Salt iodization is a highly effective fortification strategy in most countries, including many in Africa( 1 ). Ensuring high household coverage with adequately iodized salt is a key to success of these programmes( 1 ). However, in some countries, in areas where there are multiple scattered small-scale salt producers, it is difficult to establish the infrastructure for salt iodization. This is the case in several countries in West Africa, including Senegal and Ghana( Reference Nyumuah, Hoang and Amoaful 2 ). In Ghana, since 2011, household coverage of adequately iodized salt (≥15 ppm) has been 35–48 %, while the proportion of household salt with any concentration of iodine has been 66–68 %( 3 , 4 ) (J Knowles, unpublished results).
In many industrialized countries, processed foods account for more than 80 % of dietary salt intake( Reference Zimmermann 5 ). Salt used in those foods needs to be iodized if salt iodization programmes are to provide sufficient iodine to target populations( Reference Zimmermann 5 ). Similarly, in low- and middle-income countries with growing processed food consumption( Reference Spohrer, Larson and Maurin 6 ), iodizing household salt alone may not be sufficient to ensure adequate population iodine status. In West Africa, processed foods provide increasing amounts of dietary salt, especially in urban areas( Reference Spohrer, Knowles and Jallier 7 ). One important processed food product is bouillon cubes. Bouillon cubes are typically composed of 50–70 % salt, along with dehydrated stock, solid vegetable fat, flavour enhancers and spices( Reference Mejia, Aguilera-Gutiérrez and Martin-Cabrejas 8 ); they are marketed in a variety of flavours such as vegetable, seafood, chicken and beef. Bouillon cubes can be ‘indirectly’ fortified with iodine through the use of iodized salt in their production( Reference Spohrer, Knowles and Jallier 7 ). Consumption of bouillon cubes containing iodized salt may be increasing in countries in Central and Western Africa, Asia and, more recently, in the Caribbean and Latin America( Reference Spohrer, Knowles and Jallier 7 , Reference Mejia, Aguilera-Gutiérrez and Martin-Cabrejas 8 ).
In Africa, bouillon cubes are widely available in both rural and urban markets and at a low cost that makes them accessible to poorer subgroups of the population( Reference Spohrer, Knowles and Jallier 7 ). In Senegal, the estimated per capita daily consumption of bouillon cubes is 4·3 g in rural areas( Reference Spohrer, Knowles and Jallier 7 ). Therefore, the use of iodized salt in bouillon could be an effective vehicle and strategy to complement household iodized salt. A potential advantage of the use of iodized salt in bouillon cubes within West Africa is that the bouillon industry is consolidated, in contrast to small-scale salt production, making regulation and monitoring more feasible.
Current legislation on salt iodization in Ghana stipulates that all food-grade salt sold in the country must be fortified with iodine at a manufacturer level of 50 ppm( Reference Nyumuah, Hoang and Amoaful 2 ). Many bouillon cube manufacturers supplying the Ghanaian market, located both within and outside Ghana, reported complying with this legislation (A-R Abizari, unpublished results). Despite low household coverage with iodized salt in northern Ghana, the recent national survey of iodine nutrition based on median urinary iodine concentration (UIC) found adequate iodine intakes in school-aged children, with the median UIC greater than 150 µg/l (J Knowles, unpublished results). We therefore conducted a cross-sectional survey to assess iodine from different dietary sources and hypothesized that iodized salt in bouillon cubes is the major source of iodine in rural diets of school-aged children in northern Ghana.
Methods
Study site and participants
The current cross-sectional survey was conducted in Bole and Sawla communities, located in two neighbouring districts of the western corridor of Northern Region, Ghana. The region is within the Savannah agro-ecological zone and largely rural. Most available food is produced through subsistence farming. In each community, one public primary school was selected to participate. Primary schools were selected in collaboration with the district education directors in order to include children representative of the usual sociodemographic characteristics of the communities. We selected schools that were not participating in the national school feeding programme, to maximize the chances that most of the children’s meals were consumed at home. The primary schooling rate estimated from the 2010 population and housing census was 51 % in Bole district( 9 ) and 59 % in Sawla-Tuna-Kalba district( 10 ).
Pupils in lower primary (classes 1–4, age 6–13 years) were selected to participate in the survey. The a priori decision was for 250 pupils to be included in the survey, in order to provide a population-level estimate of iodine status in this age group. In each school, the class registers were pooled to form one sampling frame. Simple random sampling was used to select 125 pupils from each school. The study was conducted in April 2014 with a follow-up in April 2015.
Data collection
A short questionnaire (pertaining to age, sex, class, date of birth, weight and height) was completed for each selected pupil in school. Each pupil collected a midstream urine sample into a 20 ml sterile screw-top urine container and the samples were transported to the Public Health Laboratory of the Tamale Teaching Hospital, Ghana, where they were aliquoted into 2 ml tubes and stored at −20°C. Of the pupils who returned a spot urine sample, 100 were randomly selected and given containers to bring salt samples from their households the next day. The salt samples were stored in opaque polyethylene bags until analysis.
An additional 100 pupils were randomly selected and followed home to complete a questionnaire on household utilization of bouillon cubes. Mothers/caregivers of the pupils, who had primary responsibility for cooking meals, were asked whether they add bouillon cubes to the foods the pupils eat at home. A 24 h recall of bouillon utilization was conducted. Mothers/caregivers were asked to recall whether bouillon cubes were used for each cooking moment within the 24 h preceding the interview. The number of bouillon cubes used per cooking moment and the frequency of cooking moments per day were recalled. To have an idea of how regularly the households used bouillon cubes, respondents were asked to indicate the usual frequency of bouillon utilization in a week. Respondents also provided information about the favourite brands of bouillon cubes used by their households.
Samples (n 34) of the different brands of bouillon cubes available in the communities were bought from different retailers at the respective markets. We sampled the three major brands produced in Ghana (Brands A, B and C; n 27) and seven minor brands (n 9). Household water from different sources was collected (all, n 17): borehole (n 5); pipe borne (n 7); well (n 3) and dam (n 2). Milk products (n 7) sold to children (locally made on a small scale) were collected. Urine, salt, food and water samples were sent to the Human Nutrition Laboratory of the ETH Zurich, Switzerland for analysis.
Anthropometry
Weight and height of children were measured according to standard procedures( Reference Cogill 11 ). Height was measured to the nearest 0·1 cm with a microtoise (Bodymeter 208; Seca GmbH, Germany). Weight was measured with a calibrated electronic scale (UNIscale; Seca GmbH) to the nearest 0·1 kg. Both weight and height were measured twice for each child and the average of the two measurements was taken. Age was calculated using verifiable records (birth certificate, health record, class register) or, in a few cases, estimated based on another child’s record or event on a traditional calendar.
Laboratory analyses
UIC was measured at ETH Zurich using the Pino modification of the Sandell–Kolthoff reaction( Reference Pino, Fang and Braverman 12 ). The iodine laboratory at the ETH Zurich participates successfully in the EQUIP programme of the US Centers for Disease Control and Prevention, Atlanta, GA, USA. Iodine in household water samples and salt aliquots dissolved in ultrapure water (18 MΩ cm) was measured using a modification of the Sandell–Kolthoff reaction; at iodine concentration of 30 µg/g salt, the inter-assay CV in our laboratory is 7 %. The iodine concentration of local milk products and bouillon cubes was analysed in duplicate by inductively coupled plasma–mass spectrometry (ICP-MS). Bouillon cubes were dissolved in 400 ml ultrapure water (18 MΩ cm) using an ultrasonic bath. Sample preparation of the milk product and dissolved bouillon cubes was done using tetramethylammonium hydroxide for iodine extraction at 90°C( Reference Fecher, Goldmann and Nagengast 13 ). We used a Finnigan NEPTUNE high-resolution double-focusing multicollector ICP-MS (Thermo Scientific, Waltham, MA, USA) and applied isotope dilution analysis with 129I for quantification and tellurium for mass bias correction, as recently described( Reference Dold, Baumgartner and Zeder 14 ).
Data management and statistical analysis
Microsoft® Excel 2010 and IBM SPSS Statistics Version 22.0 were used for the data analysis. Total daily household bouillon cube utilization was estimated by multiplying the quantity of bouillon cube per cooking moment by the number of usual cooking moments in the household. The household daily total consumption of bouillon cubes was divided by the corresponding household size to derive the individual consumption by school-aged children. Estimated iodine intake from bouillon cubes was calculated for each child by multiplying the calculated daily per capita bouillon cube intake by the mean concentration of iodine in the preferred brand of bouillon cube in the child’s household. For the estimations of individual-level intakes of bouillon we assumed that: (i) iodine in the bouillon cubes is stable during storage; (ii) there is no loss of iodine during cooking; and (iii) all foods prepared at home and containing bouillon cubes were completely consumed. Estimated total daily iodine intake was calculated from the UIC and body weight of each child using the following US Institute of Medicine( Reference Trumbo, Yates and Schlicker 15 ) formula:
Evaluation of Q–Q plots, the Kolmogorov–Smirnov test and the Shapiro–Wilk test were used to test normality of data. In descriptive analyses, normally distributed data were presented as mean and standard deviation, and non-normally distributed data as median and interquartile range (IQR). UIC, salt iodine concentration (SIC), estimated iodine intake from UIC and the content of iodine in the cubes were not normally distributed. In the case of non-normality, the data were log transformed and rechecked for normal distribution. If data were still not normal after log transformation, non-parametric tests were used for analysis of the non-transformed data. Spearman’s correlations were conducted since data were not normally distributed. The level of significance was set at P<0·05.
Results
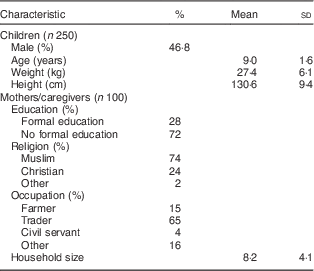
The background characteristics of the children and their caregivers are shown in Table 1. Among the children (n 250), mean (sd) age was 9 (1·6) years, 46·8 % were male, and fifty-seven (22·8 %), seventy-three (29·2 %), seventy-eight (31·2 %) and forty-two (16·8 %) were from primary school classes 1, 2, 3 and 4, respectively. Mean (sd) household size was 8 (4), 74 % were Muslim, and the literacy rate among the caregivers was 28 %.
Table 1 Characteristics of northern Ghanaian schoolchildren and their caregivers, April 2014
The SIC in household samples and the children’s UIC values are shown in Table 2. The median SIC was only approximately 2 µg/g: 72 % of samples had SIC <5 µg/g, 6 % had SIC between 5 and 15 µg/g, 4 % between 15 and 40 µg/g, and 18 % had SIC >40 µg/g. The median (IQR) UIC of the schoolchildren was 242 (163–365) µg/l (Table 2).
Table 2 Household salt iodine concentrations and iodine status of northern Ghanaian schoolchildren, April 2014
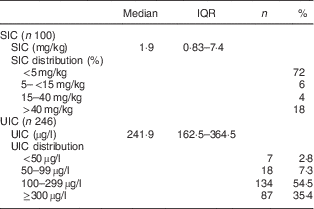
IQR, interquartile range; SIC, salt iodine concentration; UIC, urinary iodine concentration.
The iodine concentrations in household water and local milk products were low. The median (IQR) iodine concentration in household water (n 17) was 0·0 (0·0–3·0) µg/l; one water sample from a borehole contained 35 µg/l and one well water sample contained 27 µg/l; all other samples contained <5 µg/l. The median (IQR) iodine concentration in milk products (n 7) was 9·0 (8·3–9·0) ng/g.
Except for Brand A, which was packed as 12 g/cube, all other brands of bouillon cube were packed as 10 g/cube. Among the study households, there was a clear preference for Brand A cubes (used by 75 % of households), while 22 % of households used Brand B cubes and only 3 % preferred other brands. The overall median (IQR) concentration of iodine in bouillon cubes (n 34), weighted by frequency of brand use, was 31·8 (26·8–43·7) µg/g. By brand, the median (IQR) concentration of iodine in the three most popular brands of bouillon cubes was: Brand A (n 9), 32·0 (27·2–47·5) µg/g; Brand B (n 8), 30·6 (25·6–31·5) µg/g; and Brand C (n 8), 27·6 (27·0–29·2) µg/g (Table 3). The distribution of iodine concentrations (µg/g) among all samples of bouillon cubes is shown in Fig. 1.
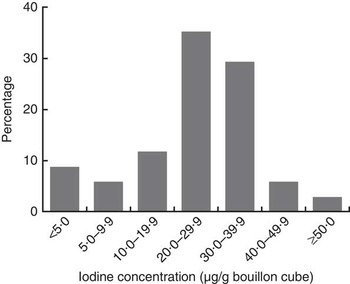
Fig. 1 Distribution of iodine concentration in bouillon cubes sold in northern Ghana, April 2014
Table 3 Iodine concentration of bouillon cubes (n 34) sold in northern Ghana, April 2014

IQR, interquartile range.
* Weighted by frequency of consumption of brands.
Among the households surveyed (n 100), the median (IQR) quantity of bouillon cubes used daily was 20·0 (12·0–24·0) g per household and the per capita daily bouillon cube intake was estimated at 2·4 (1·5–3·3) g. Weighted by frequency of use of the different brands among the households (above), we calculated the estimated median (IQR) per capita iodine intake from bouillon cubes as 88·3 (50·9–10·4) µg/d. Median (IQR) daily iodine intake projected from the children’s UIC and body weight was 129·3 (85·1–220·9) µg (Table 4). There was no significant correlation between SIC and UIC, after controlling for iodine intake from bouillon cubes, but there was a significant positive correlation between bouillon cube iodine content and UIC (r=0·36, P=0·031).
Table 4 Household bouillon cube use and estimated contribution of bouillon cubes to daily iodine intake in northern Ghana, April 2014 (n 100)

IQR, interquartile range; UIC, urinary iodine concentration.
Discussion
Our findings suggest that in this region of northern Ghana the major source of iodine in school-aged children’s diets is iodized salt used in the manufacture of bouillon cubes. Despite very low coverage of households with iodized salt, iodine intakes were adequate, as the median iodine intake, derived from the UIC of the children, was 129 µg/d, just above the recommended daily iodine intake of 120 µg/d for this age group( 1 , Reference Trumbo, Yates and Schlicker 15 ). We estimate that iodine from bouillon cubes was contributing over two-thirds of dietary iodine in the children’s diets. In this inland area far from the Atlantic coast, intakes of iodine-rich seafood and ocean fish are negligible, and the groundwater contains very little iodine. Therefore, iodized table salt would be expected be the primary source of dietary iodine. However, our data suggest only 22 % of households were using adequately iodized salt (as defined by SIC ≥15 mg/kg)( 1 ). Milk products can be a rich source of iodine in countries where diets are rich in dairy products, such as the USA, Switzerland and Australia( Reference Zimmermann 5 ). However, dairy products are consumed only rarely by rural children in northern Ghana, and the concentrations of iodine in milk drinks for sale in the study area were negligible.
Because their main ingredient is salt, bouillon cubes (and other seasoning powders) could be a good fortification vehicle for iodine if they contained iodized salt and their consumption by target groups is sufficiently high. Hess et al.( Reference Hess, Brown and Sablah 16 ) summarized the findings on consumption of bouillon cubes from the Fortification Rapid Assessment Tool (FRAT) surveys conducted in sub-Saharan Africa. Five of the FRAT surveys included bouillon cubes as a food vehicle of interest( Reference Hess, Brown and Sablah 16 ). In Mali, approximately 98 % of all women reportedly consumed bouillon cubes in the previous 24 h( Reference Hess, Brown and Sablah 16 ). In Burkina Faso in 1999 (n 840), 79 % of women consumed bouillon cubes in the past week, the mean number of days bouillon cubes were consumed per week was 5·4, and the median amount of bouillon cube consumed on the previous day among consumers was 2·6 g( Reference Hess, Brown and Sablah 16 ). For Niger in 2001 (n 840), the respective numbers were 88 %, 5·6 and 1·7 g/d( Reference Hess, Brown and Sablah 16 ). For Senegal in 2006 (n 827), the respective numbers were 99 %, 6·8 and 3·5 g/d( Reference Hess, Brown and Sablah 16 ). Finally, for Cameroon (n 912), the respective numbers were 96 %, 13·8 times per week and 1·9 g/d( Reference Engle-Stone, Ndjebayi and Nankap 17 ). In our study population, the estimated median (IQR) per capita bouillon cube intake was 2·4 (1·5–3·3) g/d, comparable to these previous studies in West Africa.
In our study area, over 70 % of households were using the Brand A of bouillon cube, while about 20 % were using the Brand B. These two popular brands had a median (IQR) iodine concentration of 32·0 (27·2–47·5) and 30·6 (25·6–31·5) µg/g, respectively, and bouillon cubes are usually ≈50 % salt( Reference Spohrer, Knowles and Jallier 7 , Reference Mejia, Aguilera-Gutiérrez and Martin-Cabrejas 8 ). This suggests that adequately iodized salt, likely close to the Ghanaian standard of 50 µg iodine/g, was being used in their manufacture. A recent study in Senegal estimated that, based on the measured iodine content of bouillon cubes and average daily bouillon cube consumption of 4·3 g in rural areas, bouillon cubes could deliver between 3 and 57 % of the recommended adequate daily iodine intake in adults depending on the brand of bouillon cube consumed( Reference Spohrer, Knowles and Jallier 7 ). That study suggested that bouillon cubes containing salt iodized close to the level stipulated in Senegalese legislation (30 µg/g)( 18 ) could potentially cover 46–57 % of an adult’s recommended daily iodine intake of 150 µg/d( Reference Spohrer, Knowles and Jallier 7 ). It should be mentioned, however, that only two out of thirteen brands of bouillon cubes the authors worked with appeared to have been made from adequately iodized salt. Nevertheless, this indicates that bouillon cubes, if they contain well-iodized salt, can be a substantial contributor to iodine adequacy among vulnerable population groups in West Africa such as children and women of reproductive age.
This substantial contribution of ‘hidden’ iodine in bouillon cubes and other seasoning powders to dietary iodine intakes may explain the discrepancy in many low- to middle-income countries between data suggesting, on the one hand, low coverage of households with adequately iodized salt, but on the other, adequate iodine intake based on the median UIC. Iodine sufficiency in populations as indicated by a median UIC of ≥100 μg/l in school-aged children( 1 ) has been reported from many countries with poor household iodized salt coverage. For example, in Senegal, household coverage of adequately iodized salt was ≈56 % but median UIC among school-aged children was in the adequate range (104 µg/l)( 18 ). In the Philippines, household coverage with adequately iodized salt was only 25 % but median UIC in school-aged children was adequate, at 132 µg/l( 19 ). Recognizing this issue, the WHO has stated that countries that focus on iodization of table salt alone may not achieve optimal iodine nutrition and it is necessary to include iodized salt in processed foods( 20 ). This statement by WHO is a reminder to stay true to universal salt iodization, which in principle means that all edible salt, including that used in processed foods, be adequately iodized.
Stability of iodine in poor-quality, damp salt is a concern, and may be partly responsible for the large proportion of inadequately iodized salt at the household level( Reference Diosady, Alberti and Venkatesh Mannar 21 ) in counties where there is a high percentage of raw, poor-quality salt. Due to the relatively long shelf-life of bouillon cubes and other seasoning powders, similar concerns about iodine losses may also apply to these products albeit the consistency of bouillon cubes is different from moist salt. There is little information on iodine losses in cubes; however, recent studies in Senegal( Reference Spohrer, Knowles and Jallier 7 ) have shown that iodine concentration in bouillon cubes is minimally affected by prolonged storage (average loss of iodine in bouillon cubes was 13·6 % over 6 months) and is virtually unaffected by cooking conditions. This suggests that if producers used adequately iodized salt, bouillon cubes could serve a predictable and stable source of dietary iodine to households. However, data from the bouillon cubes analysed indicated that there are wide differences (up to two- to threefold differences) in iodine concentration within the same brand. Also, there was often disagreement between the analysed values and the concentration stated on the product label. Therefore, there is a need for improved quality control and labelling, and the iodine content in salt used in the preparation of the bouillon cubes will need to be monitored carefully so that its contribution as part of national programmes can be tracked with some certainty.
Fortification of bouillon cubes with iodized salt is a relatively new concept in iodine nutrition programmes. Whether this strategy is successful will depend on the enforcement and willingness of domestic and multinational manufacturers to use iodized salt in their bouillon cubes. Some companies have already voluntarily introduced bouillon cubes with added iodine and iron in Ghana and other parts of Africa. One large manufacturer has released a fortified bouillon product in multiple West African countries (Benin, Togo, Cameroon, Côte d’Ivoire, Nigeria, Senegal, Ghana, Niger and Guinea), which is currently fortified with iodine as well as iron( 22 ). Discussions by the authors with large bouillon cube producers in Ghana revealed that although there are no factory-level iodine fortification protocols, the national regulatory reference of 50 mg/kg guides the procurement of salt they use in bouillon production, and they are willing to comply with legislation regarding iodine fortification. In a recent review, Mejia and Bower( Reference Mejia and Bower 23 ) recommend that countries with existing voluntary fortification programmes covering bouillon cubes and seasonings revise and convert them into mandatory regulations, to ensure that the products comply with fortification standards and guidelines. Also, government monitoring and enforcement need to be integrated into such mandatory policy guidelines to ensure adherence and compliance( Reference Spohrer, Knowles and Jallier 7 ). Improved labelling would also be valuable: typical packaging refers to the amount of sodium per cube, but does not indicate whether that sodium comes from monosodium glutamate or salt. Therefore, without the product recipe or conducting laboratory analysis, it is impossible to calculate the potential amount of iodine from iodized salt contained per cube solely from the amount of sodium( Reference Spohrer, Knowles and Jallier 7 ). However, the two major brands of bouillon in the current survey listed ‘iodated salt’ as an ingredient and indicated the amount of iodine per cube. For example, the label of the Brand A bouillon cube package states that 12 g of the product (1 cube) contains 370 µg iodine and that of the Brand B bouillon cube states that 10 g of the product (1 cube) contains 80 µg iodine.
Our study findings have limitations. Estimation of daily intake of bouillon cube per capita was based on the assumption that each meal prepared at the household was completely served to all members of the household and that each schoolchild completely finished his/her portion. Dividing the total quantity of household bouillon cubes used by the household size presumes equal sharing of food but portion sizes usually vary for different individuals in the household, with children receiving smaller portions. This is likely to lead to overestimation of bouillon intake. Nevertheless, our estimate for daily bouillon intake is similar to that derived from a quantitative dietary assessment for schoolchildren within the same region (A-R Abizari, unpublished results). Also, we studied children attending primary schools and only 50–60 % of children in this region are attending school; it is possible that families of children not attending school have different dietary patterns and our findings may not apply to them.
Conclusion
In conclusion, bouillon cubes are an important source of dietary iodine in northern Ghana and possibly other West African countries. Growing use of bouillon cubes in rural Africa will likely reduce the quantity of table salt used by households during cooking. This could decrease delivery of iodine by household salt even if it is well iodized, and reinforces the need to use adequately iodized salt in the production of bouillon cubes. Future research should determine the potential impact of iodine in bouillon cubes not only on school-aged children in other settings and diets, but also on other target populations of iodine programmes such as pre-school children (who eat less foods from the table) and young women (whose iodine requirements are higher).
Acknowledgements
Acknowledgements: The authors thank all the children and their mothers/caregivers who participated in the study. They are grateful to the teachers of DA Primary School (Bole and Sawla) for their role in facilitating data collection. They thank Jerry Alagpulinsa, Francis Gakpetor, Mohammed Shaibu Osman, Aboku Osman and Suara Sufyan for helping with data collection and processing of samples, and are thankful to Annica Jucker and Timo Christ for assistance with laboratory analyses. The authors also thank Jacky Knowles for her support and for providing relevant information concerning iodine nutrition in the study area. Financial support: This work was funded by the Global Alliance for Improved Nutrition (GAIN), Geneva, Switzerland; UNICEF, New York, USA; and the Human Nutrition Laboratory, ETH Zurich, Switzerland. The funders had no further role. Conflict of interest: R.K. is a UNICEF staff member. The opinions and statements in this article are those of the author and may not reflect official UNICEF policies. Authorship: A.-R.A. and M.B.Z. designed the research. A.-R.A. conducted and supervised the fieldwork. A.-R.A., S.D. and M.B.Z. analysed the data. A.-R.A. and M.B.Z. wrote the first draft of the paper. All authors made critical comments during the preparation of the manuscript. All authors edited and approved the final version of the manuscript. Ethics of human subject participation: The parents/caregivers of the pupils gave verbal informed consent. The verbal consent was witnessed and formally recorded. The review boards of the ETH Zurich, Switzerland and the Tamale Teaching Hospital in Ghana gave ethical permission for the study.








