Inflammation is a response due to repeated ‘injury’, e.g. from cigarette smoking, infection or hypertension, and evidence is accumulating on the role of chronic inflammation in cancer( Reference Keibel, Singh and Sharma 1 ), with colon cancer being the most well described( Reference Terzic, Grivennikov and Karin 2 ). The inflammatory microenvironment includes production of cytokines and chemokines that also promote tumour initiation, growth and invasion( Reference Jackson and Evers 3 ).
The acute-phase protein C-reactive protein (CRP) is produced in response to stimulation by interleukins, such as IL-6( Reference Erlinger, Platz and Rifai 4 ). Although used as a marker of inflammation in such conditions as rheumatoid arthritis for many decades, the more recent development of a high-sensitivity C-reactive protein (hs-CRP) assay permitted the detection of inflammation at the vascular level( Reference Ridker, Glynn and Hennekens 5 ). Many studies have shown that CRP is associated with a number of CVD end points( Reference Ridker, Rifai and Rose 6 ). In addition, CRP and inflammatory cytokines such as IL-6 and TNF-α are increased among obese individuals, positively correlated with BMI, and central adiposity has been shown to be associated with increased inflammation( Reference Hermsdorff, Zulet and Puchau 7 ). Higher levels of IL-6 among obese individuals are associated with insulin resistance( Reference Bastard, Jardel and Bruckert 8 ). Ridker et al.( Reference Ridker, Buring and Cook 9 ) found that each component of the metabolic syndrome (obesity, hypertriacylglycerolaemia, low HDL-cholesterol, hypertension, abnormal glucose metabolism) is significantly associated with higher levels of hs-CRP.
Diet is known to play a major and significant role in the regulation of chronic inflammation. Previous research has shown an inverse association between fruit and vegetable consumption and inflammatory markers such as CRP, IL-6 and TNF-α( Reference Hermsdorff, Zulet and Puchau 10 ). The ‘Western’ diet, which is high in red meat, high-fat dairy products, refined grains and simple carbohydrates, has been associated with higher levels of CRP and IL-6( Reference Esmaillzadeh, Kimiagar and Mehrabi 11 ). On the other hand, the Mediterranean diet, which is high in whole grains, fish, fruit and green vegetables, with moderate alcohol and olive oil intakes, and low in red meat and butter, has been associated with lower levels of inflammation( Reference Dalziel, Segal and de Lorgeril 12 – Reference Serrano-Martinez, Palacios and Martinez-Losa 16 ). Diets high in fruits and vegetables have been associated with lower levels of CRP( Reference Gao, Bermudez and Tucker 17 – Reference Esmaillzadeh, Kimiagar and Mehrabi 19 ). Specific nutrients such as n-3 PUFA( Reference Ferrucci, Cherubini and Bandinelli 20 – Reference Gunter, Stolzenberg-Solomon and Cross 25 ), fibre( Reference Bo, Durazzo and Guidi 26 – Reference Ma, Griffith and Chasan-Taber 30 ), moderate alcohol intake( Reference Avellone, Di Garbo and Campisi 31 – Reference Imhof, Froehlich and Brenner 33 ), vitamin E( Reference Gunter, Stolzenberg-Solomon and Cross 25 , Reference van Herpen-Broekmans, Klopping-Ketelaars and Bots 34 – Reference Murphy, Foley and Tome 40 ), vitamin C( Reference van Herpen-Broekmans, Klopping-Ketelaars and Bots 34 , Reference Chien, Chang and Chen 41 – Reference Wannamethee, Lowe and Rumley 43 ), β-carotene( Reference Gunter, Stolzenberg-Solomon and Cross 25 , Reference van Herpen-Broekmans, Klopping-Ketelaars and Bots 34 , Reference Folsom, Desvarieux and Nieto 44 – Reference Kritchevsky, Bush and Pahor 46 ) and magnesium( Reference Bo, Durazzo and Guidi 26 , Reference King, Mainous and Geesey 47 – Reference Song, Ridker and Manson 49 ) also have consistently been shown to be associated with lower levels of inflammation.
Over the past three years, we have developed a Dietary Inflammatory Index (DII) that can be used in different data sets across diverse population in order to predict levels of inflammatory markers and related health outcomes( Reference Shivappa, Steck and Hurley 50 ). We have substantially modified the DII since the original publication in 2009( Reference Cavicchia, Steck and Hurley 51 ). As described in a companion article( Reference Shivappa, Steck and Hurley 50 ), this moves beyond typical dietary indices in the rigour applied to reviewing the literature (nearly 2000 articles were read and scored) and ‘anchoring’ this to what people actually eat (the final DII is based on actual food consumption data from eleven populations around the world). In this paper, we describe results of fitting this DII to two different sources of dietary intake information in order to predict hs-CRP in a longitudinal study of diet and inflammation.
Materials and methods
Study design
Briefly, the Seasonal Variation of Blood Cholesterol Study (SEASONS) was a prospective observational study. A total of 641 healthy participants were followed for 1 year, with data obtained at baseline and then every 3 months, within a 3-week window on either side of the individual's quarterly appointment date, to the 1-year anniversary point (total of five assessments). Eligibility criteria included being a resident of Worcester County (MA, USA), age 20–70 years and having telephone service. Study participants were not taking cholesterol-lowering medications (e.g. statins) and were not actively on lipid-lowering or weight-control diets, did not have possible causes of secondary hyperlipidaemia, had not been diagnosed as having CHD, and were free of life-threatening illness. Individuals were recruited between December 1994 and February 1997 and enrolment occurred throughout the calendar year. The Institutional Review Boards of the Fallon Healthcare System and the University of Massachusetts Medical School approved all subject recruitment and data collection procedures. Each individual signed an approved informed consent form before entering the study.
The SEASONS data set was selected for testing the DII for two reasons: (i) it had extremely high-quality data on individual-level exposure to food parameters; and (ii) it had hs-CRP data corresponding to each dietary measure. Details of the SEASONS are provided elsewhere( Reference Merriam, Ockene and Hebert 52 , Reference Ockene, Chiriboga and Stanek 53 ).
Eligible participants were scheduled for an in-clinic appointment. At the first visit, previously completed questionnaires were obtained, anthropometric measurements were taken and a fasting blood sample was drawn. Follow-up appointments were scheduled every 3 months for 1 year, for a total of five appointments. There was a 6-week window on both sides of each participant's quarterly appointment during which blood samples were obtained. Considerable information was collected on each participant. Questionnaire-derived data included: demographics, psychosocial measures, social desirability and approval measures, seasonal patterns in mood and behaviour, dietary information in the form of validated 7 d dietary recalls (7DDR)( Reference Hebert, Ockene and Hurley 54 ) and 24 h recalls (24HR), and stress measures. Anthropometric measurements included height, weight, waist circumference and hip circumference. Blood pressure also was measured, as were serum hs-CRP and lipids.
Diet and physical activity assessment
Diet and physical activity were measured using the 24HR method. A set of three 24HR was administered on randomly selected days representing two weekdays and one weekend day at baseline and at each subsequent quarter. All dietary 24HR data were entered and analysed using the Nutrition Data System software (NDS V2·3). Values from the three dietary 24HR were averaged and these were used to calculate DII, thereby resulting in a single DII score for each individual at baseline and in each quarter. Participants also provided dietary data using the 7DDR. This structured instrument, consisting of questions on the amount (i.e. size and frequency) of consumption of 118 food and thirteen beverage items, was developed by Hebert et al.( Reference Hebert, Ockene and Hurley 54 ) for use in the Worcester Area Trial for Counseling in Hyperlipidemia (WATCH) study, which was conducted in Greater Worcester, the same region in central Massachusetts in which SEASONS participants were recruited( Reference Hebert, Ebbeling and Ockene 55 , Reference Ockene, Hebert and Ockene 56 ). While the focus was primarily on parameters that would affect blood lipids, the validation of the instrument indicated that it provides long-term estimates of diet across a wide variety of nutrients( Reference Hebert, Ockene and Hurley 54 ). The 7DDR is an optically scannable form that is filled out in less than 25 min. The form along with appropriate instructions was mailed to individuals prior to each of the five visits. For the 24HR, we were able to obtain intake values for forty-four of the forty-five food parameters required for DII calculation with the exception of trans-fat, because the version of NDS that was used did not calculate intake of trans-fat. However, owing to limited representation of dietary information on any structured questionnaire, data were available on twenty-eight of the forty-five food parameters for the 7DDR( Reference Hebert, Ockene and Hurley 54 ). Physical activity was measured as part of the 24HR interview process using a previously validated method( Reference Matthews, Hebert and Freedson 57 ), and output as energy expenditure overall and by domain as total metabolic equivalents of task (MET).
Serum collection and laboratory measurements
Blood was drawn at baseline and quarterly for up to 1 year (a total of five assessment periods). There was a 6-week window around the quarterly appointment to obtain serum samples from participants. This window spanned from 3 weeks before to 3 weeks after the scheduled appointment. Serum hs-CRP was analysed in the laboratory of Dr Nader Rifai at the Children's Hospital, Harvard Medical School, Boston, MA. The methodology was described previously( Reference Ockene, Matthews and Rifai 58 ). Inter-assay and intra-assay CV for hs-CRP were in compliance with the ranges accepted by the US Centers for Disease Control and Prevention (CDC). Assays for total cholesterol, HDL-cholesterol and TAG were conducted in a CDC Standardized Laboratory( Reference Ockene, Chiriboga and Stanek 53 ). LDL-cholesterol was calculated by the Friedewald formula( Reference Friedewald, Levy and Frederickson 59 ). When TAG exceeded 400 mg/dl, LDL-cholesterol was not calculated. Healthy male and female participants who had at least one hs-CRP measurement over her/his entire 1-year participation were included in the analysis.
Statistical analyses
Summary statistics were used to describe the study population at baseline separately for both 24HR and 7DDR subsets (as the numbers of participants with complete data from each were unequal; n 495 and n 559, respectively). Comparisons of baseline characteristics by sex were made using χ 2 tests for categorical variables and two-sample t tests for continuous variables. DII was converted to tertiles and tests for trend across DII tertiles were carried out for age, smoking status, hs-CRP, BMI, MET/d, LDL-cholesterol and HDL-cholesterol. Generalized linear mixed models (proc GLIMMIX in SAS) were used for more complex analyses. Here, we used a compound symmetry covariance matrix to account for the dependence of observations made on the same individuals. The primary outcome variable for this analysis was hs-CRP, which was dichotomized to ≤3 mg/l and >3 mg/l, and the odds of elevated hs-CRP (>3 mg/l) was determined. Values of hs-CRP >10 mg/l were excluded from the total number of observations because this may be a result of acute inflammation; only sixty-five such values (3 % of the total) were excluded from the total of 2165 available hs-CRP measures as a consequence of this( Reference Pearson, Mensah and Alexander 60 ). The primary independent variable was the score obtained from the DII and tertiles of DII. Both unadjusted and adjusted analyses were carried out. We also tested for effect modification between DII score and categories of BMI, age and infection status by including interaction terms in the model. Variables controlled in analyses were age, sex, race, BMI, smoking status, alcohol consumption status, physical activity, marital status, HDL-cholesterol, total cholesterol, anti-inflammatory medication use, light season, herbal supplement use, and a variable indicating if the participant had an infection during the study quarter. Race was dichotomized into ‘White’ and ‘Other’ because 90 % of the study population was White. BMI was categorized into normal weight (18·5 to <25·0 kg/m2), overweight (25·0 to <30·0 kg/m2) and obese (≥30·0 kg/m2). Participants considered underweight (<18·5 kg/m2) were excluded from analysis. Smoking status was dichotomized as yes/no. Level of education was categorized into high-school graduate or less, vocational/trade and some college, and college graduate or more. Marital status was categorized into single, married, living with a partner, separated, divorced or widowed. Total cholesterol and HDL-cholesterol were left as continuous variables. Seasons were categorized using the ‘light season’ definition centred at the equinoxes/solstices (winter: 6 November to 4 February; spring: 5 February to 6 May; summer: 7 May to 5 August; and autumn: 6 August to 5 November). Participants who reported having arthritis were excluded from analysis. Also, observations missing hs-CRP were excluded from analysis. All data analyses were performed using the SAS® statistical software package version 9·2.
Results
A total of 519 participants for 24HR and 586 for 7DDR had at least one clinic visit with hs-CRP data available. After excluding participants with hs-CRP >10 mg/l, arthritis, BMI <18·5 kg/m2 and those missing any of the measurements for the covariates entered in the model, the final sample size for the analysis was 495 for the 24HR and 559 for the 7DDR with baseline data. The number of follow-up quarterly measurements available for the 24HR totalled 1672 (an average of ≈3·4 per participant), and for the 7DDR 1839 (an average of ≈3·3 per participant). Most participants (nearly 75 %) had both an hs-CRP measurement and at least one 24HR at three or more measurement points: 12 % had such paired data at one point, 14 % at two points, 20 % at three, 26 % at four and 28 % had paired data at all five points. For hs-CRP and 7DDR, the percentages with both were similar: 14 % with both at one point, 16 % with two, 20 % with three, 26 % with four and 24 % with both at all five points.
Baseline characteristics of the participants are presented by sex (Table 1). The majority of the participants were White, married and working full time. The mean age was 49 (sd 12) years. Compared with females, males were more likely to be overweight, married, working full time and to have a higher level of education.
Table 1 Baseline characteristics of the participants (categorical variables); Dietary Inflammatory Index Development and Testing Study, Columbia, SC, USA, 2011–2012
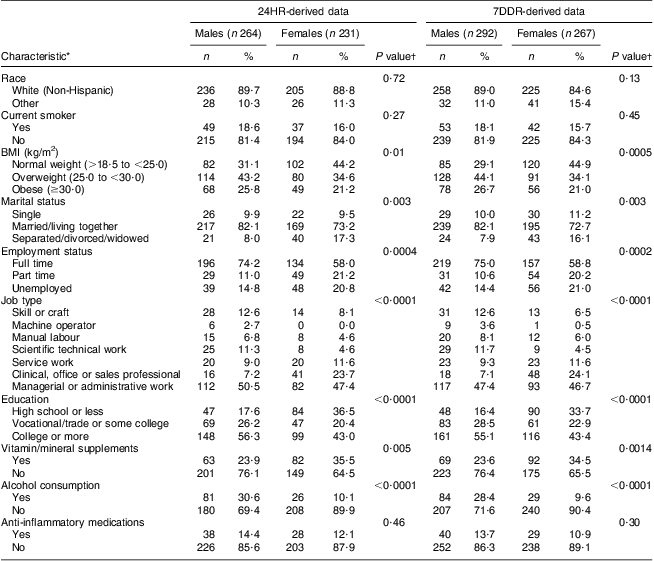
24HR, 24 h diet recall interviews; 7DDR, 7 d diet recall.
*Some of the categories do not sum to the total because of missing data.
†P value for the χ 2 test of the hypothesis that there is no difference between genders.
Energy intake was higher among males than females (difference of 2812 kJ/d (672 kcal/d) for 24HR and 1456 kJ/d (348 kcal/d) for 7DDR; Table 2). For most nutrients, males had a significantly higher intake compared with females, largely because of their higher energy intake. Females had a significantly higher intake of vitamin or mineral supplements (Table 1). No difference was found in herbal supplement use and very few participants consumed fish-oil supplements (three participants in total). Approximately 13·5 % of the study population was using anti-inflammatory drugs at baseline in the 24HR subset compared with 12·5 % of those in the 7DDR subset (this was controlled for during analysis). Across all time points, the DII score ranged from 5·8 to −5·4 for the 24HR subset and from 4·3 to −5·3 for the 7DDR subset, which represents about 67 % and 57 %, respectively, of the range observed in the eleven-country data set used as our standard referent( Reference Shivappa, Steck and Hurley 50 ). Mean DII score for the 24HR subset was 0·18 (sd 2·18) and the mean DII score for the 7DDR subset was 0·84 (sd 2·0).
Table 2 Baseline characteristics of the participants (continuous variables); Dietary Inflammatory Index Development and Testing Study, Columbia, SC, USA, 2011–2012
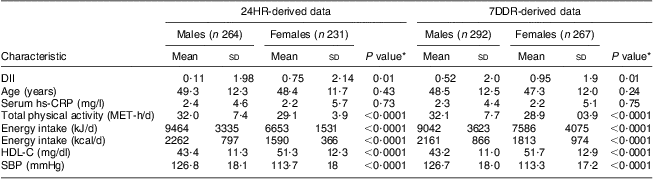
24HR, 24 h diet recall interviews; 7DDR, 7 d diet recall; DII, Dietary Inflammatory Index; hs-CRP, high-sensitivity C-reactive protein; MET, metabolic equivalents of task (a physiological measure expressing the energy cost of physical activities); HDL-C, HDL-cholesterol; SBP, systolic blood pressure.
*P value for the two-sample t test of the hypothesis that there is no difference between genders.
As presented in Table 3, tests for trends across DII tertiles revealed significant increasing trends for hs-CRP (P < 0·0001 in both 24HR and 7DDR subsets), BMI (P < 0·0001 in the 24HR subset and P = 0·002 in the 7DDR subset) and serum LDL-cholesterol (P = 0·009 for 24HR and P < 0·0001 for 7DDR) while a significant decreasing trend was observed for total physical activity (MET-h/d) in the 24HR subset (P < 0·0001).
Table 3 Characteristics of the participants according to DDI tertile; Dietary Inflammatory Index Development and Testing Study, Columbia, SC, USA, 2011–2012
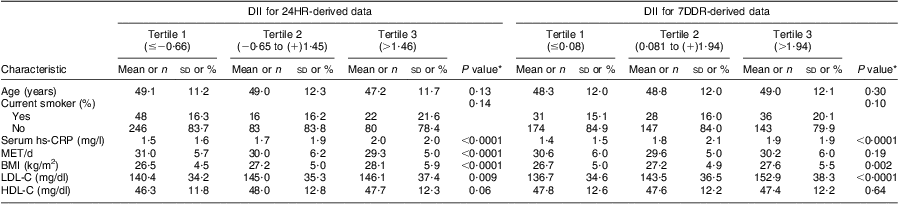
DII, Dietary Inflammatory Index; 24HR, 24 h diet recall interviews; 7DDR, 7 d diet recall; hs-CRP, high-sensitivity C-reactive protein; MET, metabolic equivalents of task (a physiological measure expressing the energy cost of physical activities); LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol.
Data are presented as mean and standard deviation except for current smoker, which is presented as number and percentage.
*P value for trend across tertiles of DII.
Analysis of high-sensitivity C-reactive protein as dichotomous
As recommended by the CDC and the American Heart Association, we dichotomized hs-CRP at the level of 3 mg/l, considering measurements greater than this level at higher CVD risk( Reference Pearson, Mensah and Alexander 60 ). A total of 302 observations (18 % of the total 1672 observations) for 24HR and 323 observations (18 % of the total of 1839 observations) for 7DDR had an elevated hs-CRP; i.e. >3 mg/l. Unadjusted analysis with continuous DII as the independent variable yielded significant results in both the 24HR and 7DDR subsets (OR = 1·06; 95 % CI 1·00, 1·12 for 24HR and OR = 1·10; 95 % CI 1·03, 1·17 for 7DDR). Results from adjusted analysis revealed a significant association between DII score and elevated hs-CRP; a 1-point increase in score was associated with an increased odds of elevated hs-CRP for both subgroups (OR = 1·08; 95 % CI 1·01, 1·16 for 24HR and OR = 1·10; 95 % CI 1·02, 1·19 for 7DDR; Table 4). Analysis with DII as tertiles revealed significant associations between DII in tertiles 3 and 2, compared with tertile 1 (tertile 3 v. 1, OR = 1·47; 95 % CI 1·03, 2·12; tertile 2 v. 1, OR = 1·35; 95 % CI 1·01, 1·81) for the 24HR subset, whereas for the 7DDR subset a significant association was seen between tertile 3 and tertile 1 (OR = 1·61; 95 % CI 1·15, 2·27). In addition, age, HDL-cholesterol and absence of an infection during the study quarter were significantly associated with hs-CRP in both subsets. Overweight and obese individuals had significantly higher odds of an elevated hs-CRP compared with normal-weight individuals. No other variable entered into the model was significantly associated with hs-CRP. We examined the association between DII score and the dichotomous hs-CRP with stratification by infection status. The effect of the DII score was not different among those with v. without an infection; therefore, we reported only the overall odds ratios here. A 5-point increase in the 24HR-derived DII score was associated with ≈50 % increase in the odds of an elevated hs-CRP (OR = 1·47; 95 % CI 1·03, 2·10), while a 10-point increase more than doubled the odds (OR = 2·15; 95 % CI 1·06, 4·40). Similarly, a 5-point increase in the 7DDR-derived score was associated with a 60 % increase in the odds of an elevated hs-CRP (OR = 1·60; 95 % CI 1·09, 2·36), while a 10-point increase more than doubled the odds ratio (OR = 2·56; 95 % CI 1·18, 5·56). This new population-based DII was more highly correlated with hs-CRP (r = 0·11, P < 0·0001) than was the old DII (r = 0·04, P = 0·08).
Table 4 Summary of logistic regression analysis based on hs-CRP as a dichotomous variable (≤3 mg/l, >3 mg/l); Dietary Inflammatory Index Development and Testing Study, Columbia, SC, USA, 2011–2012

hs-CRP, high-sensitivity C-reactive protein; 24HR, 24 h diet recall interviews; 7DDR, 7 d diet recall; DII, Dietary Inflammatory Index.
*Results (odds ratios and associated 95 % confidence intervals) shown are obtained from the logistic regression model, which controlled for all variables shown with additional control for MET (metabolic equivalents of task), gender, light season, race, marital status, serum total cholesterol, employment status, anti-inflammatory medication use, alcohol status and herbal supplement use.
Discussion
Updating the literature database and refinement of the scoring algorithms for the DII appeared to increase construct validation in SEASONS, a study ideally suited for this purpose because of carefully collected dietary exposure (i.e. multiple 24HR and 7DDR) and hs-CRP measures demarcating the four seasons of the year in adult female and male participants. We were able to predict hs-CRP levels ≤3 mg/l v. >3 mg/l using the DII applied to the dietary assessment methods in SEASONS.
Because an hs-CRP value of >3 mg/l is recommended as a relevant clinical cut-off point for identifying individuals at high CVD risk( Reference Pearson, Mensah and Alexander 60 ), we were particularly interest in the association between the DII and hs-CRP as a dichotomous variable. Using multiple 24HR-derived data, we found that a pro-inflammatory diet, as defined by the DII, was associated with an increase in the odds of an elevated hs-CRP (>3 mg/l). We also were very interested in whether there would be a large drop-off in predictive ability in going from multiple 24HR, which are too expensive to be used in most epidemiological studies, to use of a structured questionnaire typical of larger-scale studies. Even though the total number of food parameters used to compute the DII for the 7DDR is less than that used for the DII applied to the 24HR (twenty-eight v. forty-four), there was uniform distribution of pro- and anti-inflammatory food parameters in the available data and, most importantly, virtually no degradation in predictive ability. In all models, we controlled for age( Reference Khor, Muhlestein and Carlquist 61 ) and BMI( Reference Ma, Chiriboga and Pagoto 62 , Reference Wee, Mukamal and Huang 63 ), which are known predictors of CRP.
Despite its racial homogeneity (consisting of ∼90 % non-Hispanic Whites), there are a number of strengths in using SEASONS. It is one of the few studies where both multiple-day 24HR and structured questionnaire were used to collect dietary data. The 24HR is the most accurate method for measuring macronutrient and micronutrient intakes, owing to its ability to assess intake of foods, such as spices, that are not commonly found on structured instruments but which may have a substantial effect on inflammation despite that they tend to be consumed in small quantities in the USA( Reference Hebert, Hurley and Chiraboga 64 ). Like most such structured instruments, the 7DDR, with its focus on long-term intake of macronutrients especially dietary fat, did not measure such foods. Still, its predictive ability seems unimpaired; perhaps owing to the infrequency of consuming these foods in this population.
Another strength of the SEASONS is that the sample size is large for both data subsets, thus providing a robust estimate of the association between the DII and hs-CRP. Within SEASONS, there also is a wealth of information collected at each time point. This allowed appropriate control for a number of other variables, which in turn decreased the possibility of uncontrolled confounding as an explanation for our results.
It is important to note that SEASONS is an observational study; therefore, it is remarkable to observe significant prediction of interval changes in hs-CRP by the DII in the absence of an intervention. Despite that it focuses mainly on White Americans, the values obtained from the 24HR and the 7DDR in SEASONS represent a wide range of the global range of the DII (i.e. 67 % and 57 %, respectively)( Reference Shivappa, Steck and Hurley 50 ).
In summary, we found that the DII was able to predict odds of having an elevated hs-CRP in SEASONS, a longitudinal study with high-quality dietary and inflammation-related data obtained at baseline and at the end of each of four seasons over a year, using both the 24HR- and 7DDR-derived dietary data.
Acknowledgements
Sources of funding: This work was supported by the South Carolina Statewide Cancer Prevention and Control Program Research Fund and the National Heart, Lung, and Blood Institute (grant #1R01 HL073194, SEASONS Study; Principal Investigator I.S.O.). J.R. Hébert was supported by an Established Investigator Award in Cancer Prevention and Control from the Cancer Training Branch of the National Cancer Institute (K05 CA136975). Y.M. was supported by grant #1R01HL094575-01A1 from the National Heart, Lung, and Blood Institute. The National Heart, Lung, and Blood Institute and the National Cancer Institute had no role in the design, analysis or writing of this article. Conflicts of interest: None of the authors declares a conflict of interest. The SEASONS was approved by the University of Massachusetts Institutional Review Board but for the purpose of the current analysis the Institutional Review Board approval was exempt. Authors’ contributions: N.S. was involved in the design of the DII, analysed data from SEASONS and collaborated with J.R. Hébert in writing the original draft of the paper; S.E.S. was involved in design of the DII and provided critical input in revising drafts of the paper; T.G.H. was involved in the design of the DII, provided statistical expertise and helped to write the paper; J.R. Hussey was involved in analysing the data, provided high-level statistical expertise and input in writing the paper; Y.M. provided consultation on analysing the SEASONS data and participated in writing the paper; I.S.O. provided high-level consultation on the SEASONS and input in writing the paper; F.T. provided consultation to N.S. in fitting the models and input in writing the paper; J.R. Hébert devised the initial DII concept, guided all phases of the design of the DII, supervised the analysis of the data used in validating the DII and took the lead in writing the paper.






