Obesity is a rapidly worsening public health problem associated with a variety of co-morbidities including type 2 diabetes, hypertension, stroke and CVD( 1 ). Obesity is impacting heavily upon health-care systems around the world, including Luxembourg, where 21 % of the population is obese( Reference Alkerwi, Sauvageot and Donneau 2 ). Obesity occurs in the context of a variety of interrelated demographic, socio-economic and lifestyle factors; nutrition is coming to the fore as a major modifiable determinant. Nutritional epidemiology has produced evidence that an energy-dense and high-fat diet, concomitant with physical inactivity, are independent risk factors for weight gain and obesity( Reference Astrup 3 – Reference Mendoza, Drewnowski and Christakis 5 ).
The literature surrounding the effects of protein intake and particular sources of protein on body composition is unclear. Three recent meta-analyses have been conducted, comparing higher- v. lower-protein diets on health outcomes, including body composition( Reference Santesso, Akl and Bianchi 6 – Reference Wycherley, Moran and Clifton 8 ), with conflicting conclusions. One indicates that a high protein intake in the context of an energy-restricted diet provides greater improvement in body composition compared with standard protein diets, matched for energy intake( Reference Wycherley, Moran and Clifton 8 ). Another systematic review concluded that higher-protein diets probably improve adiposity than lower-protein diets, but the effect is small( Reference Santesso, Akl and Bianchi 6 ). The most recent meta-analysis, which included studies using both energy-restricted and non-energy-restricted diets, reported no added benefit for body weight or body composition from a high-protein diet v. lower-protein diets( Reference Schwingshackl and Hoffmann 7 ). The reason for these different findings is not clear, but suggests that higher protein intakes may only benefit body composition when consumed as part of a weight-loss diet as there is no convincing evidence linking dietary protein intake and body weight under conditions of weight maintenance( Reference Hite, Feinman and Guzman 9 ). Nevertheless, protein-rich diets could have potential beneficial effects by increasing satiety and thermogenesis( Reference Paddon-Jones, Westman and Mattes 10 ), and other studies have suggested that replacing carbohydrate with protein from meat, poultry and dairy foods may have beneficial metabolic effects( Reference Farnsworth, Luscombe and Noakes 11 ) and help reduce abdominal obesity( Reference Merchant, Anand and Vuksan 12 ).
Evidence is emerging that the type of dietary protein consumed can elicit different health effects and play an important role in disease aetiology( Reference Velasquez and Bhathena 13 ). In general, plant proteins have been related to health benefits more than animal proteins( Reference Ashton, Dalais and Ball 14 – Reference Lin, Bolca and Vandevijvere 17 ). While vegetable protein intakes have been found to be inversely associated with blood pressure( Reference Elliott, Stamler and Dyer 18 ), a high consumption of red and/or processed meat has been associated with a number of adverse cardiovascular health outcomes such as higher systolic blood pressure( Reference Tzoulaki, Brown and Chan 19 ), increased risk for type 2 diabetes( Reference Song, Manson and Buring 20 – Reference Pan, Sun and Bernstein 22 ), ischaemic stroke( Reference Chen, Lv and Pang 23 ), global and central obesity( Reference Wagemakers, Prynne and Stephen 24 , Reference Wang and Beydoun 25 ) and weight gain( Reference Xu, Yin and Tong 26 , Reference Vergnaud, Norat and Romaguera 27 ).
Although the adverse effects of red and processed meat consumption are well documented, there is still lack of firm evidence regarding the effect of other sources of animal protein intake (fish, eggs and milk products) on health outcomes, including body weight status( Reference Hite, Feinman and Guzman 9 , Reference Clifton 28 ). The present study aimed to examine the association of animal protein intake and more specifically, intakes of animal protein derived from different sources, i.e. meats (red meat, poultry), fish and shellfish, eggs and milk products, with global and abdominal obesity in a nationally representative sample of adult participants in the Observation of Cardiovascular Risk Factors in Luxembourg (ORISCAV-LUX) survey in Luxembourg. It was hypothesized that animal protein would be positively associated with obesity, but the association may vary according to protein source. The findings will contribute to current knowledge on the influence of animal protein intake on body weight status and help guide the development of future prevention/weight control intervention studies.
Methods
Study population
The study material consisted of individuals from the national ORISCAV-LUX study, a cross-sectional, population-based survey among adults aged 18–69 years. The data collection, sample design and representativeness have been reported in detail elsewhere( Reference Alkerwi, Sauvageot and Donneau 2 , Reference Alkerwi, Donneau and Sauvageot 29 , Reference Alkerwi, Sauvageot and Couffignal 30 ). Briefly, a stratified random sample of 1432 non-institutionalized individuals was enrolled between November 2007 and January 2009, with a participation rate (32·2 %) that corresponded to the theoretically expected rate upon which the sample size was calculated( Reference Alkerwi, Sauvageot and Couffignal 30 ). Participants with missing data (i.e. at least two pages of the FFQ were uncompleted) or implausible dietary data (i.e. with values of nutrient intakes outside the 1st and 99th percentiles of the distribution) were excluded (n 85). Those who reported currently being on diet for weight loss or for chronic CVD (diabetes, hypertension, dyslipidaemia) were also removed (n 195) from the analyses. Hence the final sample size used in the analysis was 1152 individuals.
The ORISCAV-LUX study received the approval of the Luxembourg national ethical committee and the national commission for private data protection. All participants provided written informed consent.
Data collection
Participant data were collected from three sources: (i) a self-administered questionnaire, including information on demographics, socio-economics, smoking history, diet and physical activity; (ii) anthropometric measurements; and (iii) blood sampling. Extensive quality control measures for the completeness and integrity of dietary and non-dietary information were applied with the help of trained research nurses.
Anthropometric measures and obesity assessment
Height, body weight and waist circumference (WC) were measured in light clothing without shoes, according to previously published methods( Reference Alkerwi, Sauvageot and Donneau 2 ). BMI was calculated as weight in kilograms divided by the square of height in metres (kg/m2). WC (centimetres) was measured at the level midway between the twelfth rib and the uppermost lateral border of the iliac crest at the end of normal expiration. Study participants were classified as normal weight (BMI<25·0 kg/m2), overweight (BMI≥25·0 to <30·0 kg/m2) or obese (BMI≥30·0 kg/m2)( Reference Chu, Rimm and Wang 31 ). Global obesity was defined as BMI≥30·0 kg/m2, while abdominal obesity was defined as WC≥102 cm for men and ≥88 cm for women( 32 ).
Dietary intakes
Dietary intake was assessed by means of a 134-item semi-quantitative FFQ, self-administered and then verified with the participant by trained staff. The overall validity of the FFQ examined against nutritional biomarkers( Reference Sauvageot, Alkerwi and Albert 33 ) and a 3 d dietary record( Reference Sauvageot, Alkerwi and Adelin 34 ) showed a satisfactory performance in detecting and ranking micro- and macronutrients. Participants were required to assign the frequency and quantity of foods and beverages habitually consumed during the preceding 3 months. Food intakes were calculated by multiplying the self-reported food portion by the frequency of consumption. Energy and nutrient intake data were compiled using a French food composition table( Reference Hercberg, Arnault and Astorg 35 ). To account for energy intake, macronutrients were adjusted for total daily energy intake and expressed as percentages of total energy intake (%E). Mathematically, a given intake was first divided by daily energy intake, then multiplied by 400 for protein and carbohydrate intake and by 900 for fat intake( Reference Willett, Howe and Kushi 36 ).
Animal protein sources
Consistent with the guidelines of the US Department of Agriculture( 37 ), protein derived from animal foods was divided into four broad categories: (i) meat; (ii) fish and shellfish; (iii) eggs; and (iv) milk products. The meat group was defined as the sum of the following types of meats: processed and unprocessed red meat including beef, pork, lamb, veal, game and poultry (chicken and turkey). Fish and shellfish included white fish, fatty fish such as salmon, canned fish such as tuna and seafood such as shrimp and squid. Milk products included milk, yoghurt and cheese (whole fat and reduced fat). The fraction of animal protein (8·59 %) derived from meat, fish and shellfish, eggs or dairy products mixed in prepared dishes (e.g. soup with pieces of meat, paella with meat or fish and shellfish, quiche with meat, eggs or cheese) was not included in the calculation of total animal protein.
Potential confounding factors
Based on an extensive literature review, several sociodemographic, lifestyle and dietary confounding factors were considered. Smoking status was dichotomized as ‘non-smoker’ and ‘smoker’. Physical activity was calculated as the total amount of time engaged in physical activity per week. Education status was classified into primary, secondary or tertiary level. The dietary factors were total fat (g/d), total carbohydrate (g/d), total fibre (g/d) and fruit and vegetable intake (g/d). These variables have been described in detail elsewhere( Reference Alkerwi, Donneau and Sauvageot 29 ).
Statistical analysis
Quantitative data were expressed as mean and standard deviation. Frequency tables were used for categorical findings. Participants’ demographic and dietary characteristics according to global obesity (normal weight, overweight, obese) and abdominal obesity (absent, present) were compared by ANOVA or the χ 2 test for contingency tables.
Total protein intake (animal and vegetal), total animal protein intake and protein intake from meats, fish and shellfish, eggs and milk products were used as the independent (explanatory) variables in the statistical analyses. All of these variables were expressed in g/d and in percentage of total daily energy intake (%E). Binary logistic regression was applied to assess the odds for global obesity (BMI≥30·0 kg/m2) and for abdominal obesity (WC≥102 cm for men and ≥88 cm for women), according to protein intakes. Three models were actually designed: model I adjusted for age and gender; model II further adjusted for education, smoking status, physical activity and intakes of total fat, carbohydrate, fibre, and fruit and vegetables; model III took also total vegetal protein into account. Finally, for each animal protein source, model III also made adjustment for protein intake from other animal sources. Results were expressed as odds ratios and 95 % confidence intervals. All main effect tests were two-sided at the 5 % critical level (P<0·05). Statistical calculations were done using the statistical software package IBM SPSS Statistics version 21.
Results
Participant characteristics according to animal protein intake
Animal protein intake varied considerably according to gender (P=0·039), with more men than women in each quartile of intake except the first. The intake of animal protein also varied significantly between normal-weight, overweight and obese participants (P<0·001); likewise in participants with abdominal obesity (Table 1).
Table 1 Participants’ characteristics according to animal protein intake, Observation of Cardiovascular Risk Factors in Luxembourg (ORISCAV-LUX) study (n 1152)
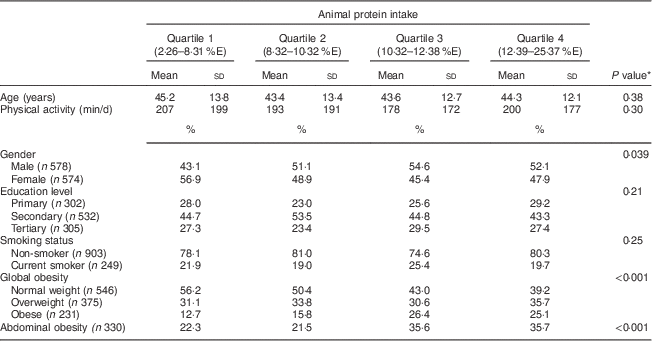
%E, percentage of total daily energy intake.
* P values are from ANOVA (continuous variables) or the χ 2 test (categorical variables).
Protein intakes
Participants with global or abdominal obesity exhibited higher total protein intake (%E) than those without the disorder (both P<0·001; Table 2). Total animal protein intake and protein intakes from meat and from fish and shellfish all increased significantly with body weight status (all P<0·0001; Table 3). Similarly, total animal protein intake, protein intake from meat and protein intake from fish and shellfish (all measured in g/d or as %E) were significantly higher in participants with abdominal obesity (all P<0·01). Protein derived from eggs or milk products did not differ according to body weight status.
Table 2 Participants’ total energy and macronutrient intakes according to global and abdominal obesity, Observation of Cardiovascular Risk Factors in Luxembourg (ORISCAV-LUX) study (n 1152)
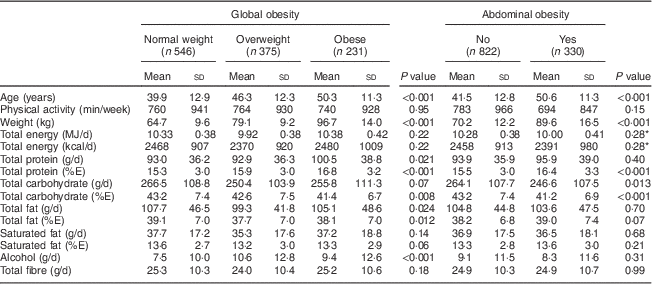
%E, percentage of total daily energy intake.
* Total energy intake was positively correlated with waist circumference (as a continuous variable).
Table 3 Participants’ total vegetal and animal protein intakes and protein intakes from main animal sources, according to global and abdominal obesity status, Observation of Cardiovascular Risk Factors in Luxembourg (ORISCAV-LUX) study (n 1152)
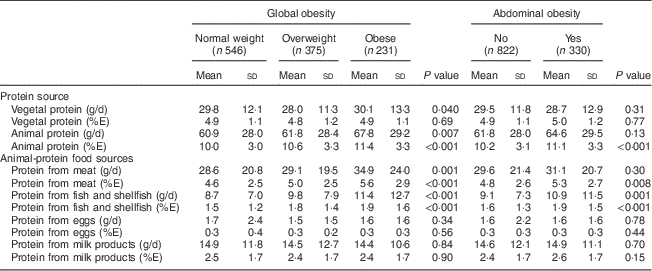
%E, percentage of total daily energy intake.
Multivariate modelling of global and abdominal obesity
The major findings obtained from the multivariate modelling (models I–III) of global and abdominal obesity with respect to total protein, total animal protein and animal protein derived from each dietary source are displayed in Table 4. For model I (age- and gender-adjusted), total protein and total animal protein intakes (as %E) were found to be associated with increased odds for both disorders (all P<0·001). These associations remained significant when adjusting for education, smoking, physical activity and total fat, total carbohydrate, total fibre, fruit and vegetable intakes (model II).
Table 4 Multivariate modelling (models I–III) of global and abdominal obesity with respect to intakes of total protein, total animal protein and protein from main dietary sources based on 1152 participants from the Observation of Cardiovascular Risk Factors in Luxembourg (ORISCAV-LUX) study
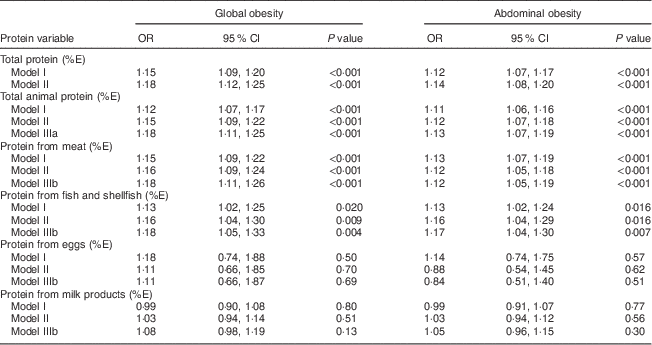
%E, percentage of total daily energy intake.
Model I: adjusted for age and gender.
Model II: adjusted for age, gender, education, smoking, physical activity and intakes of total fat, total carbohydrates, total fibre, and fruit and vegetables.
Model IIIa: adjusted for covariates in model II and total vegetal protein (%E).
Model IIIb: adjusted for covariates in model II, total vegetal protein (%E) and protein from other animal sources (%E).
When examining the individual dietary sources of animal protein and controlling for other animal protein sources, protein derived from meat was independently related to global obesity (model III: OR=1·18; 95 % CI 1·11, 1·26) and abdominal obesity (OR=1·12; 95 % CI 1·05, 1·19). Likewise, a significant independent positive association was observed between protein derived from fish and shellfish and global obesity (model III: OR=1·18; 95 % CI 1·05, 1·33) and abdominal obesity (model III: OR=1·17; 95 % CI 1·04, 1·30). Protein consumption from eggs and milk products was unrelated to global and abdominal obesity.
Discussion
The present study has demonstrated a positive independent association between animal protein intake and both global and abdominal obesity in presumably healthy adults in Luxembourg. Higher animal protein intake was associated with higher odds of global and abdominal obesity, specifically from meat (red meat, poultry) and fish and shellfish consumption, but not eggs or milk products. The odds of global obesity increased by 18 % for every 1 % increase in total energy intake derived from meat products or from fish and shellfish (both P<0·01). Our results indicate that the four selected sources of animal protein may have a differential effect on obesity, independent of age, gender, education level, lifestyle behaviours and other dietary factors.
Although the literature on the animal protein intake–obesity relationship is not abundant, our data are consistent with a recent cross-sectional study conducted in a Belgian population( Reference Lin, Bolca and Vandevijvere 17 ), which showed positive associations between animal protein intake and BMI and WC in males, but not in females. To further investigate our data, a separate gender-specific sensitivity analyses showed similar positive significant relationships for both men and women with animal protein. These findings are also in line with a recently published prospective study, the Chicago Western Electric Study( Reference Bujnowski, Xun and Daviglus 15 ), which examined the association between protein intake and obesity in over 1000 American men over 7 years. Animal protein intake was positively associated with overweight and obesity, independent of energy, carbohydrate and fat intakes. Both abovementioned studies( Reference Bujnowski, Xun and Daviglus 15 , Reference Lin, Bolca and Vandevijvere 17 ) also found an inverse association between vegetable protein intake and obesity; no such associations were observed in the present study. We believe we have added to the current literature by evaluating the relationship between specific animal-protein food sources and obesity. The present findings concur with positive associations observed in other cross-sectional studies between red meat consumption and BMI( Reference Wang and Beydoun 25 , Reference Okubo, Sasaki and Murakami 38 , Reference Maskarinec, Novotny and Tasaki 39 ), WC( Reference Wang and Beydoun 25 , Reference Azadbakht and Esmaillzadeh 40 ) and the metabolic syndrome( Reference Azadbakht and Esmaillzadeh 40 , Reference Babio, Sorli and Bullo 41 ). Total meat consumption has also been positively associated with weight gain in both normal-weight and overweight adults over a 5-year follow-up period in a large European cohort( Reference Vergnaud, Norat and Romaguera 27 ).
Although a consensus exists that energy restriction promotes weight loss, the effect of varying the macronutrient composition of the diet (fat, protein, carbohydrates) on weight loss has been debated( Reference St Jeor, Howard and Prewitt 42 ). Several intervention trials indicate that low-carbohydrate diets promote a greater degree of weight loss in the short term than a conventional high-carbohydrate, low-fat diet( Reference Foster, Wyatt and Hill 43 – Reference Stern, Iqbal and Seshadri 45 ), even when energy intake is matched( Reference Halyburton, Brinkworth and Wilson 46 ), but over the long term weight loss is similar to other that achieved with energy-restricted dietary patterns( Reference Brinkworth, Noakes and Buckley 47 ). Other studies have demonstrated that high-protein diets with concomitant decreases in energy intake may result in sustained weight loss or improved health( Reference Halton and Hu 48 , Reference Skov, Toubro and Ronn 49 ). In high-protein diets, weight loss is initially high due to fluid loss related to reduced carbohydrate intake, overall energy restriction and ketosis-induced appetite suppression( Reference St Jeor, Howard and Prewitt 42 ).
The biological mechanisms that might explain the adverse relationship between animal protein intake and the risk of obesity are still unclear. The high cholesterol, Fe and C-reactive protein levels in red meat may have detrimental effects on body weight and health outcomes( Reference Azadbakht and Esmaillzadeh 40 ). In addition, high-protein foods of animal sources (in particular red meats) are energy-dense, high-fat foods, particularly rich in SFA. This macronutrient composition may contribute to the adverse effect on body weight and energy regulation. Energy density may be a key element in body-weight regulation as it may alter appetite control signals (i.e. hunger and satiety)( Reference Vergnaud, Estaquio and Czernichow 50 ).
Most of the randomized controlled trials investigating protein consumption on health outcomes have focused on adjusting total protein in relation to other macronutrients in the diet, rather than on the types of protein or specific sources. As recognized by others( Reference Santesso, Akl and Bianchi 6 ), few randomized controlled trials examining protein intake and health outcomes have reported protein sources (animal v. vegetable). Including different food sources of protein within a high- or low-protein diet may contribute to the conflicting results among studies regarding the animal protein–obesity association. The present findings confirm our initial hypothesis regarding the varying effects according to animal dietary sources. The nutritional content of different protein sources included in the diet may have different influences on body weight and therefore help to explain some of the disparate findings. This will be an important distinction to make in future dietary trials. In addition, the diversity of study designs (cross-sectional, longitudinal, clinical trials) and differential control for confounders may explain the inconsistent findings.
Our contemporary nationwide database( Reference Alkerwi, Sauvageot and Donneau 2 , Reference Alkerwi, Sauvageot and Couffignal 30 ) constitutes an opportunity to examine associations between animal protein and anthropometric measures, with a focus on different animal protein sources, namely meat, fish, eggs and dairy products. As the ORISCAV-LUX measured a large set of potential dietary and non-dietary confounders, we trust that our ‘European’ findings contribute to a growing body of evidence indicating that high intakes of meat may not have a favourable effect on body composition. As in similar population-based studies, the ORISCAV-LUX survey has some limitations, related mainly to the current absence of a gold standard for dietary assessment. Food group and nutrient intakes were estimated by self-reported data. It is well known that overweight/obese people under-report their dietary intake to a greater extent than normal-weight people( Reference Macdiarmid and Blundell 51 ). Despite intensive efforts to minimize dietary reporting inaccuracies through extensive control procedures( Reference Alkerwi, Sauvageot and Donneau 2 ), diet is a complex exposure factor with measurement being subject to imprecision and a wide range of errors and biases. Two extensive validation studies( Reference Sauvageot, Alkerwi and Albert 33 , Reference Sauvageot, Alkerwi and Adelin 34 ) have been performed to examined the performance of the FFQ, showing that it performed well in assessing intakes of several foods and micronutrients and the observed correlations were within the range noted by other investigators. However, the complexity and expense to perform N analyses in 24 h urine collections (gold standard recovery biomarker to validate protein intake) may constitute a drawback in the present study.
The cross-sectional design of the study did not allow conclusions on causal relationships to be made. It precludes inferences about long-term dietary effects on obesity. However, it is less plausible that obese participants have altered their diet by consuming more meats and fish, since those who are currently on diet to reduce their weight or for a cardiovascular health problem (diabetes, hypertension, lipid disorders) were excluded from the analyses. The fact that there was no difference in physical activity between those who were normal weight and those who were obese (the same goes for abdominal obesity) suggests that the majority of those who were obese are trying to resist the state that they are in and perhaps may explain why they are eating more protein-rich foods and less carbohydrates. Unfortunately, we were not able to distinguish the individual associations between particular types of meat (red meat or poultry), as the FFQ was unable to capture this difference. The meat group included a wide range of meats, such as pork, beef, lamb and poultry, and cold processed meats, such as salami. Similarly, a variety of fish types were included within ‘fish and shellfish’ and distinctions between, for example, fatty fish and canned fish were unable to be made due to few cases.
Meat intake can be linked to adverse effects on adiposity through plausible mechanisms and the results from other previous prospective studies lend support to our findings. Along with the effect of meat consumption on the risk of other diseases, such as CVD, diabetes, metabolic syndrome and colorectal cancer, our findings build an added argument against adopting a high-animal-protein diet, specifically from meat, to maintain healthy weight. These findings may have practical implications for public health dietary recommendations. Notwithstanding, it should be kept in mind that protein is one of the three major macronutrients and an important source of energy, needed for both younger and elderly age groups( Reference Chernoff 52 ). In the present studied population, total protein intake contributed 15·8 % to total energy intake. Two-thirds of total protein intake (mean intake of 94·5 g/d) was derived from animal protein (mean intake of 62·6 g/d) and one-third was from vegetable protein (mean intake of 29·3 g/d; data not shown).
Conclusion
Consumption of animal protein, particularly that derived from meat products, showed a positive association with adiposity measures among presumably healthy adults in Luxembourg, independently of gender, age, educational level, smoking status, physical activity and intakes of fibre, fat and carbohydrates. The consumption of meat, a major source of animal protein, plays a vital role in providing a diversified and nutritious diet. Animal products are major sources of a wide range of essential micronutrients; in particular, vitamin A and minerals such as Fe and Zn. Any emphasis placed on the need to reduce animal protein intake in the diet of apparently healthy people should be seen in the sense of opting for other sources of animal protein, such as eggs or dairy products, rather than increasing carbohydrate or fat consumption. In the scarcity of robust evidence from long-term, high-cost prospective and interventional trials, our findings may constitute a relevant contribution to the prevention and control of obesity.
Acknowledgements
Financial support: This research work was supported by a research grant from the National Fund of Research (Fond National de Recherche (FNR)); project DIQUA-LUX, 58 70 404. The FNR had no role in the design, analysis or writing of this article. Conflict of interest: None. Author contributions: Both M.G. and G.E.C. contributed equally to the supervision. A. Alkerwi was involved in the conception and design of the ORISCAV-LUX survey, coordinated the field data collection, conceived the present research, contributed to data analyses and drafted the manuscript. N.S. conducted the statistical analyses and contributed to data interpretation. A.-F.D. participated in the statistical analyses. J.D.B. and A. Albert contributed to the critical revision of the manuscript and intellectual content. G.E.C. and M.G. provided expertise and oversight throughout the process. All of the authors reviewed drafts and approved the final version of the manuscript. Ethics of human subject participation: The ORISCAV-LUX study received the approval of the Luxembourg national ethical committee and the national commission for private data protection. All participants provided written informed consent.







