With breast-feeding rarely being used for children after 1 year of age, breast milk substitutes are required to ensure the nutritional requirements of young children. In this regard, many child health professionals, not to mention many parents, consider cows’ milk (CM) adequate and believe that a diet based on CM provides all nutritional requirements with the exception of Fe and vitamin D. For some 20 years now, an alternative to CM has been available. The so-called ‘growing-up milks’ (GUM) are not defined in any European Regulation or Directive, or in any Codex Alimentarius standard. GUM are intended for children after 1 year of age (up to 3 years of age in many European countries). They have a lower protein content than CM and are supplemented with several nutrients of interest including Fe and essential fatty acids (EFA), as well as vitamins D and E. Directive 2009/39/EC of the European Parliament and of the Council of 6 May 2009 classifies these products as a ‘foodstuff intended for particular nutritional uses’ and states that ‘the composition of the products shall be such that the products are appropriate to the nutritional use intended’(1). However, although the commercialization of GUM continues to increase in many countries worldwide, particularly in Europe, their benefits are still a matter of debate. This controversy arises because the possible nutritional risks associated with the use of CM and the expected benefits from the use of GUM have not been clearly demonstrated. To gain further information on this important issue in paediatric nutrition, individual dietary data from a food consumption survey performed in France were retrospectively analysed to estimate the nutritional adequacy of the diets of children aged 1–2 years as a function of their milk intake, i.e. CM or GUM.
The objectives of the present study were to answer the following two questions:
1. Does the use of CM in young children aged 1–2 years represent a risk of inadequate coverage of the nutritional requirements currently defined in France for this population?
2. If CM does not adequately meet these nutritional requirements, should GUM be used in preference by this population?
Experimental methods
Study sample
A cross-sectional food consumption survey involving 713 children aged from 15 d to 36 months was performed by the Department of Physiology of the University of Burgundy, Dijon, France. The results of this survey, sponsored by the Syndicat français des aliments de l'enfance (French Association of Baby Food Industries), were published in 2008(Reference Fantino and Gourmet2). According to French legislation, this type of nutritional survey does not need to be approved by an institutional review board. For the purpose of the present study, only data from 132 children aged 12–24 months (seventy-one boys) were taken into account. They were all full-term babies with birth weights of over 2500 g. At the time of the study, none of the children was breast-fed. The parents did not receive specific recommendations prior to the study, which reflects the daily food consumption of the children according to their parents’ choices. Two groups were defined according to their type of milk intake. Group CM comprised the sixty-three children who received a daily intake of CM of at least 250 ml (70 % as semi-skimmed milk) and who did not receive GUM or follow-on formula (FOF) or dairy products based on GUM or FOF (Fig. 1). Group GUM comprised the fifty-five children who received a daily intake of GUM of at least 250 ml (Fig. 1). This minimal value of 250 ml/d for milk consumption was retained a priori since it corresponds to one daily bottle consumption.

Fig. 1 Flow chart representing how Group CM and Group GUM were constituted (CM, cows’ milk; GUM, growing-up milk; FOF, follow-on formula)
Data acquisition
Parents participating in the survey were recruited from all regions of France (excluding overseas territories) by the polling organization TNS/SOFRES®, according to a proportional sampling technique that took into account the population of each region, the age of the children, the professional status of the mother and the socio-economic level of the family. The distribution was adjusted to that of the general French population as defined by INSEE (Institut National de la Statistique et des Etudes Economiques/National Institute of Statistics and Economic Studies) on the basis of the 2002 census. None of the families in the study population was defined as economically precarious. With the consent of both parents, the usual caregiver of the child in the family environment was asked to record in the diary provided all foods and beverages ingested by the child over three consecutive days. The study period usually included one weekend day. The caregiver was instructed to note the time of each meal, the weight (or volume) of all the ingested foods and a precise description of them, including trade names, together with the methods of their preparation and detailed recipes for all home prepared foods. All foods and beverages were weighed on kitchen scales (accuracy ±1 g), measured by mass or volume from the information on the packaging, or estimated from photographs of calibrated portions especially prepared for this purpose. Quantitative dietary records, together with the weights of any leftovers, were recorded in the diaries by the parents, then verified and if necessary clarified by especially trained researchers recruited by TNS/SOFRES.
Analysis of food dairies
The nutritional analysis was performed using tables that listed the compositions of 1260 foodstuffs in current use in home cooking in France (‘current foods’) and of all food items especially manufactured and intended for infants and young children (less than 3 years of age) available on the market in France at the time of the data acquisition (850 ‘baby foods’). The nutritional analysis of the ‘current foods’ was performed using a composition table provided by AFSSA (Agence Française de Sécurité Sanitaire des Aliments/French Food Safety Agency) and another composition table that was used for the SU.VI.MAX study (a French study on supplementation in vitamins and antioxidant minerals)(3). The compositions of very few foods (less than ten) could not be determined and these were therefore deduced from the mean compositions of similar products. The compositions of all ‘baby foods’ were provided by the manufacturers. Dietary intake was calculated for each child with the especially developed software NUTRI 7®. Each diary was encoded and verified after data entry. Energy and nutrient intakes were calculated for each child by summing his/her reported consumption over the 3 d of the study. The child's intake was reduced to a daily rate. These data were then aggregated for all children in each study group (Group CM and Group GUM). Besides the total energy intake, the nutrients considered were: protein, lipids, total carbohydrates (excluding fibre), EFA (linoleic acid and α-linolenic acid), Na, Ca, P, Mg, Zn, Fe, vitamins B1, B2, B3, B5, B6, B9, B12, C, D (exclusively of food origin, referred to hereafter as ‘alimentary vitamin D’), E (expressed as α-tocopherol equivalents), total vitamin A (expressed as retinol equivalents), retinol and carotenoids (expressed as β-carotene equivalents). In France, children aged 1 to 2 years are frequently prescribed vitamin D and fluoride, rarely Fe and multivitamin supplements. These supplementary intakes were not taken into account in the current study because of the unreliable reporting of therapeutic compliance.
Analytical methods
The means, errors and standard deviations of the daily energy and nutrient intakes were calculated for the two groups. They were compared with the French Recommended Daily Allowances (RDA), Estimated Average Requirements (EAR) and Adequate Intakes (AI)(Reference Beaufrère, Briend and Ghisolfi4). Because the weight of each infant was reported by the person filling out the diary and was not measured as part of the study, the daily intake was not calculated per unit body weight. The sex of the children was not considered. To express the variation in the intake of each nutrient, the first and third quartiles of intake are presented (with the 95 % confidence intervals). The daily energy and nutrient intakes of the study groups were compared using Student's t test, and differences were considered significant at P < 0·05. The proportion of children with inadequate dietary intake in each group was evaluated by estimating the percentage of individuals whose daily intake was less than the EAR (equivalent to 0·77 of the RDA) for the nutrient under consideration(5–Reference De Lauzon, Volatier and Martin7). However, because there are no RDA for EFA, the lower limit of the AI(5) was used for these nutrients (linoleic acid and α-linolenic acid). The 95 % confidence intervals for these threshold values are indicated. To estimate the contribution to the global nutritional intake of various food groups, five categories of ‘current foods’ were distinguished: (i) milk; (ii) other dairy products; (iii) meat, fish and eggs; (iv) other foods (mainly cereals, fruits and vegetables); and (v) vegetable fats. Similarly, for foods specifically intended for infants younger than 3 years, three categories were defined: (i) GUM and GUM/FOF-based dairy products; (ii) meat and fish; and (iii) other ‘baby foods’. The contribution of these food categories to each nutrient intake was expressed as daily amounts. The effect of variations in daily milk consumption on the intake of eight nutrients of interest (protein, Fe, linoleic acid, α-linolenic acid, retinol, vitamin C, vitamin E and alimentary vitamin D) was evaluated by establishing a relationship between, on the one hand, either the daily consumption of CM and CM-based products or the daily consumption of GUM and GUM-based products (including FOF) and, on the other, the total daily intake of each of these eight nutrients, using the Pearson correlation coefficient. Statistical analysis was performed with the software Number Cruncher Statistical System version 2000 for Windows.
Results
Group CM
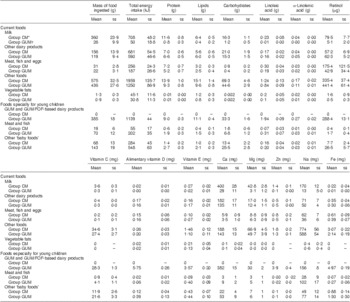
Mean age of the sixty-three children included in Group CM was 606 (sd 13) d (19·9 (sd 0·4) months). Their diet was characterized by (Tables 1 and 2): (i) a protein intake well above the French RDA(5); (ii) a low lipid intake; (iii) intakes under the lower limit of the French AI for linoleic acid and α-linolenic acid, and under the RDA for Fe, Zn, vitamin C, alimentary vitamin D and vitamin E. The large variations in energy and nutrient intakes were reflected in the large standard deviations and the values for the first and third quartiles (Table 1 and Fig. 2). These variations were associated primarily with the consumption of foods other than milk and dairy products (Table 3). A high proportion of these children consumed less than the lower limit of the AI for linoleic acid (51 %) and α-linolenic acid (84 %). Their daily intake was less than the EAR for Fe (59 %), Zn (56 %), alimentary vitamin D (100 %), vitamin E (94 %) and vitamin C (49 %; Table 2). Whereas CM and CM-based products represented 43 % by weight of their total food consumption and 35 % of their daily energy intake, they represented 45 % of their daily intake of protein, 21 % of Na, 17 % of linoleic acid, 25 % of α-linolenic acid, 11 % of Fe, 46 % of Zn, 8 % of vitamin C, 16 % of vitamin E and 24 % of alimentary vitamin D (Table 3). This population consumed few non-dairy manufactured foodstuffs specifically designed for children aged 1–3 years, which contributed minimally (less than 10 %) to their total nutritional intake, except for Fe (18 %), vitamin A (16 %), alimentary vitamin D (19 %), vitamin E (19 %) and vitamin C (25 %; Table 3). There was a significant relationship between the daily consumption of CM and CM-based products and daily protein intake (P < 0·001). No relationship was found between the daily consumption of CM and CM-based products and linoleic acid, α-linolenic acid, Fe, retinol, vitamin C, alimentary vitamin D or vitamin E (Fig. 2).
Table 1 Energy and nutrient daily intake distributions of French children aged 12–24 months consuming either CM or GUM

CM, cows’ milk; GUM, growing-up milk; TEI, total energy intake.
a15% of TEI; b40% of TEI; c55% of TEI; dlower limit of AI: 2% of TEI; elower limit of AI: 0·4% of TEI (from Beaufrère et al. (Reference Beaufrère, Briend and Ghisolfi4)).
*AI: Adequate Intake (from Beaufrère et al. (Reference Beaufrère, Briend and Ghisolfi4)).
†RDA: French Recommended Daily Allowance (from Beaufrère et al. (Reference Beaufrère, Briend and Ghisolfi4)).
‡Group CM: children aged 12–24 months who received a daily intake of CM of at least 250 ml and who did not receive GUM or follow-on formula (FOF) or dairy products based on GUM or FOF (n 63).
§Group GUM: children aged 12–24 months who received a daily intake of GUM of at least 250 ml (n 55).
Table 2 Percentage of individuals whose daily intakes were less than the AI or less than 0·77 of the RDA among French children aged 12–24 months consuming either CM or GUM

CM, cows’ milk; GUM, growing-up milk.
*AI or RDA: Adequate Intake calculated as the recommended contribution of the nutrient to total energy intake or Recommended Daily Allowance (from Beaufrère et al. (Reference Beaufrère, Briend and Ghisolfi4)).
†AI or 77% of the French RDA.
‡Group CM: children aged 12–24 months who received a daily intake of CM of at least 250 ml and who did not receive GUM or follow-on formula (FOF) or dairy products based on GUM or FOF (n 63).
§Group GUM: children aged 12–24 months who received a daily intake of GUM of at least 250 ml (n 55).
∥Lower limit of AI: 2% of observed total energy intake.
¶Lower limit of AI: 0·4% of observed total energy intake.

Fig. 2 Effects of daily variation of milk product intake on nutritional daily intake of eight nutrients of interest (iron, protein, linoleic acid, α-linolenic acid, retinol, vitamin C, vitamin E, alimentary vitamin D) among French children aged 12–24 months. ![]() $$$$, Daily nutritional intake as a function of milk consumption by sixty-three children who received at least 250 ml of cows’ milk (CM) daily, and no growing-up milk (GUM) or follow-on formula (FOF), or dairy products based on GUM or FOF (Group CM);
$$$$, Daily nutritional intake as a function of milk consumption by sixty-three children who received at least 250 ml of cows’ milk (CM) daily, and no growing-up milk (GUM) or follow-on formula (FOF), or dairy products based on GUM or FOF (Group CM); ![]() $$$$, daily nutritional intake as a function of milk consumption by fifty-five children who received at least 250 ml of GUM daily (Group GUM)
$$$$, daily nutritional intake as a function of milk consumption by fifty-five children who received at least 250 ml of GUM daily (Group GUM)
Table 3 Contribution of various food groups to daily nutritional intake among French children aged 12–24 months consuming either CM or GUM
CM, cows’ milk; GUM, growing-up milk; FOF, follow-on formula.
*Group CM: children aged 12–24 months who received a daily intake of CM of at least 250 ml and who did not receive GUM or follow-on formula (FOF) or dairy products based on GUM or FOF (n 63).
†Group GUM: children aged 12–24 months who received a daily intake of GUM of at least 250 ml (n 55).
Group GUM
Mean age of the fifty-five children included in Group GUM was significantly lower than that of Group CM at 534 (sd 15) d (17·5 (sd 0·5) months; P = 0·003). Their diet did not differ significantly from that of Group CM in terms of the total mass of food, energy, carbohydrates, lipids, Na, Ca, P and Mg (Table 1). Their diet was also characterized by a high protein intake, 35·5 (sd 7·3) g/d, of which an average of 5·2 g was in the form of ‘current foods’ (meat, eggs and fish) and 16·4 g in the form of milk products, which was nevertheless lower than that of Group CM (Table 3). Their lipid intake was low. Their intakes of EFA, Fe, Zn, vitamins B3, B6, B9, C and E, total vitamin A, alimentary vitamin D and β-carotene were significantly higher (P < 0·001) than those of group CM (Table 1). Their consumption of GUM and GUM/FOF-based milk products represented 27 % of their total daily intake of protein, 51 % of linoleic acid, 52 % of α-linolenic acid, 96 % of Fe, 85 % of Zn, 26 % of total vitamin A, 74 % of retinol, 35 % of vitamin C, 58 % of vitamin E and 85 % of alimentary vitamin D. The contribution of non-dairy manufactured foodstuffs specifically designed for children aged 1–3 years to their total nutritional intake was minimal (less than 10 %), except for linoleic acid (13 %), α-linolenic acid (12 %), Fe (17 %), total vitamin A (24 %), vitamin E (17 %) and vitamin C (31 %; Table 3). As in Group CM, the variations in their energy and nutrient intakes were pronounced, particularly for non-dairy products (Table 3 and Fig. 2). Compared with Group CM, a far smaller percentage of children in this group had a daily intake of α-linoleic acid below the lower limit of the AI (26 %) or a daily intake less than the EAR for Zn (33 %), vitamin C (11 %), alimentary vitamin D (75 %) and vitamin E (15 %; Table 2). There was a significant relationship between their daily consumption of GUM and their daily intake of Fe (P < 0·001), linoleic acid and α-linolenic acid (P < 0·001), vitamin E (P < 0·001), vitamin C (P < 0·05) and alimentary vitamin D (P < 0·02; Fig. 2). No relationship was found between the daily consumption of GUM and protein or retinol intake.
Discussion
Based on the expected effects of the composition of CM and GUM, it is commonly believed that feeding a young child with CM can lead to a high protein intake and a deficient intake of linoleic and α-linolenic acids, Fe, Zn and most vitamins. This analysis is not supported by convincing research data arising from plasma measurements of nutrients of interest, with the notable exceptions of Fe(Reference Male, Persson and Freeman8) and vitamin D(Reference Gordon, Feldman and Sinclair9, Reference Misra, Pacaud and Petryk10). Energy and macronutrient consumption was identical in the two groups in the present study with the exception of protein, for which the consumption was higher in Group CM, cows’ milk being one possible but not exclusive causative factor. The main nutritional differences between the two groups concerned EFA, some trace elements and vitamins. In Group CM, consumption of EFA, Fe, Zn, vitamin C, alimentary vitamin D and vitamin E was below the French RDA or AI. As expected, given compositional differences between CM and GUM, consumption of these nutrients was higher in Group GUM and adequate as compared with the RDA or AI, except for alimentary vitamin D. Before concluding that these dietary inadequacies are attributable to the use of CM, it is also important to consider the role of the consumption of foods other than milk and dairy products. Our study shows that those foods represent a daily intake at least equivalent in protein and higher in EFA, Fe, Zn and many vitamins than that of a diet consisting solely of CM, and that their consumption is not sufficient to reach AI and RDA for the nutrients under consideration (Table 3). A food consumption survey performed in the USA in 2001 concerning 998 children aged 1–2 years reached similar conclusions. Eighty-five per cent of the study population was fed CM and 15 % was fed formula. A high percentage of these children had intakes of protein, Zn and vitamin A above the RDA, and low intakes of fat, Fe and vitamin E(Reference Devaney, Ziegler and Pac11). These results and the results from the present study do not allow us to conclude that the health of these infants is at risk because of a dietary insufficiency in one or more nutrients. For any individual, an intake less than the EAR (or the lower limit of the AI for EFA) does not mean per se that that individual is effectively lacking input, especially because there are great variations in individual needs and in different food items.
Reports from food consumption surveys have well-known limitations: lack of homogeneity among the study populations, approximate reporting of dietary intakes, imprecision in food composition tables, and failure to take into account the bioavailability of nutrients in relation to diet and physiological status(Reference Bender12–Reference Aggett, Bresson and Haschke14). Long-term epidemiological studies with recurrent blood sampling would be the gold standard but are very hard to deal with due to ethical considerations, high financial burden and the large number of children lost to follow-up. Paediatric EAR and RDA are not generally supported by indisputable data, whether expressed in relation to the nutritional qualities of breast milk or evaluated by factorial methods with biomarkers or by extrapolation from defined adult dietary needs secondarily adapted to the height and weight of children(Reference Allen13). At the present time, Fe and vitamin D are the only nutrients for which the determination of requirements is based on reliable data. For EFA, Zn and vitamins C and E, it is not possible to infer that diets really constitute a risk for deficiencies in these nutrients, given our very poor knowledge of children's actual needs(Reference Aggett, Bresson and Haschke14–Reference Azaïs-Braesco, Bruckert and Durier16). Taking into account the above-mentioned methodological drawbacks(Reference Carriquiry and Camano-Garcia17, 18), the only nutrients for which there is nowadays a higher risk of deficiency in relation to the consumption of CM are α-linolenic acid, Fe, vitamin C and vitamin D.
The increased risk of dietary insufficiency in several nutrients in a population of children aged 1–2 years fed CM is the main scientific justification for proposing modifications to their diet. These may include changing the intake of foods other than milk and dairy products, suggesting nutritional supplements, or recommending specific milk products, i.e. GUM. The modification of children's diets with respect to non-milk foods can be effective. The correction of an insufficiency in EFA by increasing the consumption of vegetable oils, corn, rapeseed or soya (3–5 g/d) is more strongly indicated in these young children whose total lipid intake is less than 35 % of total energy intake. An increased consumption of cereals, vegetables and fresh fruit (particularly citrus fruits) should correct any dietary insufficiency in vitamins, especially vitamin C. Conversely, correction of the dietary insufficiency in Fe by the increased consumption of meat products is not appropriate because it represents an additional daily protein intake of 10 g, leading to a very high total protein intake. The Committee on Nutrition of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) also emphasized that the only nutrients whose needs cannot be met by non-milk foods are Fe and vitamin D(Reference Agostoni, Decsi and Fewtrell19). Dietary supplements, which are very much in use in the USA, even in young children(Reference Briefel, Hanson and Fox20), can contribute to the correction of the insufficiencies observed in the paediatric population. In France, their use in young children, which seems to be increasing, is discouraged by most health professionals because they can lead to excessive micronutrient intake(Reference Briefel, Hanson and Fox20), the consequences of which are unknown. GUM have now been available for about 20 years, their alleged purpose being to avoid the nutritional imbalance that may arise from giving children CM after 10–12 months of age. The present study confirms that the protein intake was lower in children fed GUM than in children fed CM, partly because the protein content of GUM is lower than that of CM, but predominantly because children in Group CM were fed more non-milk foods rich in protein. The present study also indicates that GUM plays a beneficial role by improving the intake of EFA, Fe and vitamins C and D in these children. The ingestion of at least 250 ml GUM/d supplies the RDA levels of the nutrients of interest, notably α-linolenic acid and Fe, for the majority of children, with the exception of vitamin D.
There is no nutritional risk at the levels of use normally recommended for GUM (250–500 ml/d). Devaney et al. have stressed the risk in this population of an excessive intake of Zn and vitamin A(Reference Devaney, Ziegler and Pac11). We saw no evidence of this with respect to Zn in either of our two study groups. However, Allen has reported that an excessive intake of Zn is very difficult to prove, given the lack of knowledge about the risk threshold for this micronutrient(Reference Allen13). In our study, vitamin A intake exceeding the upper recommended limit for infants aged 1–3 years (600 or 800 μg/d depending on the reference source)(21, 22) was observed in infants in Group GUM, but also in Group CM. Such an elevated intake cannot be explained by the retinol content of GUM. It seems to be associated, in a few cases, with an unusual intake of retinol (two children in the study displayed a very high intake of retinol, nearly 3000μg/d, associated with the consumption of calf's liver during the 3 d study period) and above all carotenoids in non-milk foods. The same question can also be raised with respect to linoleic acid, Fe and vitamins B1, B3, and C, the intake of which was also elevated in several children. Carriquiry and Allen have demonstrated that this discussion about the excessive intake of most nutrients is at present fruitless because we lack knowledge of the risk thresholds, there are methodological uncertainties, and food consumption surveys report information derived from disciplined, regular meals, rather than from day-to-day life(Reference Carriquiry6, Reference Allen13).
Conclusion
The results of the present study confirm that the daily consumption of ≥250 ml CM by young children aged 1–2 years partly increases the risk of a high protein intake and significantly increases the risk of an insufficient intake of nutrients of paramount importance, namely α-linolenic acid iron, vitamin C and vitamin D. The daily use of ≥250 ml GUM as a substitute for CM can prevent these nutritional risks, except for vitamin D. Randomized clinical trials comparing plasma levels of Fe, vitamin C, vitamin D and α-linolenic acid in young children fed CM or GUM are needed.
Acknowledgements
The present study received no financial support from any baby food company or from the French Association of Baby Food Industries or any other public or private support. The declaration of interests of the authors is as follows: J.G., none; M.F., Lactalis (funding for the research unit of CREABio); D.T., Danone (funding for the university research lab), Mead Johnson, Nestlé, United Pharmaceuticals (refund for travel expenses or congress expenses); G.P.d.C., none; M.V., none. Author contributions: J.G. wrote the largest part of the paper and performed data analyses; M.F. organized the logistics of the study, reviewed data questionnaires and performed data analyses; D.T. and M.V. performed critical revision of the paper for important intellectual content; G.P.d.C. assisted with interpretation of data.







