowing and SSB consumption (using the statistical software package IBM SPSS Statistics version 22·0). Differences in SSB consumption between time points were determined with paired t tests. Linear regression analysis (enter method) was used to assess the association of SSB consumption with post-exercise airway narrowing, controlling for confounding factors (fruit and vegetable consumption, body fat percentage and VO2max). Significance was set at P<0·05 for all analyses.
Results
Table 1 presents participant anthropometric and outcome variables at pre- and post-puberty. Body fat percentage was significantly greater in girls compared with boys both pre- and post-puberty (P<0·05) and girls increased in body fat from pre- to post-puberty (change: 7·2 (sd 5·3) %; P<0·05). There were no differences in fruit and vegetable intake between boys and girls pre- or post-puberty, and fruit and vegetable intake did not change from pre- to post-puberty in either boys or girls (P>0·05). VO2max was significantly greater in boys both pre- and post-puberty (P<0·05), with no increase in VO2max from pre- to post-puberty in either sex (P>0·05). There were no sex differences in SSB consumption either pre-puberty or post-puberty (P>0·05). There were no differences from pre- to post-puberty in SSB consumption in boys, girls or combined (P>0·05).
Table 1 Anthropometric and outcome measures at pre- and post-puberty among ten boys and ten girls, Manhattan, KS, USA
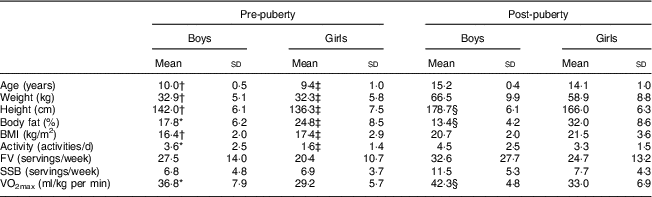
FV, fruit and vegetable intake; SSB, sugar-sweetened beverage consumption; VO2max, maximal aerobic capacity.
* Pre-puberty boys significantly different from pre-puberty girls (P<0·05).
† Pre-puberty boys significantly different from post-puberty boys (P<0·05).
‡ Pre-puberty girls significantly different from post-puberty girls (P<0·05).
§ Post-puberty boys significantly different from post-puberty girls (P<0·05).
Figure 1 and Table 2 display the relationship between SSB intake and post-exercise airway narrowing (ΔFEV1 from pre- to post-exercise). SSB consumption (boys and girls combined) was not significantly correlated with ΔFEV1 pre-puberty (r=−0·35, P>0·05), but the relationship was significant post-puberty (r=−0·60, P=0·005; Fig. 1(a)), indicating that with increased SSB consumption there was greater narrowing of the airways post-exercise. Change in SSB consumption from pre- to post-puberty was significantly associated with ΔFEV1 post-puberty (r=−0·61, P=0·010; Fig. 1(b)) and change in ΔFEV1 from pre- to post-puberty (r=−0·45, P=0·048; Fig. 1(c)), indicating greater post-exercise airway narrowing both post-puberty and across puberty with increased SSB consumption across puberty.

Fig. 1 Relationship between sugar-sweetened beverage (SSB) consumption and post-exercise airway narrowing (determined as the percentage change in forced expiratory volume in 1 s from pre- to post-exercise, ΔFEV1) across puberty among ten boys and ten girls (aged 9·7 (sd 0·9) years at baseline and 14·7 (sd 0·9) years at follow-up approximately 5 years later), Manhattan, KS, USA. At post-puberty, there was a significant correlation (r=−0·60, P=0·005) between SSB consumption and ΔFEV1 (a). This relationship held when considering the change in SSB consumption from pre- to post-puberty v. post-puberty ΔFEV1 (r=−0·61, P=0·01; (b)), as well as the change in both variables from pre- to post-puberty (r=−0·45, P=0·048; (c))
Table 2 Pearson correlations assessing the relationship between sugar-sweetened beverage (SSB) consumption and post-exercise airway narrowing (determined as the percentage change in forced expiratory volume in 1 s from pre- to post-exercise, ΔFEV1) across puberty among ten boys and ten girls (aged 9·7 (sd 0·9) years at baseline and 14·7 (sd 0·9) years at follow-up approximately 5 years later), Manhattan, KS, USA
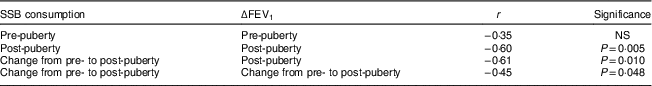
In general, greater SSB consumption was associated with greater post-exercise airway narrowing. As post-exercise airway narrowing is described via decreases in ΔFEV1, the r values are negative. In each analysis except pre-puberty, SSB consumption is significantly associated (P<0·05) with more post-exercise airway narrowing; see Fig. 1 for visual representation.
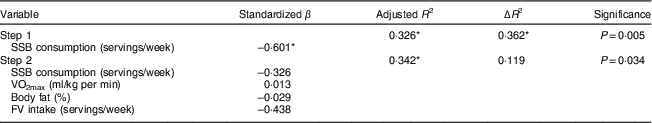
Linear regression was used to test the association between SSB consumption and ΔFEV1 post-puberty, while controlling for potential confounding factors including fruit and vegetable intake, body fat percentage and aerobic capacity (Table 3). A significant standardized β coefficient was found for SSB consumption alone (β=−0·60, P=0·005) but not within the adjusted model (β=−0·33, P=0·211; full model: adjusted R 2=0·34, P=0·03).
Table 3 Linear regression testing the association between sugar-sweetened beverage (SSB) consumption and post-exercise airway narrowing (determined as the percentage change in forced expiratory volume in 1 s from pre- to post-exercise, ΔFEV1) post-puberty, while controlling for potential confounding factors including aerobic capacity (VO2max), body fat percentage and fruit and vegetable intake (FV), among ten boys and ten girls (aged 9·7 (sd 0·9) years at baseline and 14·7 (sd 0·9) years at follow-up approximately 5 years later), Manhattan, KS, USA
Discussion
The primary findings of the present study were that: (i) SSB consumption was significantly positively correlated with post-exercise airway narrowing (a predictor of future asthma development) in post-puberty boys and girls; and (ii) the change in SSB consumption from pre- to post-puberty was significantly associated with both post-puberty post-exercise airway narrowing and the change in post-exercise airway narrowing from pre- to post-puberty. These findings suggest that increased SSB consumption may be a risk factor for future asthma development risk both post-puberty and across puberty.
Our results are in agreement with recent cross-sectional data which have suggested a link between SSB consumption and poor airway health in both children and adults. Specifically, a study in nearly 17 000 Australian adults revealed a significant association between SSB intake and asthma( Reference Shi, Dal Grande and Taylor 7 ). Also, utilizing a multivariate analysis, the authors found the odds of having asthma were 26 % greater in adults who consumed more than half a litre of SSB daily, as compared with those who did not consume SSB( Reference Shi, Dal Grande and Taylor 7 ). With regard to children, Stensson et al.( Reference Stensson, Wendt and Koch 17 ) investigated the relationship between oral health and asthma in 127 asthmatic and 117 matched, non-asthmatic pre-school children. An intriguing finding of the study was that asthmatic children consumed significantly higher levels of SSB than non-asthmatic children. While both of the aforementioned studies are cross-sectional, along with the present study, they suggest a potential link between SSB consumption and asthma.
There are a few potential mechanisms that might explain the link between SSB consumption and asthma. For instance, asthma is associated with inflammation. Consumption of certain foods promotes inflammation and oxidative stress that over time could precipitate the development of asthma. The large amount of added sugar in SSB may particularly induce this chain of events, as sugar consumption increases susceptibility to allergic airway inflammation and triggers the innate immune response in the lung( Reference Kierstein, Krytska and Kierstein 28 ). One study revealed that mice fed a sugar-rich diet had twice as much airway inflammation as mice that consumed a control diet( Reference Kierstein, Krytska and Kierstein 28 ). It is also possible that the link between SSB consumption and asthma is moderated by excess adiposity, as SSB intake increases the risk of obesity( Reference Malik, Popkin and Bray 15 ) and obesity is highly correlated with asthma( Reference Sin and Sutherland 29 ). However, the study by Shi et al.( Reference Shi, Dal Grande and Taylor 7 ) found that the relationship between SSB consumption and asthma held even when controlling for body composition. Thus, there appear to be other important factors besides obesity that may explain the connection between SSB and asthma development. The concept of non-obesity-related influences playing a role in the aetiology of poor airway health is in agreement with the results of the present study, as body fat percentage was not a significant predictor of post-exercise airway narrowing in the linear regression analysis, nor was body fat percentage associated with post-exercise airway narrowing either pre- or post-puberty. Therefore, the likely driver in the relationship between SSB intake and asthma development is the inflammatory and oxidative stress response, although obesity may also play a role. However, more research is needed to better understand the important link between SSB consumption and asthma aetiology.
Experimental considerations
Limitations of our study should be considered when interpreting findings. Our dietary assessment may not have been as vigorous as what is typically used in the adult literature. For example, a common method for assessing dietary intake in adults is a food log in which the participant records all food and beverages consumed over a set amount of time. The present investigation utilized a 7 d dietary recall specifically pertaining to fruits, vegetables and beverages( Reference Cullen, Baranowski and Baranowski 27 ), so it may not have been a comprehensive assessment of dietary habits. However, this dietary assessment method was chosen because it has been used as an age-appropriate assessment of nutrition status in children( Reference Cullen, Baranowski and Baranowski 27 ). It should also be pointed out that the BS-FJV-FFQ( Reference Cullen, Baranowski and Baranowski 27 ) does not include energy drinks (as consumption of energy drinks was not as prevalent during the first time point of the study), which may have led to underestimates in SSB consumption.
The present study was a part of a larger study investigating cardiopulmonary limitations to exercise tolerance from pre- to post-puberty( Reference Emerson, Kurti and Rosenkranz 26 ). The design of the larger study excluded asthmatic children and as a result, to answer the research question of the present study (i.e. is there a link between SSB intake and asthma development?), our best option was to use post-exercise airway narrowing as a predictor of future asthma development. Thus, while we did not specifically document the development of asthma from pre- to post-puberty by nature of the study design, we did describe an important response (i.e. post-exercise airway narrowing) that is indicative of future asthma development in healthy individuals( Reference Hopp, Townley and Biven 24 ).
Conclusions and significance
The consumption of SSB has been on the rise in recent decades, with SSB consumption per capita being highest in young adults( Reference Bleich, Wang and Wang 13 ). With this high consumption of SSB combined with rising asthma prevalence in both children and adults( Reference Masoli, Fabian and Holt 2 ), it is important to be aware of health behaviours that may increase the likelihood of asthma development. Our findings indicate a statistically significant relationship between SSB consumption and post-exercise airway narrowing across puberty. Public health professionals may consider reduction in SSB consumption as one possible means to diminish deleterious airway outcomes during this important period of human development.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None of the authors claim any known or perceived conflict of interest. Authorship : S.R.E. helped develop the study design, helped collect the data, analysed the data and wrote the manuscript. S.K.R. helped develop the study design, helped collect the data and edited the manuscript. R.R.R. helped interpret the data and edited the manuscript. S.P.K. helped collect the data and helped edit the manuscript. C.A.H. helped develop the study design and helped edit the manuscript. Research took place in the laboratory of C.A.H. Ethics of human subject participation: All research components were reviewed and approved by the Institutional Review Board of Human Subjects at Kansas State University.







