Dietary pattern may be a more accurate representation of the dietary exposure of free-living adults and better predict disease risk than single nutrients or foods( Reference Kant 1 – Reference Bhupathiraju and Tucker 4 ). Dietary patterns have traditionally been identified through empirical methods on collected dietary information (e.g. factor and cluster analysis) as well as a priori dietary indices based on dietary recommendations( Reference Hu 2 , Reference Kant 3 ). The Western dietary pattern has been characterized by higher intakes of red or processed meats, butter, high-fat dairy products, eggs, refined grains and sugars, fried potatoes, fast-food items (FFI) and desserts, as well as sugar-sweetened drinks (SSD)( Reference Hu 2 ). While associations between the Western dietary pattern and increased risk of cardiovascular and total mortality are mostly consistent across multiple populations( Reference Zazpe, Sanchez-Tainta and Toledo 5 – Reference Martinez-Gonzalez, Zazpe and Razquin 9 ), associations with cancer mortality are less clear( Reference Kant 3 , Reference Yang, Kenfield and Van Blarigan 8 , Reference Fung, Kashambwa and Sato 10 ). However, the inclusion of food items which comprise this dietary pattern as well as definitions for FFI and SSD often vary across studies, which limits the interpretability of findings.
Given strong associations of both FFI and SSD with risks of obesity( Reference Rosenheck 11 – Reference Alkerwi, Crichton and Hebert 17 ), CVD( Reference Bahadoran, Mirmiran and Golzarand 18 – Reference Cahill, Pan and Chiuve 20 ) (the leading cause of death in the USA( Reference Jemal, Ward and Hao 21 )) and some cancers( Reference Chandran, McCann and Zirpoli 22 , Reference Stott-Miller, Neuhouser and Stanford 23 ), it is possible that intake of FFI and/or SSD may contribute to the increased mortality outcomes associated with Western dietary patterns. Yet, studies which actually link the intake of FFI( Reference Odegaard, Koh and Yuan 24 ) or SSD( Reference Fung, Kashambwa and Sato 10 , Reference Singh, Micha and Khatibzadeh 25 , Reference Fuchs, Sato and Niedzwiecki 26 ) to total or disease-specific mortality outcomes are relatively few( Reference Martinez-Gonzalez, Zazpe and Razquin 9 ). Recent guidelines to reduce fast food and sugared drink consumption as a cancer preventive strategy have been issued( 27 ), despite limited information as to whether this action may impact disease burden. Furthermore, understanding how the intake of FFI and SSD relates to mortality outcomes may be salient to socio-economic and racial disparities given greater availability( Reference James, Arcaya and Parker 28 ) and higher intake of these items within disadvantaged communities( Reference Boone-Heinonen, Gordon-Larsen and Kiefe 29 , Reference Larson and Story 30 ). This information may serve to support systemic efforts to promote healthy nutrition environments to facilitate individual behaviour change as a strategy to reduce obesity-related health disparities( Reference Thow, Downs and Jan 31 ). Therefore the purpose of the current analysis was to determine whether intake of FFI and SSD influences total and disease-specific mortality among a large, well-characterized sample of adults.
Methods
Study cohort
The Vitamins and Lifestyle (VITAL) study is a prospective study designed to determine whether vitamin and mineral supplementation and other lifestyle factors are associated with cancer risk. Men and women between the ages of 50 and 76 years residing in Western Washington State were eligible to participate and names were acquired through purchased mailing lists. Baseline surveys querying supplement use, health history, cancer risk factors, diet and demographic information were completed by 77 718 individuals who were enrolled in the study between 2000 and 2002. Details of the study design and sample characteristics are reported elsewhere( Reference White, Patterson and Kristal 32 ).
Dietary behaviours were assessed via a semi-quantitative FFQ; participants were excluded from the analysis if they reported gastrointestinal malabsorption disorders or gastric bypass surgery (n 45) or if they failed more specific FFQ quality control checks (i.e. did not complete at least five items per page of the FFQ, had an estimated daily energy intake below 3347 kJ (800 kcal) for men or 2510 kJ (600 kcal) for women or above 20 920 kJ (5000 kcal) for men or 16 736 kJ (4000 kcal) for women; n 7178). Finally, participants were also excluded if they died (or were lost to follow-up) within the first year of follow-up (n 913) as the diet of these individuals reported in the last year of their life may have been influenced by their health conditions (i.e. reverse causation) and be less representative of their long-term diet. The final sample included 69 582 individuals. All study protocols were reviewed and approved by the Fred Hutchinson Cancer Research Center Institutional Review Board.
Intake of fast-food items and sugar-sweetened drinks
Diet over the year previous to baseline was assessed using a 120-item semi-quantitative FFQ adapted from the Women’s Health Initiative with special emphasis on the measurement of dietary fats( Reference Patterson, Kristal and Tinker 33 ). Adjustment questions on portion sizes as well as types of foods and preparation techniques (e.g. use of low-fat v. whole-fat foods) were also used. Nutrient values from the Minnesota Nutrition Data System for Research were used in the FFQ analytic program( Reference Schakel, Buzzard and Gebhardt 34 ). FFI were selected a priori, representing foods typically served at fast-food restaurants including: hotdogs, hamburgers, fried chicken, fried fish or fish sandwiches, pizza and French fries( Reference Heidemann, Schulze and Franco 6 , Reference Odegaard, Koh and Yuan 24 ), and were expressed as servings per week. SSD (servings per week) were selected a priori and included servings of: sugar-sweetened soda (not diet), fruit drinks not including juice (e.g. Hi-C, Gatorade, lemonade)( Reference Emond, Patterson and Jardack 35 ) and cranberry juice as it is highly sweetened to be palatable( Reference Wilson, Singh and Vorsa 36 ) and commonly consumed among people of this age who are at higher risk for urinary tract infections( Reference Jepson, Williams and Craig 37 ).
Mortality assessment
There were a total of 4187 deaths that occurred among study participants from one year post-baseline through 31 December 2008. Information on deaths was gathered from Washington State death records (n 4161), the Social Security Death Index (n 22) and the Western Washington Surveillance Epidemiology and End Results cancer registry (n 4) through linkage based on participant identifiers. Cause of death was obtained through the Washington State death records using codes of the International Classification of Diseases, Tenth Revision, but was not available via other reporting sources. Causes of death were categorized as due to CVD (I00–I99) or cancer (C00–D48).
Covariates
Covariates were collected via baseline survey and selected for model inclusion based on a priori associations with total mortality or specific cause of death. Demographic, anthropometric and lifestyle characteristics included in models of total mortality were age and sex as well as race, marital status, education, income, BMI at age 45 years, average yearly change in BMI from age 45 years, alcohol intake (g/d), morbidity score, self-rated health, medication use, hormone therapy use and age at menopause (for women), age at death of parents, average physical activity in 10 years before baseline (MET×h/week, where MET is metabolic equivalents of task), smoking status, history of cancer screening, fruit and vegetable consumption (servings/d) and total daily energy intake for fully adjusted models. The morbidity score was generated by including twenty-five health conditions (listed in the table footnotes) in sex-specific models predicting mortality and then creating a score for each participant based on his/her health conditions. Medication use included non-steroidal anti-inflammatory drugs, aspirin and cholesterol-lowering medication, while hormone therapy use included oestrogen only as well as oestrogen plus progestin preparations (for women). History of cancer screening included separate variables for reported mammogram (for women) and prostrate-specific antigen screening (for men) in the last 2 years, as well as report of sigmoidoscopy in the last 10 years for men and women. A selection of the previously described covariates was included in all models of cause-specific mortality in addition to corresponding cardiovascular or cancer risk factors, as listed in the footnotes of Table 4.
Statistical analysis
We used Cox proportional hazards regression with age as the time metric to estimate risk of mortality associated with consumption of FFI and SSD. Participants were censored at the age they died (n 4187), moved out of Washington State (n 3183), withdrew from the study (n 16) or participated through 31 December 2008 (n 62 196). Participants were divided into quartiles of intake and hazard ratios (HR) were calculated using the lowest quartile of intake as the referent group. Tests for trend were performed by modelling quartiles of intake linearly in a separate Cox model. Separate models were generated for total mortality, deaths due to CVD and deaths due to cancer. Deviation from the proportional hazards assumption was assessed graphically by plotting scaled Schoenfeld residuals v. time and by a statistical test of interaction of the exposure variables with baseline age category (50–59, 60–69, 70–76 years), as age was the time metric for the Cox models. The proportional hazards assumption was not rejected.
We also tested for interaction between exposure variables and socio-economic status (i.e. education and income), morbidity score, sex, as well as baseline age. Interaction terms were generated by multiplying categories of exposure by variable categorizations as presented in Table 1. All analyses were conducted using the statistical software package Stata SE version 13.0.
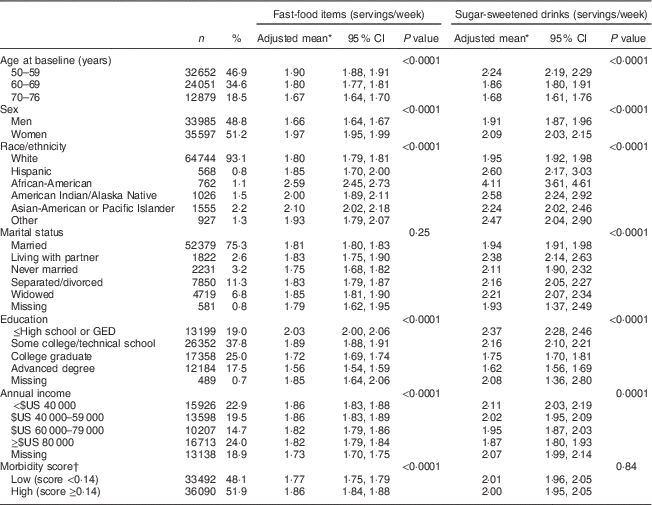
Table 1 Demographic and health characteristics of participants: older adults (n 69 582) enrolled in the Vitamins and Lifestyle (VITAL) study in 2000–2002
GED, General Educational Development.
* Means for each variable adjusted for all variables in the table in addition to BMI at age 45 years (<18·5 kg/m2, 18·5–24·9 kg/m2, 25·0–29·9 kg/m2, ≥30·0 kg/m2, missing), average yearly change in BMI from age 45 years, physical activity in 10 years before baseline (tertiles of MET×h/week, where MET is metabolic equivalents of task), smoking (never, former, current), average alcohol intake (tertiles of g/d), number of servings/d of fruits (quartiles), number of servings/d of vegetables (quartiles) and total daily energy intake (continuous).
† Using Cox regression, the following conditions (yes/no) were modelled simultaneously in sex-specific and age-adjusted models to obtain a continuous morbidity score: current use of medication for depression or anxiety; current use of blood pressure medication; history of cancer of the lung, colon, bladder, pancreas, breast, cervix, uterus, ovaries, and all other sites combined except non-melanoma skin cancer (all as separate variables); IHD (defined as history of heart attack, coronary bypass surgery, angioplasty, or diagnosis of angina); stroke; congestive heart failure; rheumatoid arthritis; diabetes; viral hepatitis; cirrhosis of the liver; other chronic liver disease; emphysema, chronic obstructive pulmonary disease; kidney disease; ulcerative colitis or Crohn’s disease; Parkinson’s disease; and osteoporosis in women.
Results
Table 1 presents demographic characteristics of the study population and their associations with intake of FFI and SSD. Almost all demographic characteristics (each adjusted for other characteristics listed in Table 1) were significantly associated with the number of weekly servings of FFI and SSD reported by individuals. Specifically, individuals who were younger, female, African-American, American Indian or Alaska Native, Asian-American or Pacific Islander, of lower educational attainment, and of lower income were more likely to consume more servings of FFI and SSD per week at baseline (P<0·0001 for all). A total of 4187 (6·02 %) participants died during a mean of 6·9 years of follow-up (8·73 deaths per 1000 person-years) including 1066 deaths due to CVD and 1933 deaths due to cancer.
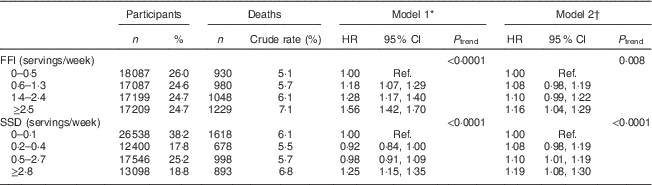
Table 2 gives the risk of subsequent total mortality associated with baseline consumption of FFI and SSD. Higher intake of both FFI and SSD was associated with greater risk of mortality in both minimally and fully adjusted models. Specifically, individuals in the highest quartile of intake for FFI were 56 % more likely to die compared with individuals in the lowest quartile when only age and sex were adjusted for (P<0·0001) and remained 16 % more likely to die (P=0·004) in the fully adjusted model. Similarly, individuals in the highest quartile of intake for SSD were 25 % (P<0·0001) and 19 % more likely to die (P<0·0001) compared with individuals in the lowest quartile in the minimally and fully adjusted models, respectively.
Table 2 Hazard ratios (HR) for total mortality associated with intake of fast-food items (FFI) and sugar-sweetened drinks (SSD) among older adults (n 69 582) enrolled in the Vitamins and Lifestyle (VITAL) study in 2000–2002
Ref., referent category.
* Adjusted for age and sex.
† Model 1 plus race/ethnicity, marital status (married/living together, never married, separated/divorced, widowed), education (≤high school graduate, some college, college/advanced degree), annual income (<$US 40 000, $US 40 000–59 999, $US 60 000–79 999, ≥$US 80 000, missing), BMI at age 45 years (<18·5 kg/m2, 18·5–24·9 kg/m2, 25·0–29·9 kg/m2, ≥30·0 kg/m2, missing), average yearly change in BMI from age 45 years, morbidity score Footnote ‡, self-rated health (excellent, very good, good, fair, poor), current use of cholesterol-lowering medication (yes/no), aspirin use in last 10 years (none, low, high, missing), non-aspirin non-steroidal anti-inflammatory drug use in last 10 years (none, low, high, missing), years of oestrogen therapy (none, <5, 5–9, ≥10), years of oestrogen plus progestin therapy (none, <5, 5–9, ≥10), age at menopause (<40, 40–44, 45–49, 50–54, ≥55 years), age at death of father (<60, 60–69, 70–79, 80–89, ≥90 years), age at death of mother (<60, 60–69, 70–79, 80–89, ≥90 years), average physical activity in 10 years before baseline (tertiles of MET×h/week, where MET is metabolic equivalents of task), smoking status (never, former, current), average alcohol intake (tertiles of g/d), mammogram in past 2 years (yes/no), prostrate-specific antigen test in the last 2 years (yes/no), sigmoidoscopy in the last 10 years (yes/no), number of servings/d of fruits (quartiles), number of servings/d of vegetables (quartiles) and total daily energy intake (continuous).
‡ Using Cox regression, the following conditions (yes/no) were modelled simultaneously in sex-specific and age-adjusted models to obtain morbidity score: current use of medication for depression or anxiety; current use of blood pressure medication; history of cancer of the lung, colon, bladder, pancreas, breast, cervix, uterus, ovaries, and all other sites combined except non-melanoma skin cancer (all as separate variables); IHD (defined as history of heart attack, coronary bypass surgery, angioplasty, or diagnosis of angina); stroke; congestive heart failure; rheumatoid arthritis; diabetes; viral hepatitis; cirrhosis of the liver; other chronic liver disease; emphysema, chronic obstructive pulmonary disease; kidney disease; ulcerative colitis or Crohn’s disease; Parkinson’s disease; and osteoporosis in women.
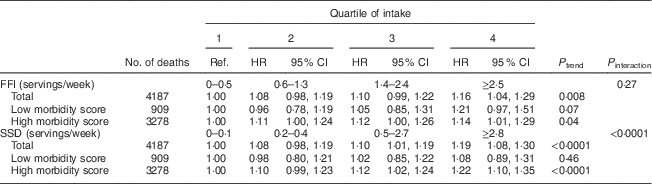
Of the tests for interaction in the association between FFI and SSD and mortality outcomes, only terms including morbidity score were significant in some models. Table 3 gives associations with total mortality stratified by above v. below the median morbidity score (0·14) in the study population at baseline. The association between FFI intake and mortality was not dependent on morbidity status at baseline, while the association between SSD intake and total mortality was stronger among those with greater morbidity at baseline (P interaction<0·001). There were no significant interactions by age, sex, income or education.
Table 3 Hazard ratios (HR)Footnote * for total mortality associated with intake of fast-food items (FFI) and sugar-sweetened drinks (SSD), stratified by morbidity scoreFootnote † at baseline, among older adults (n 69 582) enrolled in the Vitamins and Lifestyle (VITAL) study in 2000–2002
Ref., referent category.
* All models adjusted for age, sex, race/ethnicity, marital status (married/living together, never married, separated/divorced, widowed), education (≤high-school graduate, some college, college/advanced degree), annual income (<$US 40 000, $US 40 000–59 999, $US 60 000–79 999, ≥$US 80 000, missing), BMI at age 45 years (<18·5 kg/m2, 18·5–24·9 kg/m2, 25·0–29·9 kg/m2, ≥30·0 kg/m2, missing), average yearly change in BMI from age 45 years, morbidity score †, self-rated health (excellent, very good, good, fair, poor), current use of cholesterol-lowering medication (yes/no), aspirin use in last 10 years (none, low, high, missing), non-aspirin non-steroidal anti-inflammatory drug use in last 10 years (none, low, high, missing), years of oestrogen therapy (none, <5, 5–9, ≥10), years of oestrogen plus progestin therapy (none, <5, 5–9, ≥10), age at menopause (<40, 40–44, 45–49, 50–54, ≥55 years), age at death of father (<60, 60–69, 70–79, 80–89, ≥90 years), age at death of mother (<60, 60–69, 70–79, 80–89, ≥90 years), average physical activity in 10 years before baseline (tertiles of MET×h/week, where MET is metabolic equivalents of task), smoking status (never, former, current), average alcohol intake (tertiles of g/d), mammogram in past 2 years (yes/no), prostrate-specific antigen test in the last 2 years (yes/no), sigmoidoscopy in the last 10 years (yes/no), number of servings/d of fruits (quartiles), number of servings/d of vegetables (quartiles) and total daily energy intake (continuous).
† Using Cox regression, the following conditions (yes/no) were modelled simultaneously in sex-specific and age-adjusted models to obtain a continuous morbidity score: current use of medication for depression or anxiety; current use of blood pressure medication; history of cancer of the lung, colon, bladder, pancreas, breast, cervix, uterus, ovaries, and all other sites combined except non-melanoma skin cancer (all as separate variables); IHD (defined as history of heart attack, coronary bypass surgery, angioplasty, or diagnosis of angina); stroke; congestive heart failure; rheumatoid arthritis; diabetes; viral hepatitis; cirrhosis of the liver; other chronic liver disease; emphysema, chronic obstructive pulmonary disease; kidney disease; ulcerative colitis or Crohn’s disease; Parkinson’s disease; and osteoporosis in women.
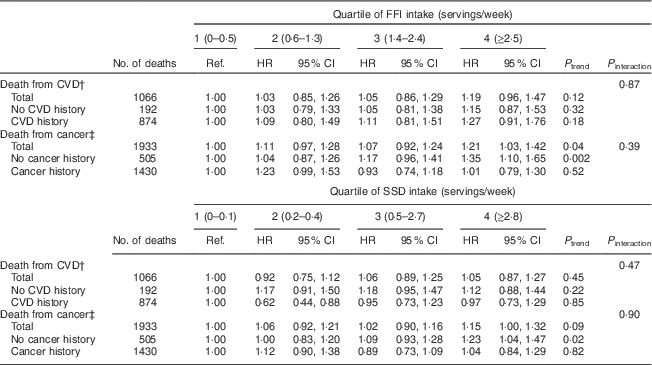
Associations between intake of FFI and SSD and cause-specific mortality are given in Table 4. Intake of FFI was significantly associated with total deaths from cancer. Specifically, those in the highest quartile of FFI intake were 21 % more likely to die from cancer (P=0·04) compared with individuals in the lowest quartile of intake (Table 4). Risk of CVD death was also highest among those in the highest quartile of FFI intake, although this trend was not statistically significant. With respect to SSD, greater intake may be associated with total deaths from cancer, although this trend was also not significant. There was no evidence of an association between intake of SSD and risk death from CVD.
Table 4 Hazard ratios (HR)Footnote * for cause-specific mortality associated with quartiles of intake of fast-food items (FFI) and sugar-sweetened drinks (SSD) among older adults (n 69 582) enrolled in the Vitamins and Lifestyle (VITAL) study in 2000–2002
Ref., referent category.
* All models adjusted for age, sex, race/ethnicity, marital status (married/living together, never married, separated/divorced, widowed), education (≤high-school graduate, some college, college/advanced degree), annual income (<$US 40 000, $US 40 000–59 999, $US 60 000–79 999, ≥$US 80 000, missing), BMI at age 45 years (<18·5 kg/m2, 18·5–24·9 kg/m2, 25·0–29·9 kg/m2, ≥30·0 kg/m2, missing), average yearly change in BMI from age 45 years, self-rated health (excellent, very good, good, fair, poor), current use of cholesterol-lowering medication (yes/no), aspirin use in last 10 years (none, low, high, missing), non-aspirin non-steroidal anti-inflammatory drug use in last 10 years (none, low, high, missing), average physical activity in 10 years before baseline (tertiles of MET×h/week, where MET is metabolic equivalents of task), smoking status (never, former, current), average alcohol intake (tertiles of g/d), mammogram in past 2 years (yes/no), prostrate-specific antigen test in the last 2 years (yes/no), sigmoidoscopy in the last 10 years (yes/no), number of servings/d of fruits (quartiles), number of servings/d of vegetables (quartiles) and total daily energy intake (continuous).
† Also adjusted for history of CVD (yes/no, defined as history of heart attack, coronary bypass surgery, angioplasty, stroke, congestive heart failure, or diagnosis of angina), family history of heart attack (number of relatives, 0, 1, ≥2), current use of blood pressure medication (yes/no), years of oestrogen therapy (none, <5, 5–9, ≥10) and years of oestrogen plus progestin therapy (none, <5, 5–9, ≥10).
‡ Also adjusted for history of cancer other than non-melanoma skin cancer (yes/no), family history of cancer (number of relatives, 0, 1, ≥2), years of oestrogen therapy (none, <5, 5–9, ≥10), years of oestrogen plus progestin therapy (none, <5, 5–9, ≥10), age at menopause (<40, 40–44, 45–49, 50–54, ≥55 years) and age at menarche (<12, 12, 13, ≥14 years).
Discussion
In the present large prospective study of adults, we found significant associations between intake of FFI and SSD and total mortality, even after adjustment for numerous factors that correlate with a healthy lifestyle. While the magnitude of these associations is modest, these findings still have large public health implications given the ubiquity of FFI and SSD intake and outlets in the US food environment, especially among socio-economic and racially disadvantaged groups( Reference Boone-Heinonen, Gordon-Larsen and Kiefe 29 , Reference Larson and Story 30 ). Furthermore, FFI associations with mortality were not significantly different across strata of age, sex, socio-economic status or morbidity score, which further underscores the significance of these findings. Morbidity status, however, does appear to play a role in associations of SSD and mortality. Specifically, the association between SSD and total mortality was primarily evidenced among those with high morbidity at baseline. To further investigate the relationships of FFI and SSD with mortality outcomes, we evaluated associations with risk of death due to CVD and total cancer. Here, we found that associations were more strongly evidenced for cancer-specific death.
To our knowledge, only one other study has compared specific intake of FFI with any mortality outcome (i.e. deaths due to CHD)( Reference Odegaard, Koh and Yuan 24 ) while ours is the first study which has evaluated the associations of FFI intake with total mortality and total cancer mortality. In the study by Odegaard and colleagues, greater intake of FFI was associated with greater risk of dying of CHD (HR=1·56; 95 % CI 1·18, 2·06) among Chinese adults( Reference Odegaard, Koh and Yuan 24 ). Despite differences in study populations, our suggestive but non-significant findings are still consistent. While we found no statistically significant association between greater intake of FFI and CVD mortality, our outcome included other cardiovascular outcomes (e.g. stroke, heart failure, arrhythmia and heart valve problems) in addition to CHD, which may have limited our ability to detect an association.
The evidence supporting associations between SSD and mortality is only slightly larger( Reference Fung, Kashambwa and Sato 10 , Reference Singh, Micha and Khatibzadeh 25 , Reference Fuchs, Sato and Niedzwiecki 26 , Reference Odegaard, Koh and Yuan 38 ). In a large pooling study by Singh and colleagues, global cause-specific population-attributable fractions for SSD were calculated and multiplied by cause-specific mortality estimates which resulted in an estimated 184 000 total deaths, 45 000 CVD deaths and 6450 cancer deaths per year due to SSD intake( Reference Singh, Micha and Khatibzadeh 25 ). Studies which have evaluated baseline intake of SSD with subsequent mortality outcomes among individuals are few( Reference Fung, Kashambwa and Sato 10 , Reference Fuchs, Sato and Niedzwiecki 26 , Reference Odegaard, Koh and Yuan 38 ). Our positive finding for SSD and total mortality is not consistent with a similar study which found no association with total, CVD- or cancer-specific mortality( Reference Odegaard, Koh and Yuan 38 ), although this could be due to differences in study populations. We also found a positive association between SSD and total mortality only among individuals with greater morbidity at baseline. Interestingly, these findings are consistent with the positive association found with total cancer mortality( Reference Fuchs, Sato and Niedzwiecki 26 ) among cancer survivors. Clearly, more research is needed to understand these complex relationships.
Other lines of inquiry also support our results. There are numerous studies which report associations of FFI and SSD with obesity( Reference Rosenheck 11 , Reference Ruff, Akhund and Adjoian 13 – Reference Alkerwi, Crichton and Hebert 17 ), CVD risk( Reference Bahadoran, Mirmiran and Golzarand 18 – Reference Cahill, Pan and Chiuve 20 ) and, to a lesser degree, cancer risk( Reference Chandran, McCann and Zirpoli 22 , Reference Stott-Miller, Neuhouser and Stanford 23 ) which may underpin the associations with mortality reported here. Evidence is also growing that foods purchased and consumed away from home (e.g. fast food or restaurant meals) contribute to obesity in adults( Reference Kim, Lee and Han 39 ) and children( Reference Altman, Cahill Holland and Lundeen 40 ) and are of poorer dietary quality compared with meals prepared and consumed in the home. Additional evidence comes from studies that compare the distribution of fast-food and convenience outlets (which also sell SSD) and mortality across neighbourhoods. Specifically, studies have noted increased risk of total and CVD mortality with greater availability of fast-food outlets in a neighbourhood after adjustment for area- and individual-level demographic characteristics( Reference Daniel, Paquet and Auger 41 , Reference Alter and Eny 42 ).
The strengths of the present analysis include its large sample size as well as robust tests for interactions and adjustment for numerous covariates. It should be noted that most of the adjustment factors (e.g. income) are potential confounders, while some covariates (e.g. fruit and vegetable intake, body mass and change in body mass) could be confounders or possibly mediators of the FFI–mortality and SSD–mortality associations. Nevertheless, the associations with total mortality remained after adjustment for twenty-five covariates plus a morbidly score of twenty-five health conditions. To reduce possible reverse causality, analyses did not include deaths occurring within the first year after baseline. Additionally, FFI and SSD were quantified using an FFQ rather than a single item and were selected a priori based on foods typically served in chain fast-food restaurants as well as drinks with added sugar. Limitations of the analysis include a lack of generalizability given the sample was of mostly white, educated, older adults. It is possible that the interactions tested in these analyses may be evidenced in a more representative sample. Also, there is possible exposure measurement error given that we do not know whether food was prepared or cooked at home v. in restaurants( Reference Altman, Cahill Holland and Lundeen 40 , Reference Cohen and Bhatia 43 ). Finally, although the sample size was large, the number of CVD-specific deaths was relatively small compared with the number of total and cancer-specific deaths; it is possible those analyses were not powered to detect a true association.
Conclusion
In conclusion, although additional studies are still needed, the present analyses provide a link between intake of FFI, SSD and subsequent mortality outcomes among older individuals. Furthermore, limiting consumption of FFI may have more far-reaching public health implications given that associations with mortality outcomes did not vary by age, sex, socio-economic status or morbidity status. These findings could be used to support systemic efforts to promote healthy food environments in addition to guiding individual dietary choice to reduce obesity-related health disparities.
Acknowledgements
Financial support: This work was supported by the National Cancer Institute (W.E.B., grant number R25CA094880) and by the National Cancer Institute and Office of Dietary Supplements (E.W., grant number K05CA154337). The funders had no role in the design, analysis or writing of this article. Conflict of interest: The authors have no conflicts of interest to disclose. Authorship: W.E.B. and E.W. contributed equally to the work resulting in this manuscript. W.E.B. and E.W. contributed to all aspects of study design, data analysis, interpretation and manuscript preparation. Ethics of human subject participation: The Fred Hutchinson Cancer Research Center Institutional Review Board reviewed and approved all study protocols.







