Globally, childhood obesity rates have increased dramatically over the past few decades(Reference Kumar and Kelly1). Epidemiological evidence has found childhood overweight and obesity to be a growing problem in Mediterranean countries, with the prevalence of obesity in the southern European region to be among the highest in Europe(Reference Kumar and Kelly1,Reference Manios, Androutsos and Katsarou2) . Greece, in particular, has been found to have the highest prevalence, of overweight and obesity, at approximately 20 %(Reference Manios, Androutsos and Katsarou2). It is well established that increased visceral fat mass levels and central adiposity present in obesity are associated with metabolic syndrome (MetS) which composes a cluster of risk factors including insulin resistance (IR), hypertension and dyslipidaemia(Reference Kim, Lee and Lim3). Evidence also suggests that childhood obesity and related cardiometabolic risk factors are very likely to track into adulthood, thus increasing the risk for Type 2 Diabetes Mellitus and CVD(Reference Kim, Lee and Lim3).
Unhealthy lifestyle habits, including physical inactivity, sedentary behaviour and poor diet quality, have been identified as the most important behavioural risk factors for obesity, MetS and its individual components(Reference Mirmiran, Ziadlou and Karimi4). Conversely, there is strong evidence surrounding the cardioprotective effect of the Mediterranean diet (MedDiet), with higher adherence demonstrating improved longevity and associations with reduced rates of atherosclerosis, MetS, CHD and inflammation in adults(Reference Estruch, Martínez-González and Corella5). Studies assessing dietary intake within Mediterranean populations indicate that the traditional MedDiet is seldom being adhered to(Reference Da Silva, Bach-Faig and Quintana6). The change in dietary patterns, including straying from the traditional Mediterranean way of eating and its effect on health outcomes including obesity and related co-morbidities have not been well elucidated, especially in children. Furthermore, although adherence to the MedDiet has previously been reported to be poor in children in Greece(Reference Farajian, Risvas and Karasouli7), the association of this poor adherence to the MedDiet with the MetS and its components has not been established.
The aim of the present study was to determine the adherence to the MedDiet using the KIDMed score, in 9–13-year-old schoolchildren in Greece, and to examine the association between the level of adherence to the MedDiet and obesity, IR, MetS and its components in this population.
Methods
Sampling
The Healthy Growth Study was a large-scale cross-sectional epidemiologic study initiated in May 2007 and completed in June 2009. The study population was composed of 9–13-year-old schoolchildren, attending the fifth and sixth grades of primary schools located in municipalities within the counties of Attica, Aitoloakarnania, Thessaloniki and Iraklio. The sampling of schools was random, multistage and stratified by parents’ educational level and total population of students attending schools within municipalities of these counties (based on Census data), as described in more detail elsewhere(Reference Moschonis, Tanagra and Vandorou8). The study population was regionally representative of the 9-13-year-old school children living in the four counties under study. Nonetheless, these counties are scattered throughout the Greek territory, covering the northern (i.e. Thessaloniki), central (i.e. Attica), western (i.e. Aitoloakarnania) and southern (i.e. Iraklio-Crete) parts of Greece. This combined with the random, multistage and stratified sampling procedures followed to recruit our sample are indicative of the representativeness of our population. The sampling procedure yielded seventy-seven primary schools, representative of the total number of schools in the counties under study, which responded positively when they were invited to participate in the study. An extended letter explaining the aims of the study and a consent form for taking full measurements were provided to all parents or guardians having a child in these schools. Signed parental consent forms were collected for 2656 children (response rate 64·1 %), whose parents allowed their participation to the study. Assessment between responders and non-responders based on initial screening data indicated that there were no significant differences between groups for children’s weight and parental education level (data not shown).
Dietary intake
Dietary intake data were obtained by trained dietitians and nutritionists via morning interviews with the children at the school site, using a twenty-five item FFQ and three 24-h recalls. More specifically, children’s habitual dietary intake was assessed with an FFQ, which included food items from all food groups, as well as discretionary foods that are usually consumed by children in this age group. The 24-h recalls were collected through face-to-face interviews from all study participants. Children were asked to describe the type and amount of foods and beverages consumed, during the previous 24 h for two consecutive weekdays and one weekend day. The main prerequisite for the 24-h recalls was that the previous day was a usual day according to the participant’s perception. To improve the accuracy of food description, standard household measures (cups, tablespoons, etc.) and food models were used to define amounts. At the end of each interview, the interviewers, who were dietitians rigorously trained to minimise the interviewer’s effect, reviewed the collected data with the respondent in order to clarify entries, servings and possible forgotten foods. Food intake data were analysed using the Nutritionist V diet analysis software (version 2.1, 1999, First Databank), which was extensively amended to include traditional Greek recipes(Reference Trichopoulou9). The database was updated with nutritional information for foods available in Greece including processed foods provided by independent research institutes, food companies and fast-food chains.
Physical activity
Physical activity was assessed using a standardised questionnaire validated with accelerometers by a subsample of the Healthy Growth study(Reference Manios, Androutsos and Moschonis10). In this study, children completed the questionnaire for two consecutive weekdays and one weekend day. Reported activities were assessed as moderate-to-vigorous physical activity provided that they were of intensity higher than four metabolic equivalents and included activities such as bicycling, rhythmic-gymnastics, dancing, basketball, soccer, athletics, tennis, swimming, jumping rope and general participation in active outdoor games. Given the age group, moderate-to-vigorous physical activity was defined as continuous physical activities causing sweating and heavy breathing for periods longer than 15 min, but with occasional breaks in intensity, rather than the strict aerobic definition of twenty continuous minutes appropriate for adults(Reference Baranowski, Hooks and Tsong11).
KIDMed score
Children’s adherence to the MedDiet was assessed using the KIDMed score (see online Supplementary Table 1)(Reference Serra-Majem, Ribas and Ngo12), via a combination of data stemming from the FFQ and the 24-h recalls. The score combined sixteen questions that summarise the characteristics of the Mediterranean dietary pattern. A score of +1 was used for ‘yes’ responses to positive questions, and a score of −1 was given to ‘yes’ responses to negative questions; 0 was given to ‘no’ responses. Total possible scores ranged between −4 and 12. The data derived from the FFQ were used for the calculation of the sub-scores of those KIDMed components related to children’s habitual consumption of fruits and fruit juice, vegetables, fish, fast food, legumes, pasta or rice, breakfast cereals or grains, nuts, olive oil, yogurt and/or cheese, sweets and candy. Considering that the information derived from the FFQ was adequate to cover thirteen out of sixteen components for the calculation of the KIDMed score, the information for the remaining three components resulted from the data stemming from the three 24-h recalls. In this regard, the 24-h recall data related to skipping breakfast and consumption of dairy products and baked goods or pastries for breakfast were extracted from the 24-h recalls.
Total KIDMed scores equal or lower than 3 were indicative of ‘poor’ adherence to the MedDiet, scores between 4 and 7 reflected ‘medium’ adherence, while scores higher than 8 were indicating ‘high’ adherence(Reference Serra-Majem, Ribas and Ngo12). For the purpose of this study and to ensure a more meaningful analysis, moderate and high adherence were merged as there were a very small number of children with high adherence.
Anthropometry and physical examination
Participants underwent a physical examination by two trained members of the research team. The protocol and equipment used were the same in all schools. Weight was measured to the nearest 10 g using a Seca digital scale (Seca Alpha, Model 770). Students were weighed without shoes in the minimum clothing possible. Height was measured to the nearest 0·1 cm using a commercial stadiometer (Leicester Height Measure, Invicta Plastics) with the participant standing barefoot, keeping shoulders in a relaxed position, arms hanging freely and head in Frankfurt horizontal plane. Weight and height were used to calculate BMI using Quetelet’s equation (weight (kg)/height (m)2). The International Obesity Task Force cut-off points(Reference Cole, Flegal and Nicholls13,Reference Cole, Bellizzi and Flegal14) were used to categorise participants as ‘underweight’, ‘normal weight’, ‘overweight’ or ‘obese’. Waist circumference (WC) was measured in triplicate to the nearest 0·1 cm with the use of a non-elastic tape (Hoechstmass) with the student standing, at the end of a gentle expiration. If the three measurements were within 5 % of each other the mean was used, if not the mean of two of the three values within 5 % was reported. The measuring tape was placed around the waist, half-way between the lower rib margin and the iliac crest. Blood pressure was recorded after 5 min of rest, from the right arm while in a sitting position. A valid automatic Omron M6 Blood Pressure Monitor (Omron Healthcare Europe BV) was used. The measurement was taken twice with a 2-min interval between readings. A third measurement was taken if there was a difference of over 10 mmHg between the previous two measurements. The average value of the measurements taken was used. Systolic and diastolic blood pressures were recorded.
Furthermore, one well-trained and experienced paediatrician in each prefecture determined pubertal maturation (Tanner stage) after thorough visual inspection of breast development in girls and genital development in boys(Reference Tanner15).
Biochemical indices
Blood samples were obtained for biochemical and haematological screening tests between 08·30 and 10·30 after a 12-h overnight fast. Reminders were distributed the previous day to both parents and children to ensure compliance with fasting. Professional staff performed venipuncture to obtain a maximum of 23 ml blood. Part of the blood was collected in test tubes with no added anticoagulant, where it was allowed to clot for approximately 2 h, as this was designated for serum separation. Clotted blood was centrifuged at 3000 rpm for 15 min, and the collected serum was divided into aliquots and stored at −80 °C. All serum samples were transported in dry ice to the Laboratory of Nutrition and Clinical Dietetics at Harokopio University, where central storage of back-up samples at –80 °C took place.
Total cholesterol, HDL-cholesterol and TAG were determined in duplicate using commercially available enzymatic colorimetric assays (Roche Diagnostics SA) on an automated analyser (Roche/Hitachi Modular). LDL-cholesterol was calculated by the Friedewald equation(Reference Friedewald, Levy and Fredrickson16).
Plasma glucose was determined using commercially available enzymatic colorimetric assays (Roche Diagnostics SA). Serum insulin was determined by a Chemiluminescence immunoassay (Kyowa Medex Ltd, for Siemens Diagnostics USA). IR was measured through homoeostasis model assessment (HOMA-IR)(Reference Matthews, Hosker and Rudenski17). This index was calculated using fasting glucose and fasting insulin as follows: HOMA-IR = (fasting insulin (μunits/ml) x fasting glucose (mmol/l)/22·5. HOMA-IR > 3·16(Reference Keskin, Kurtoglu and Kendirci18) was used as a cut-off point to define insulin-resistant schoolchildren. HOMA-IR has been validated as a surrogate measure of IR in non-diabetic children, with studies showing correlations as high as 0·91 with the hyperinsulinemic-euglycemic clamp, a frequently sampled intravenous glucose tolerance test(Reference Conwell, Trost and Brown19).
MetS was defined using the criteria proposed by the International Diabetes Federation for children and adolescents. Children with MetS had abdominal obesity (WC ≥ 90th percentile by age and sex) and the presence of two or more of the following clinical features: elevated TAG (≥ 1·7 mmol/L); low HDL-cholesterol (<1·03 mmol/L); high blood pressure (systolic blood pressure ≥ 130 mm Hg and/or diastolic blood pressure ≥ 85 mm Hg and/or diagnosis of hypertension) and elevated plasma glucose (≥ 5·6 mmol/L and/or diagnosis of type 2 diabetes)(Reference Zimmet, George and Alberti20).
Socio-economic and other information collected from the parents
Data on the socio-economic background of the families having at least one child participating in the study were collected from the parents (most preferably from the mother) during scheduled face-to-face interviews at school. For those parents not able to attend the meetings (approximately 5 % of the total sample), data were collected via telephone interviews. All interviews were conducted by research team members who were rigorously trained to minimise interviewer’s effect by using a standardised questionnaire. More specifically, the data collected by primary caregivers included demographic characteristics such as parent’s age and nationality; socio-economic characteristics such as educational level (years of education) and mean annual family income over the last 3 years (€/year). Lifestyle characteristics such as smoking habits and anthropometric characteristics such as mother’s height and weight were also collected.
Statistical analysis
Continuous variables were summarised as mean (sd), while categorical variables were presented as frequencies (n) and percentages (%). The Kolmogorov–Smirnov test was used to examine the normality of the distribution of all continuous variables. Differences of mean values between groups were examined using Students’ t-test (or the non-parametric Mann–Whitney test in the case of non-normally distributed continuous variables), while associations between categorical variables were examined by using the χ 2 test and the two-sample z-test for proportions whenever appropriate. Logistic regression analyses were performed to evaluate the associations between KIDMed score with MetS, MetS components, IR and obesity in children. More specifically, two different models were applied: model 1 – unadjusted and model 2 – adjusted for children’s sex, Tanner stage, urbanisation degree (i.e. urban, semi-urban or rural), maternal educational level, physical activity and dietary daily energy intake. A post hoc power calculation based on available sample size showed that there was more than 80 % statistical power to detect effect sizes in the multivariable logistic regression models. The results of logistic regression models were presented as OR and 95 % CI. The level of statistical significance was set at P < 0·05, while all reported P-values were two-tailed. All statistical analyses were conducted using the Statistical Package for Social Sciences (SPSS) software for Windows (version 24.0, SPSS Inc).
Results
Table 1 presents the main descriptive characteristics of the study population. The total number of children with full data, included in the current statistical analysis, was 1972, with approximately 50 % of children being girls. The mean age of the study participants was 11·1 (0·6) years, with the vast majority of children (65·2 %) living in urban areas. Regarding sex differences, dietary energy intake and physical activity levels were significantly higher in boys compared with girls (P < 0·001). In addition, BMI, WC, serum LDL-cholesterol and plasma glucose levels were also higher in boys compared with girls (P < 0·05), while girls had higher levels of serum TAG, serum insulin, HOMA-IR and diastolic blood pressure compared with boys (P < 0·001). A similar pattern was also observed for the level of biological maturation, since the majority of girls were categorised at higher Tanner stages compared with boys (P < 0·001). When comparisons between poor and medium and high adherence to MedDiet were carried out, the results indicated that there was a higher energy intake with better compliance to MedDiet (P < 0·001), while higher adherers to the MedDiet were more physically active (P < 0·001). They also had significantly lower BMI and WC and more favourable lipid profiles (Table 1).
Table 1 Descriptive characteristics of the examined population (total sample and by sex)

* P-values were derived from Student’s t-test in the case of normally distributed continues variables and the χ 2 test in the case of categorical variables.
† P-value was derived from the non-parametric Mann–Whitney test. MVPA = -moderate-to-vigorous intensity physical activity; HOMA-IR = homoeostatic model assessment insulin resistance.
Figure 1 depicts the percentages of children, in the total sample and by sex, with ‘poor’, ‘medium’ and ‘high’ adherence to the MedDiet based on the KIDMED score. According to these percentages, 64·8 %, 34·3 % and 1 % of children were found to have ‘poor’, ‘medium’ and ‘high’ adherence to the MedDiet, respectively. As there was such a low frequency of ‘high’ adherence to the MedDiet medium and high adherence groups were pooled together for analyses. Relevant percentages, as in the total sample, were also observed for boys and girls, with no statistically significant differences between sexes.

Fig. 1 Percentages of children with ‘poor’, ‘medium’ and ‘high’ adherence to the Mediterranean diet, based on the KIDMED score in the total sample and by sex. ![]() , Total sample;
, Total sample; ![]() , boys;
, boys; ![]() , girls
, girls
Table 2 summarises the prevalence of the MetS and its individual components for the total sample, by sex and by adherence to the MedDiet. The overall prevalence of the MetS was 3·4 %, while regarding its individual components, 15·8 % of children were found to have central obesity, 1·7 % hypertriglyceridaemia, 7·3 % low HDL-cholesterol concentrations, 17·7 % hyperglycaemia, 24·8 % systolic hypertension and 7·4 % diastolic hypertension. In addition, 28·8 % and 11·6 % of children were insulin resistant and obese, respectively. Regarding sex differences, a higher percentage of girls compared with boys had low HDL-cholesterol concentrations (8·8 % v. 5·9 %, P = 0·013), diastolic hypertension (8·9 % v. 5·9 %, P = 0·011) and IR (35·1 % v. 22·5 %, P < 0·001). On the contrary, a higher percentage of boys were hyperglycaemic (22·7 % v. 12·6 %, P < 0·001) and obese (13·6 % v. 9·5 %, P = 0·004) compared with girls. Regarding differences between children with different adherence to the MedDiet, the percentages of children with central obesity (17·5 % v. 12·5 %, P = 0·003), hypertriglyceridaemia (2·2 % v. 0·7 %, P = 0·015) and IR (30·8 % v. 25·1 %, P = 0·008) were higher in those with poor compared with those with medium or high adherence. Furthermore, children with medium/high adherence to the MedDiet had a higher maternal educational level (13·0 (sd 3·1) v. 12·4 (sd 3·3) years, P < 0·001), energy intake (1932·7 (sd 578·1) v. 1726·4 (sd 523·6) kcal/day, P < 0·001) and time spent doing moderate-to-vigorous physical activities (71·4 (sd 61·9) v. 62·5 (sd 60·4) min/day, P = 0·002), compared with children with poor adherence to the MedDiet. In addition, a higher percentage of children with medium/high adherence to the MedDiet were living in urban areas, compared with their peers with poor adherence to the MedDiet (74·0 % v. 60·4 %, P < 0·05). On the contrary, a lower percentage of children with medium/high adherence to the MedDiet were living in rural areas, compared with children with poor adherence to the MedDiet (11·7 % v. 24·3 %, P < 0·05) (data not shown in Tables).
Table 2 Prevalence of metabolic syndrome (MetS), MetS components, insulin resistance and obesity in the total sample, by sex and by adherence to the mediterranean diet
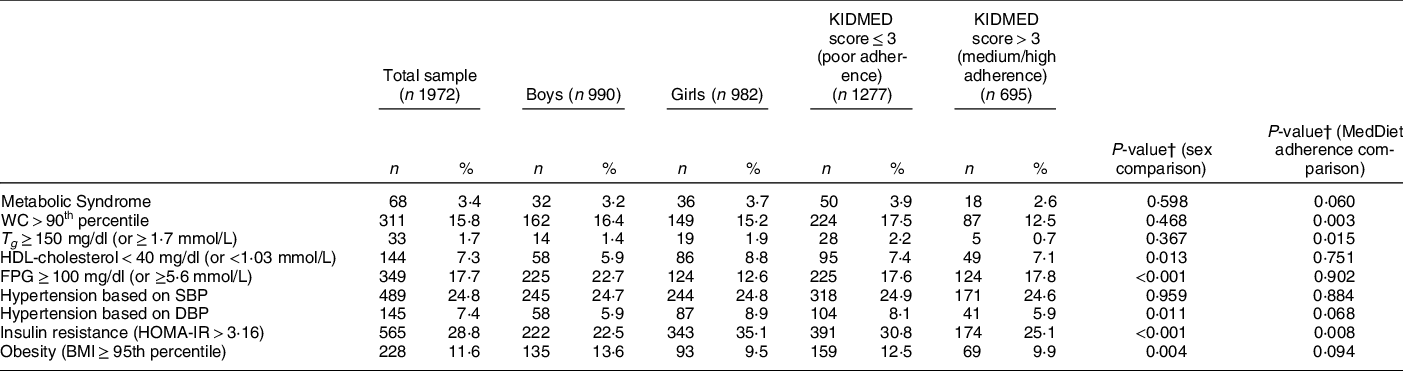
WC, waist circumference; T g , TAG; FPG, fasting plasma glucose; SBP, systolic blood pressure; DBP, diastolic blood pressure; HOMA-IR, homoeostatic model assessment insulin resistance.
† P-values were derived from the χ 2 test in the case of categorical variables.
Table 3 summarises the results from the two logistic regression models that were performed to examine the associations between KIDMed score with MetS, MetS components, IR and obesity in the examined population of children. According to the results derived from model 1 (unadjusted model), ‘poor’ adherence to the MedDiet was found to be associated with an increased likelihood for central obesity (OR 1·49; 95 % CI 1·14, 1·94), hypertriglyceridaemia (OR 3·09; 95 % CI 1·19, 8·05) and IR (OR 1·49; 95 % CI 1·33, 1·64). The significant associations observed in model 1 were also retained in model 2, after adjusting for sex, Tanner stage, physical activity levels and total dietary energy intake, family urbanisation degree and maternal educational level. More specifically in model 2 (adjusted model), ‘poor’ adherence to the MedDiet was found to increase the likelihood for central obesity, hypertriglyceridaemia and IR by 1·31 (95 % CI 1·01, 1·73), 2·80 (95 % CI 1·05, 7·46) and 1·31 (95 % CI 1·05, 1·64) times, respectively.
Table 3 Likelihood of metabolic syndrome (MetS), increased levels of MetS components, insulin resistance and obesity in Greek children (n 1972) with poor adherence to the Mediterranean diet pattern (KIDMED score ≤ 3)*

WC, waist circumference; T g , TAG; FPG, fasting plasma glucose; SBP, systolic blood pressure; DBP, diastolic blood pressure; HOMA-IR, homoeostatic model assessment insulin resistance.
* Cells in bold indicate statistically significant OR.
† Adjusted for sex and Tanner Stage (stages 1-5), maternal educational level (years), urbanisation level (rural, semi urban and rural), time spent to - - (min/d) and total dietary daily energy intake (kcal).
Discussion
The aim of the present study was to determine adherence to the MedDiet using the KIDMed score, in 9–13-year-old school children in Greece, and to evaluate the association between MedDiet adherence and obesity, IR, MetS and its individual components. Based on the findings from this study, adherence to MedDiet in this population was poor and was associated with an increased likelihood of central obesity, hypertriglyceridaemia and IR.
In this study, we focused on paediatric populations where there are currently little data assessing the effect of a MedDiet and how this impacts metabolic outcomes(Reference Idelson, Scalfi and Valerio21). An increased prevalence of obesity, and subsequently MetS in paediatric populations(Reference Kumar and Kelly1), is known to result in elevated future risk of CVD. Therefore, it is important that modifiable risk factors such as diet are explored to determine if the incidence and outcomes related to MetS can be mediated to reduce the risk of CVD. Subsequently, this may help to reduce related morbidity and mortality in adulthood. Whilst there is extensive literature to support the efficacy of the MedDiet in adults for the prevention and management of MetS(Reference Esposito and Giugliano22), the evidence is scarce in paediatric populations. The MedDiet has been shown to efficiently decrease occurrence of MetS(Reference Godos, Zappalà and Bernardini23), specifically in children and adolescents and an inverse association between adherence to the MedDiet and obesity has also been reported(Reference Fornari and Maffeis24). Given the extensive protective role of MedDiet extended upon from the results from this study, further investigation of the MedDiet within children is warranted.
To determine the level of children’s adherence to the MedDiet, the present study used the KIDMed score, which is the most widely used index in studies conducted with children and adolescents(Reference Idelson, Scalfi and Valerio21). The results from this study indicated that approximately two-thirds of children had ‘poor’ adherence to the MedDiet, while only 1 % of children had ‘high’ adherence. These results demonstrate that despite Greece being located within the Mediterranean basin, dietary intake has become Westernised, a notion that is in line with other literature reporting low adherence to the MedDiet in Greek children(Reference Idelson, Scalfi and Valerio21,Reference Farajian, Risvas and Karasouli25,Reference Kontogianni, Vidra and Farmaki26) . When comparing these results to other paediatric cohorts in the Mediterranean region, the levels of adherence to the MedDiet were lower than those reported in a study for Cypriot children, where only 6·7 % of children had ‘high’ adherence to the MedDiet, while 37 % were found to have ‘poor’ adherence(Reference Lazarou, Panagiotakos and Kouta27) A systematic literature review on children and adolescents, which included thirty-eight studies that used KIDMED score to assess adherence to the MedDiet(Reference Idelson, Scalfi and Valerio21), indicated that adherence to the MedDiet varied across Mediterranean countries(Reference Farajian, Risvas and Karasouli7) More specifically, the review indicated that across the whole population, ‘poor’ adherence was lowest in Spanish children (1·6 %)(Reference Mariscal-Arcas, Rivas and Velasco28) and highest in Greek adolescents (62·8 %)(Reference Magriplis, Farajian and Pounis29), ‘moderate’ adherence was highest in rural Italian adolescents (73·8 %)(Reference Grosso, Marventano and Buscemi30) and lowest in Greek adolescents (28·0 %)(Reference Tsartsali, Thompson and Jago31), while ‘high’ adherence was lowest in Greek adolescents (4·3 %)(Reference Farajian, Risvas and Karasouli7) and highest in Spanish children(Reference Pérez, Bayona and Mingo32). These findings confirm the results reported by the present study, highlighting that approximately two-thirds of children in Greece have a ‘poor’ adherence to the MedDiet.
Large epidemiological data from the 1999 to 2002 NHANES database, a US representative sample within a comparable demographic, indicated a prevalence of MetS in paediatric populations ranging from 2 to 9 % depending on the diagnostic criteria used(Reference Cook, Auinger and Li33). The rates of MetS reported within the present study are not dissimilar to that of the NHANES, despite the difference in locality with 3·4 % of children are diagnosed with the MetS, thus falling within the range reported in a seemingly Westernised obesogenic population. Data on MetS in previous studies have demonstrated a higher prevalence in boys compared with girls, although there was no significant difference between sexes from the results herein(Reference Friend, Craig and Turner34). However, what was alarming was a large number of children, despite not meeting the criteria for the MetS who had established MetS components. Rates of central adiposity and IR were particularly high (almost 16 % and 30 %, respectively) in the total sample, and obesity and hyperglycaemia were significantly higher in boys. Furthermore, our findings showed that IR was higher in girls compared with boys, which was expected considering the earlier biological maturation (reflected by higher Tanner Stage scores) observed in girls, which occurs during this life stage of preadolescence(Reference Nelson and Bremer35). The prevalence of IR observed in the present study is notably higher compared with the rates reported in a systematic literature review, which showed that IR (based on HOMA-IR) in children ranged from 2 to 20 %(Reference Matthews, Hosker and Rudenski17,Reference Lee, Okumura and Davis36) . These findings are concerning given that IR and subsequent hyperinsulineamia have been proposed as the underlying mechanisms driving MetS, as well as other diseases which relate to obesity and/or the MetS, including polycystic ovary syndrome in females, non-alcoholic fatty liver disease, obstructive sleep apnoea and certain types of cancer(Reference Reaven37).
There was an overall low prevalence of MetS (3·4 %) in this paediatric population which makes sense given that MetS is not an actual clinical condition in children and adolescents, thus the focus by paediatric health professionals is mainly given to the individual components of MetS (i.e. central obesity, dyslipidaemia, hyperglycaemia and hypertension). In fact that there is no consensus for the definition of the MetS in paediatric populations to date. In this cohort of children, however, there was poor adherence to the MedDiet and high rates of MetS components. The rates of unfavourable cardiometabolic risk factors were higher in children with poor adherence to the MedDiet compared with children with ‘moderate’ and ‘high’ adherence. The MedDiet is characterised by a high intake of plant-based foods featuring vegetables, fruits, wholegrains, legumes, nuts, seeds and extra virgin olive oil. Smaller amounts of fish, white meat and fermented dairy are also included with red meat and sweets consumed least often. This high fibre diet is low in saturated fat and high in monounsaturated fat, predominantly attributable to the extra virgin olive oil, which is the main culinary fat(Reference George, Kucianski and Mayr38). It is hypothesised that the phytonutrient rich, plant-based foods and high amounts of unsaturated fats included in this diet help mediate blood lipids, glycaemic control, while the anti-inflammatory and antioxidant properties of the MedDiet reduce inflammation and oxidation that also contribute to the MetS(Reference George, Kucianski and Mayr38,Reference Esposito, Ciotola and Giugliano39) . This has been demonstrated extensively in adult populations(Reference Sofi, Abbate and Gensini40), but the present study is one of few studies reporting this potential relationship in children. In this study, poor adherence to the MedDiet was associated with central adiposity and thus we hypothesise that the MedDiet may be satiating(Reference Carbonneau, Royer and Richard41). This has previously been recognised due to the palatability(Reference George, Kucianski and Mayr38) and high healthy fat composition, and as such, a higher level of adherence to the diet may regulate energy balance and thus regulate weight status.
Although there is some evidence examining the relationship between childhood obesity with adherence to the MedDiet, there are limited studies reporting associations between adherence to the MedDiet with central adiposity, IR and components of the MetS. A systematic review assessing children and adolescents included ten studies that reported significant negative associations between the adherence to the MedDiet and BMI or obesity status(Reference Idelson, Scalfi and Valerio21). Only two of these studies provided evidence of a negative association between MedDiet adherence and WC in children, thus confirming the findings observed in the present study(Reference Schröder, Mendez and Ribas-Barba42,Reference Lydakis, Stefanaki and Stefanaki43) . However, most of the relevant literature includes observational studies, while only two prospective studies examined associations between MedDiet adherence with BMI or WC in children, but they did not report any significant findings. The only known randomised intervention study was conducted in Mexico(Reference Velázquez-López, Santiago-Díaz and Nava-Hernández44) and reported a positive effect of the MedDiet on children’s BMI. Insulin resistance was also assessed in this trial, and their results indicated that MedDiet was effective at reducing fasting glucose(Reference Giannini, Diesse and D’Adamo45). Children in this study also improved their TGA levels(Reference Manios, Kourlaba and Grammatikaki46) which is consistent with the negative association between adherence to the MedDiet and hypertriglyceridaemia observed in our study findings. In another intervention study in a paediatric population after 12 months, total and LDL-cholesterol levels improved, but there was no change in the serum concentrations of TGA(Reference Manios, Moschonis and Grammatikaki47). Therefore, additional prospective and intervention studies are required to examine the potential cardioprotective effect of the MedDiet on the cardiometabolic profile in children and adolescents, to determine if there is consistency with the positive relationships observed in adults as part of intervention studies.
Strengths and limitations
The strengths of this study lie in the large epidemiological data set provided from the Healthy Growth Study, a representative sample of children from four counties within the wider region of Greece. However, there are a number of limitations associated with the KIDMed score, these include that the score was inspired by instruments developed for adults and there are no specific guidelines for the Mediterranean Diet in non-adult populations. Furthermore, while some analysis has shown that the KIDMed score is positively associated with key nutrients (Ca and vitamin C(Reference Kontogianni, Vidra and Farmaki26)), additional assessment of the validity of the score with biological outcomes is warranted. KIDMed score was calculated by combining data that were collected using information extracted on FFQ and 3 × 24 h recalls (2 week days and one week end), this represents a limitation of this study posing potential self-reporting bias, although it worth highlighting that the KIDMed score has been calculated this way in many other studies(Reference Idelson, Scalfi and Valerio21). In addition, the FFQ and food recalls are based on self-reported dietary intake and pose a potential cause of study bias. However the FFQ has not been validated but was designed to assess children’s habitual intake of the main and most popular foods and beverages consumed in this age group, while it has been repeatedly used in the past in other published work from the Healthy Growth Study, indicating reasonable and strong associations between children’s food habits and clinical and biochemical markers of nutritional status(Reference Manios, Kourlaba and Grammatikaki46-Reference Manios, Moschonis and Papandreou48). One additional limitation of our study also stems from the use of a high cut-off for defining hypertriglyceridaemia (T g >150 mg/dl or ≥ 1·7 mmol/L), since this may have resulted in an overestimation of the OR in the association of hypertriglyceridaemia with the KIDMED score. However, as the aim of the present study was to examine the associations between adherence to the MedDiet and the components of the MetS in children, the use of this specific cut-off for T g could not be avoided. Another limiting factor of this study lies predominantly in its cross-sectional nature, therefore causality cannot be inferred, and this study is also potentially susceptible to reverse causality bias. Lastly, as this study is observational, there is the potential for residual confounding by unmeasured variables that needs to be considered.
In conclusion, the present study was one of the very few to investigate adherence to the MedDiet and associations with obesity, IR, MetS and its components in a paediatric population. The results showed that approximately two-thirds of the examined population of Greek schoolchildren have ‘poor’ adherence to the MedDiet, which also leads to a higher likelihood for central obesity, hypertriglyceridaemia and IR. Additional prospective or intervention trials are needed to infer whether there is causal association between MedDiet and MetS components. If this relationship is confirmed, identifying strategies to increase adherence to the MedDiet among children in Greece may be an effective approach to tackle the cardiometabolic complications reflected in the components of the MetS in this population.
Acknowledgements
Acknowledgements: The authors would also like to thank the ‘Healthy Growth Study’ group for the valuable contribution to the completion of the study. Financial support: The Healthy Growth Study and specifically GM was co-financed by the European Union (European Social Fund – ESF) and Greek national funds through the Operational Program ‘Education and Lifelong Learning’ of the National Strategic Reference Framework (NSRF) – Research Funding Program: Heracleitus II. Investing in knowledge society through the European Social Fund. The funders had no role in the design, analysis or writing of this article. Conflict of interest: The authors declare that they have no conflict of interest. Authorship: G.M. and Y.M. were responsible for the conception, design and monitoring of the study, as well as for data acquisition. G.M., Y.M., C.M. and E.K. were responsible for data acquisition. G.M. conducted the statistical analyses of the data. E.G. and G.M. drafted the manuscript. All authors substantially contributed to the interpretation of the results, the writing, the critical review and approval of the final version of the manuscript for publication. Ethics of human subject participation: Approval to conduct the study was granted by the Greek Ministry of National Education and the Ethics Committee of Harokopio University of Athens. Written informed consent was obtained from guardians of all child participants. ‘This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the Harokopio University Ethics Committee. Written informed consent was obtained from all subjects/patients’.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980021001701







