Gestational weight gain (GWG) has been reported to be essential for pregnancy outcomes. Excessive GWG increases the risks of maternal and neonatal complications, including gestational diabetes mellitus (GDM), hypertensive disorders, caesarean section, post-partum weight retention, macrosomia, large for gestational age (LGA) infants and offspring overweight/obesity(Reference Kominiarek and Peaceman1–Reference Takmaz, Yalvac and Ozcan5). Inadequate GWG is linked with increased risks of small for gestational age (SGA) newborns, low birth weight (LBW) infants, preterm delivery and failure to initiate breast-feeding(Reference Kominiarek and Peaceman1,Reference Rogozinska, Zamora and Marlin4) .
In recent years, there are an increasing number of overweight and obesity in China and other developing Asian countries(Reference Xiao, Ding and Vinturache6). The prevalence of overweight(Reference Li, Liu and Zhang7,Reference Li, Liu and Guo8) and obesity(Reference Huang, Xiao and Hu9,Reference Fang, Zhao and Ju10) among Chinese reproductive-aged women was 10–24 % and 2·6–9·2 %, respectively. On the other hand, almost 9·0 % women in China were underweight, whereas it was only 2·0 % in the USA(Reference Chu, Bachman and Callaghan11). Evidence has shown that women with higher pre-pregnancy BMI were more likely to have a series of adverse maternal and neonatal outcomes, such as macrosomia, LGA, caesarean section, GDM and gestational hypertension(Reference Du, Ge and Zhou12–Reference Abenhaim, Kinch and Morin17). In contrast, pregnant women with lower BMI were at increased risk for LBW, SGA, intrauterine growth-restricted infants and preterm delivery(Reference Du, Ge and Zhou12,Reference Hung and Hsieh13,Reference Abenhaim, Kinch and Morin17) .
So far, there is no international consensus on the recommendation about GWG. The United States Institute of Medicine (IOM) revised new GWG guidelines according to the WHO BMI categories in 2009, considering the incidences and risks of several potential outcomes such as fetal growth, preterm birth, caesarean section, and maternal and offspring obesity(18). Due to the lack of official GWG recommendation for Chinese pregnant women, IOM 2009 GWG guideline was also used in China(19). However, the IOM guidelines are based mainly on Caucasian women, which may limit its generalisability to other populations. Moreover, the WHO BMI criterion may not be directly applicable to the Chinese populations, owing to racial difference in body composition and dietary habits between Chinese and Western populations. It has been reported that Asian women were more likely to have a lower BMI and a smaller GWG than those in Europe and North America(Reference Yazdani, Yosofniyapasha and Nasab20). Given the variations in race ethnicity, diet and other factors between Chinese and Western populations, the appropriateness of using the IOM 2009 GWG recommendations for Chinese women should be examined.
Studies conducted in Hong Kong(Reference Wong, Tang and Lau21) and New York(Reference Bracero and Byrne22) calculated proper GWG among pregnant women with good pregnancy outcomes. More studies conducted in different countries and regions(Reference Hirooka-Nakama, Enomoto and Sakamaki23–Reference Sunsaneevithayakul, Titapant and Ruangvutilert32) suggested optimal GWG with the lowest risk of various adverse pregnancy outcomes. However, to date, no study has been reported in China to create the optimal GWG considering multiple maternal and neonatal outcomes. Therefore, the purpose of this multicentre prospective study is to create the optimal GWG using a quantitative approach to estimate the total predicted probability of the composite maternal and neonatal outcomes. We aimed to define GWG ranges for Chinese women according to Chinese-specific BMI classification and to examine whether the proposed GWG ranges can improve pregnancy outcomes in comparison with IOM 2009 GWG guidelines.
Materials and methods
Study populations
This prospective study was conducted at hospitals of nine cities in mainland China, including Beijing, Wuhan, Chengdu, Shenzhen, Dongguan, Harbin, Shijiazhuang, Qingdao and Danyang, from April 2013 to December 2014. Pregnant women were considered eligible if they had regular prenatal care till delivery and maintained complete data during the study period. Inclusion criteria were as follows: women with Chinese nationality, aged 20–35 years, had a living singleton pregnancy and < 12 weeks pregnant. The subjects were excluded if they had a situation with pre-existing diabetes mellitus or hypertension, endocrine disease and other complications. Women with multiple pregnancy or with insufficient information about their height, pre-pregnancy weight and gestational weight were also excluded. A total of 3806 women were recruited. Due to the extreme small number of obese women (n 75), we restricted our analysis to underweight (n 821), normal weight (n 2503) and overweight women (n 407). Consequently, a total of 3731 women were included for the analysis (Fig. 1).
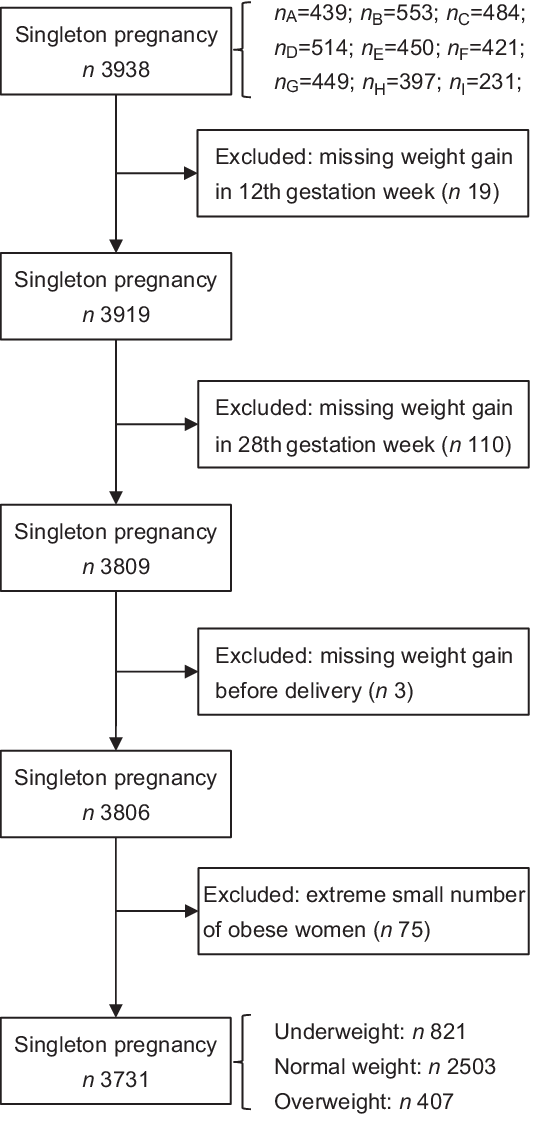
Fig. 1 Study cohort flow chart. Letters A–I represented nine cities in mainland China (A, Beijing; B, Shijiazhuang; C, Chengdu; D, Wuhan; E, Dongguan; F, Shenzhen; G, Harbin; H, Danyang; I, Qingdao)
Data collection
The baseline data were collected by trained interviewers through face-to-face interviews. A structured questionnaire was used to collect information on socio-demographic characteristics (e.g., maternal age, educational level, occupation and family income), pre-pregnancy body weight and self-reported height, lifestyle (e.g., regular and passive smoking, alcohol drinking, insomnia and physical activity), menstrual and reproductive history and history of diseases. Information on physical activity was obtained by asking subjects about their exercise frequency and classified as never, occasionally, sometimes and frequently. Insomnia disorder was defined as difficulties in initiating or maintaining sleep.
Anthropometric measurement
The trained staffs measured the participant’s weight with uniform standards at the first prenatal visit, the 12th gestational week, the 28th gestational week and prior to delivery. Pre-pregnancy BMI (kg/m2) was calculated by dividing self-reported pre-pregnancy weight in kg by the square of the height in metres. According to the ‘Guidelines for prevention and control of overweight and obesity in China’(Reference Chen and Lu33), maternal pre-pregnancy BMI was categorised as underweight (<18·5 kg/m2), normal weight (18·5–23·9 kg/m2), overweight (24·0–27·9 kg/m2) and obesity (≥ 28·0 kg/m2). Maternal GWG was calculated by subtracting the maternal pre-pregnancy weight from the last measured weight prior to delivery.
Outcomes of interest
Information on maternal outcomes (including gestational hypertension, GDM and mode of delivery) and the neonatal outcomes (including birth weight and gestational age at delivery) was obtained from the medical records. Gestational age was determined from last menstrual period which was defined by ultrasound assessments. Preterm delivery was defined as delivery occurring before 37 completed weeks of gestation(Reference Huang, Ji and Zhao34). Neonates with their birth weight < 2500 g, between 2500 and 4000 g, equal to or greater than 4000 g were classified as LBW, normal birth weight and macrosomia, respectively(Reference Yang, Peng and Wei35). SGA and LGA neonates were defined as birth weights below the 10th percentile for gestational age and above the 90th percentile, respectively(Reference Zhu, Zhang and Zhang36). Gestational hypertension was diagnosed when the pregnant woman had a blood pressure > 140/90 mmHg after 20th gestational week(Reference Lo, Mission and Caughey37). GDM was diagnosed using the International Association of Diabetes in Pregnancy Study Groups criteria(Reference Metzger, Gabbe and Persson38). The diagnosis of GDM was made when any of the following plasma glucose values are exceeded: fasting blood glucose ≥ 5·1 mmol/l, plasma glucose levels at 60 min ≥10·0 mmol/l or at 120 min ≥8·5 mmol/l following a 75 g oral glucose tolerance test at 24–28 gestational weeks. The mode of delivery was categorised as caesarean section and vaginal delivery.
Statistical analysis
The comparisons of characteristics among subjects were conducted based on pre-pregnancy BMI groups. Continuous variables were expressed as mean values and standard deviations and evaluated using ANOVA test. Categorical variables were expressed as numbers (%, percentage) and evaluated by χ 2 test.
Macrosomia, LBW, LGA, SGA, preterm delivery, caesarean section, GDM and gestational hypertension were chosen as the important pregnancy outcomes which are known to be affected by GWG. Logistic regression models were used to obtain the predicted probabilities(Reference Beyerlein, Schiessl and Lack25) of the pregnancy outcomes relating to GWG in different pre-pregnancy BMI groups. The total predicted probabilities in different BMI groups were estimated by combining the predicted probabilities of pregnancy outcomes. To determine the optimal GWG value in different pre-pregnancy BMI groups, quadratic function model was used to improve the fitness of the relationship between GWG and the total predicted probability, as reported by a previous study(Reference Morisaki, Nagata and Jwa24). The optimal GWG range was defined as the range that did not exceed a 1 % increase from the lowest predicted probability(Reference Morisaki, Nagata and Jwa24).
The IOM 2009 GWG guidelines recommended 12·5–18 kg for underweight (pre-pregnancy BMI < 18·5 kg/m2), 11·5–16·0 kg for normal weight (pre-pregnancy BMI 18·5–24·9 kg/m2), 7·0–11·5 kg for overweight (pre-pregnancy BMI 25–29·9 kg/m2) and 5–9·1 kg for obese (pre-pregnancy BMI ≥ 30·0 kg/m2) pregnant women classified by WHO BMI categories(Reference Robillard, Dekker and Boukerrou39). According to the IOM 2009 GWG guidelines and our proposed recommendations, excessive GWG group was defined as GWG above the upper range of the recommendations, whereas inadequate GWG group was defined as GWG below the lower range of the recommendations. All other women were classified as having adequate GWG.
OR and 95 % CI of the associations between pre-pregnancy BMI groups, GWG groups and pregnancy outcomes were calculated using multivariable unconditional logistic regression models. Confounding factors, selected based on the literatures and the comparison between pre-pregnancy BMI groups, included maternal age (continuous), parity (primiparous or multiparous), gestational age of delivery (for macrosomia and LBW), family history of diabetes (for GDM) or family history of hypertension (for gestational hypertension). Moreover, the comparison between the association of pregnancy outcomes with GWG categorised by our proposed recommendations and IOM 2009 GWG guidelines was performed using logistic analyses. Women with adequate GWG judged by our proposed recommendations or IOM 2009 GWG guidelines were separately served as the reference group. Positive and negative predictive values, sensitivity, specificity and AUC for detecting adverse pregnancy outcomes were calculated for our proposed recommendations and IOM 2009 GWG guidelines.
All P values are two sided, and P values of <0·05 were considered as statistically significant. Statistical analyses were performed using SPSS 25.0.
Results
The maternal and neonatal characteristics in different pre-pregnancy BMI groups are shown in Table 1. The majority of pregnant women had a normal weight (67·1 %), 22·0 % were underweight and 10·9 % were overweight. Overweight women had less GWG than underweight and normal weight women. More overweight women had a family history of diabetes or hypertension than underweight women. The proportion of primiparous women decreased with higher pre-pregnancy BMI. The prevalence of gestational hypertension, GDM, caesarean section, LGA and macrosomia increased with higher pre-pregnancy BMI. The prevalence of SGA decreased with increasing pre-pregnancy BMI. No significant differences were observed between the three groups in terms of educational level, occupation, family income, alcohol drinking, active and passive smoking, insomnia situation, physical activity and LBW (P > 0·05).
Table 1 Characteristics of the studied population according to Chinese-specific BMI categories (n 3731)

GWG, gestational weight gain.
* Continuous variables were evaluated using ANOVA test and categorical variables were evaluated by χ 2 test.
The association between pre-pregnancy BMI and maternal and neonatal outcomes is shown in Table 2. Compared with normal weight women, overweight women had an increased risk of macrosomia, LGA, preterm delivery, caesarean section, GDM and gestational hypertension, with adjusted OR of 2·07 (95 % CI 1·51, 2·84), 1·77 (95 % CI 1·34, 2·35), 1·92 (95 % CI 1·22, 3·03), 1·36 (95 % CI 1·10, 1·69), 1·40 (95 % CI 1·05, 1·88) and 1·87 (95 % CI 1·10, 3·17), respectively. By contrast, underweight women had a significantly lower risk of macrosomia (OR 0·46; 95 % CI 0·31, 0·68), LGA (OR 0·53; 95 % CI 0·38, 0·73), caesarean section (OR 0·79; 95 % CI 0·67, 0·93) and GDM (OR 0·72; 95 % CI 0·54, 0·96) compared with normal weight women.
Table 2 OR and 95 % CI of the association between pre-pregnancy BMI and maternal and neonatal outcomes (n 3731)
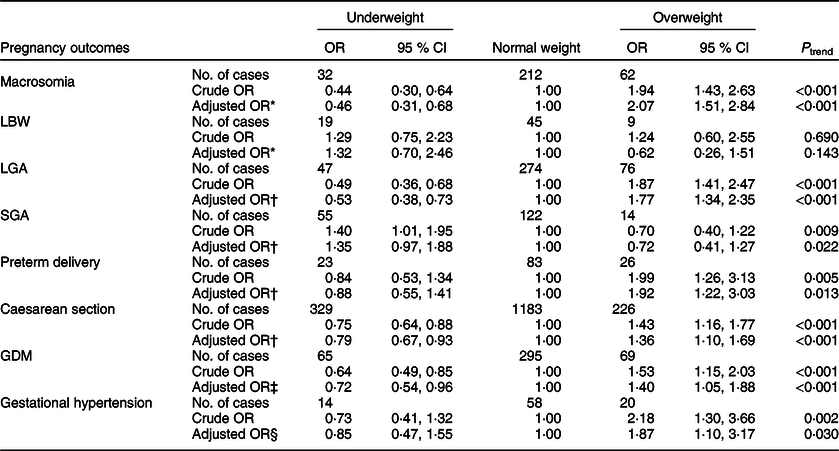
LBW, low birth weight; LGA, large for gestational age; SGA, small for gestational age; GDM, gestational diabetes mellitus.
* Adjusted for maternal age, parity and gestational age.
† Adjusted for maternal age and parity.
‡ Adjusted for maternal age, parity and family history of diabetes.
§ Adjusted for maternal age, parity and family history of hypertension.
Figure 2 shows the associations between GWG and predicted probability of maternal and neonatal outcomes in different pre-pregnancy BMI groups. Higher GWG was associated with a lower risk of LBW, SGA, preterm delivery and GDM, whereas higher GWG was associated with a higher risk of macrosomia, LGA, caesarean section and gestational hypertension.

Fig. 2 Predicted probabilities of macrosomia (![]() ), low birth weight (
), low birth weight (![]() ), preterm delivery (
), preterm delivery (![]() ), small for gestational age (
), small for gestational age (![]() ), large for gestational age (
), large for gestational age (![]() ), caesarean section (
), caesarean section (![]() ), gestational diabetes mellitus (
), gestational diabetes mellitus (![]() ) and gestational hypertension (
) and gestational hypertension (![]() ) with increasing gestational weight gain, stratified by pre-pregnancy BMI (underweight, n 821; normal weight, n 2503; overweight, n 407)
) with increasing gestational weight gain, stratified by pre-pregnancy BMI (underweight, n 821; normal weight, n 2503; overweight, n 407)
The total predicted probability of composite maternal and neonatal outcomes in relation to GWG in quadratic function model stratified by pre-pregnancy BMI categories is shown in Table 3 and Fig. 3. The predicted probability of composite adverse outcomes showed a U-shaped curve with increasing GWG for all BMI groups. The total predicted probability of composite adverse outcomes was lowest at 15·0, 14·2 and 12·6 kg, which were defined as optimal GWG values for underweight, normal weight and overweight women, respectively. The optimal GWG ranges, defined as an increased risk < 1 % compared with optimal GWG, were 12·8–17·1 kg for underweight pregnant women, 12·1–16·4 kg for normal weight pregnant women and 10·4–14·9 kg for overweight pregnant women, respectively.
Table 3 The quadratic function models and the optimal GWG corresponding to the lowest total predicted probability of combining adverse pregnancy outcomes, according to pre-pregnancy Chinese-specific BMI categories

GWG, gestational weight gain; Y, total predicted probability; x, GWG.
* The lower and upper margins of the GWG range represented that total predicted risk of composite adverse outcomes (macrosomia, LBW, LGA, SGA, preterm delivery, caesarean section, GDM and gestational hypertension) did not exceed a 1 % increase from the lowest total predicted probability.

Fig. 3 Total predicted probability of adverse pregnancy outcomes (including gestational hypertension, gestational diabetes mellitus, caesarean section, macrosomia, low birth weight, preterm delivery, small for gestational age and large for gestational age) by gestational weight gain, stratified by pre-pregnancy BMI (![]() , underweight, n 821, BMI < 18·5;
, underweight, n 821, BMI < 18·5; ![]() , normal weight, n 2503, BMI 18·5–23·9;
, normal weight, n 2503, BMI 18·5–23·9; ![]() , overweight, n 407, BMI 24·0–27·9)
, overweight, n 407, BMI 24·0–27·9)
Table 4 shows the results of the associations between multiple maternal and neonatal outcomes and GWG categorised based on our proposed recommendations and IOM 2009 GWG guidelines. Compared with pregnant women with adequate GWG judged by our proposed recommendations, excessive GWG was positively associated with the risk of macrosomia, LGA and caesarean section, with OR of 2·44 (95 % CI 1·82, 3·27), 2·04 (95 % CI 1·60, 2·62) and 1·43 (95 % CI 1·24, 1·66), respectively, whereas inadequate GWG was associated with higher risks for LBW, SGA and preterm delivery, with OR of 2·66 (95 % CI 1·34, 5·26), 2·02 (95 % CI 1·40, 2·93) and 1·56 (95 % CI 1·04, 2·34), respectively. The corresponding OR in comparison with pregnant women with adequate GWG judged by the IOM 2009 GWG guidelines were 2·63 (95 % CI 1·93, 3·60) for macrosomia, 2·26 (95 % CI 1·74, 2·93) for LGA, 1·35 (95 % CI 1·17, 1·56) for caesarean section, 2·64 (95 % CI 1·31, 5·33) for LBW, 2·03 (95 % CI 1·38, 2·96) for SGA and 1·61 (95 % CI 1·02, 2·53) for preterm delivery, respectively.
Table 4 Comparison between the association of pregnancy outcomes with GWG categorised by our proposed recommendations and IOM 2009 GWG guidelines (n 3731)

LBW, low birth weight; LGA, large for gestational age infant; SGA, small for gestational age infant; GDM, gestational diabetes mellitus.
* Adjusted for maternal age, parity, gestational age and pre-pregnancy BMI.
† Adjusted for maternal age, parity and pre-pregnancy BMI.
‡ Adjusted for maternal age, parity, family history of diabetes and pre-pregnancy BMI.
§ Adjusted for maternal age, parity, family history of hypertension and pre-pregnancy BMI.
Our proposed recommendations exhibited similar predictive values for adverse pregnancy outcomes to those of the IOM 2009 GWG guidelines. All of the AUC values of different pregnancy outcomes predicted by our cut-off value were > 0·50 (AUC ranged from 0·542 to 0·635, all P < 0·05) (Table 5).
Table 5 Predictive values of our proposed recommendations and IOM guidelines for the adverse pregnancy outcomes

IOM, Institute of Medicine; PPV, positive predictive value; NPV, negative predictive value; LBW, low birth weight; LGA, large for gestational age infant; SGA, small for gestational age infant; GDM, gestational diabetes mellitus.
Discussion
The current study examined the proper GWG for each pre-pregnancy BMI category according to Chinese-specific BMI classification. The results showed that inappropriate GWG was associated with higher risk of several maternal and neonatal outcomes. The optimal GWG (ranges) proposed in the present study was 15·0 kg (12·8–17·1) for underweight pregnant women, 14·2 kg (12·1–16·4) for normal weight pregnant women and 12·6 kg (10·4–14·9) for overweight pregnant women. The comparison between our proposed recommendations and IOM 2009 GWG guidelines showed that these two recommendations were comparable in predicting adverse pregnancy outcomes.
The distribution of pre-pregnancy BMI categories in the present study was consistent with the result of another multicentre study, with 18·9 % in the underweight and 11·1 % in the overweight, respectively(Reference Huang, Xiao and Hu9). Pre-pregnancy BMI was an independent predictor of pregnancy outcomes. In accordance with most of the previous studies(Reference Du, Ge and Zhou12–Reference Ferreira, Piccinato and Cordioli16), our study showed that a significantly higher proportion of overweight women experienced adverse maternal and neonatal outcomes, such as macrosomia, LGA, caesarean section, GDM and gestational hypertension, whereas the risk of SGA decreased with increasing pre-pregnancy BMI. No significant difference was noted in the risk of LBW in relation to pre-pregnancy BMI. This might be due to the small proportion of LBW in the subgroup population.
The GWG recommendations varied by national origin. One study including 2702 Korean women reported that the optimal GWG was considerably higher and wider than recommended by IOM 2009 GWG(Reference Choi, Lee and Kim28). However, the optimal GWG among Japanese pregnant women was narrower than that of the IOM 2009 GWG(Reference Morisaki, Nagata and Jwa24). In the present study, the suggested GWG defined for underweight and normal weight women was generally similar to IOM 2009 GWG guidelines, although it was a little narrower than that of the IOM 2009 guidelines. However, the suggested GWG defined for overweight women was considerably higher than that of the IOM 2009 GWG guidelines. In the current study, we could not determine the appropriate GWG for obese women due to the small sample size. The intrinsic ethnic, population and regional differences among Chinese women and other populations might have a potential effect on the weight gain profile.
So far, no consensus has been reached on the method of defining optimal GWG among pregnant women. Optimal GWG has been examined in some epidemiological studies, of which varied in statistical methods, selected adverse maternal and infant outcomes(Reference Bracero and Byrne22,Reference Beyerlein, Schiessl and Lack25,Reference Cedergren26,Reference Oken, Kleinman and Belfort31,Reference Langford, Joshu and Chang40–Reference Hutcheon and Bodnar43) . Recommendations made in Hong Kong(Reference Wong, Tang and Lau21) and New York(Reference Bracero and Byrne22) were derived from women with good pregnancy outcomes. However, this might be not reliable for the general population owing to the exclusion of women and infants with any adverse pregnancy outcome. Some studies(Reference Beyerlein, Schiessl and Lack25,Reference Cedergren26,Reference Sunsaneevithayakul, Titapant and Ruangvutilert32,Reference Robillard, Dekker and Boukerrou39,Reference Mestrovic, Roje and Vulic44) highlighted the importance of fetal growth to determine the GWG. But more studies(Reference Morisaki, Nagata and Jwa24,Reference Chen, Xie and Vuong27–Reference Ee, Allen and Malhotra29,Reference Voerman and Santos45) emphasised the importance of both maternal and neonatal pregnancy outcomes. The IOM 2009 GWG guidelines were established according to the lowest prevalence of the commonly pregnancy outcomes from various large studies(18). A meta-analysis of twenty-five cohort studies from Europe and North America examined optimal GWG ranges with consideration of composite adverse outcomes like preeclampsia, gestational hypertension, gestational diabetes, caesarean delivery, preterm birth, SGA and LGA(Reference Voerman and Santos45). In the present study, a quantitative approach was used to estimate the total predicted probability of the composite maternal and neonatal outcomes, including macrosomia, LBW, LGA, SGA, preterm delivery, caesarean section, GDM and gestational hypertension.
It has been suggested that different countries need to have their own BMI classification criteria based on their own morbidity and mortality data(Reference Choo46). Previous studies creating GWG are based on different pre-pregnancy BMI. The IOM 2009 GWG guidelines used WHO BMI categories to classify pregnant women to underweight (BMI < 18·5), normal weight (18·5 ≤ BMI < 24·9), overweight (25·0 ≤ BMI < 29·9) and obese (BMI ≥ 30·0) women. The pre-pregnancy BMI used in the Korean population was classified according to Asia-specific standards from the WHO as follows: underweight (BMI < 18·5), normal weight (18·5 ≤ BMI < 23), overweight (23 ≤ BMI < 25) and obese women (BMI ≥ 25)(Reference Choi, Lee and Kim28). One study in Japan was based on five pre-pregnancy BMI subgroups (17·0–18·4, 18·5–19·9, 20–22·9, 23–24·9 and 25–27·4 kg/m2)(Reference Morisaki, Nagata and Jwa24). Another study also conducted in Japan creating optimal GWG only included overweight and obese pregnant women with a BMI ≥ 25 kg/m2(Reference Hirooka-Nakama, Enomoto and Sakamaki23). Recent research claimed that Chinese people have an elevated risk for obesity-related diseases at a lower BMI than Caucasians(Reference Zhou47). The Chinese-specific BMI standard is more recommended for Chinese owing to its lower cut-off points for BMI categories(Reference Wang, Mi and Shan48) and better sensitivity and specificity for identifying risk factors of diseases(Reference Zhou47). Thus, in the present study, the Chinese-specific BMI criteria rather than WHO BMI international or Asian BMI categories were used.
Our study showed that excessive GWG is significantly associated with an increased risk of macrosomia, LGA and caesarean section, whereas inadequate GWG is significantly associated with an increased risk of LBW, SGA, preterm delivery and GDM. Consistent with our results, a meta-analysis published in 2017 concluded that inappropriate GWG was associated with higher risk of adverse pregnancy outcomes, including macrosomia, LGA, SGA, preterm birth and caesarean delivery(Reference Goldstein, Abell and Ranasinha49). Although the observation of an inverse association between GWG and the risk of GDM was consistent with several studies(Reference Yang, Han and Gao15,Reference Cho, Hur and Lee50–Reference MacDonald, Bodnar and Himes52) , it was contrary to our primary hypothesis. One plausible explanation was that pregnant women tend to adopt weight management once being diagnosed with GDM within 24–28 weeks(Reference Yang, Han and Gao15), leading to decreased weight gain in late pregnancy.
The key point of the GWG targets study is whether the new GWG targets can improve pregnancy outcomes, compared with the IOM recommendation. The results of the comparison showed that inappropriate GWG judged by our proposed recommendations was associated with higher risk of macrosomia, LBW, LGA, SGA, preterm delivery and caesarean section, which was consistent with those judged by the IOM 2009 GWG guidelines. It seems logical that our proposed GWG recommendation is comparable with the IOM 2009 GWG guidelines and both of these two recommendations are suitable for Chinese pregnant women. Moreover, all of the AUC was above 0·50. This indicated that the GWG cut-offs proposed in the present study could well predict the occurrence of pregnancy outcomes such as macrosomia, LBW, LGA, SGA, preterm delivery, caesarean section and GDM.
To our knowledge, this is the first multicentre study restricted to Chinese population to estimate optimal GWG in Chinese pregnant women by applying predicted probability of a composite adverse pregnancy outcomes. Data were precisely and prospectively collected from a nationally representative sample across multiple cities in China, which enables us to popularise the results of the current study to some extent. Some limitations need to be addressed. First, the pre-pregnancy weight was self-reported and recall bias was inevitable. Second, the current study only focused on several adverse pregnancy outcomes but ignored some rare pregnancy outcomes such as still birth, neonatal death and congenital malformation. Third, we could not determine the appropriate GWG for obese women due to the small sample size. Further studies with a larger sample size are needed to create GWG recommendation for obese women. Fourth, all maternal and neonatal outcomes were weighted equally in the present study. However, some other specific adverse outcomes such as preterm delivery and SGA would be more serious than others like LGA.
In conclusion, inappropriate GWG was associated with several unfavourable pregnancy outcomes than an adequate GWG. The optimal GWG value (ranges) for the lowest predicted probability of combining adverse pregnancy outcomes was 15·0 kg (12·8–17·1) for underweight pregnant women, 14·2 kg (12·1–16·4) for normal weight pregnant women and 12·6 kg (10·4–14·9) for overweight pregnant women.
Acknowledgements
Acknowledgements: The authors would like to thank the study participants’ participation, without them the study would not have been possible. Financial support: The study was supported by Maternal and Child Nutrition Branch of Chinese Nutrition Society. The funders had no role in the design, analysis or writing of this article. Conflict of interest: There are no conflicts of interest. Authorship: The authors’ contributions are as follows: C.X.Z.: conceptualisation (equal), formal analysis (lead), investigation (equal), writing – original draft (lead), writing – review and editing (lead); J.Q.L.: conceptualisation (equal), funding acquisition (equal), methodology (equal), resources (lead), supervision (equal), writing – review and editing (equal); K.Y.L.: formal analysis (lead), methodology (equal), visualisation (equal), writing – original draft (lead); N.H.Y.: conceptualisation (equal), investigation (equal), writing – review and editing (equal); G.Z.: conceptualisation (equal), investigation (equal), writing – review and editing (equal); L.M.M.: conceptualisation (equal), investigation (equal); Z.N.L.: conceptualisation (equal), investigation (equal); Y.T.: conceptualisation (equal), investigation (equal); W.X.: conceptualisation (equal), investigation (equal); N.D.: conceptualisation (equal), investigation (equal); Z.X.W.: conceptualisation (equal), funding acquisition (equal), investigation (equal), project administration (equal), supervision (equal), writing – review and editing (equal); Y.X.S.: conceptualisation (lead), funding acquisition (lead), project administration (lead), resources (equal), supervision (lead), writing – review and editing (equal). Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the Biomedical Ethical Committee of Chinese Nutrition Society (CNS-2012-002). Written informed consent was obtained from all subjects.











