Severe acute malnutrition (SAM) remains a major killer of children, as mortality rates in children with severe wasting – the most widespread form of SAM – are nine times higher than in well-nourished children( Reference Black, Allen and Bhutta 1 ). Current global recommendations indicate that children aged 6–59 months with a mid upper-arm circumference (MUAC) <115 mm should be admitted to a programme for the management of SAM( 2 ). The latest data available indicate that at any point in time, an average of 8 million Indian under-5s (i.e. an estimated 6·4 % of children 0–59 months old) are severely wasted( 3 , 4 ). This is the largest pool of severely wasted children worldwide.
In this context, India’s Ministry of Health has endorsed the use of MUAC<115 mm as a screening and admission criterion for the management of children with SAM( 5 ). However, some argue that this cut-off may not be appropriate for Indian children( Reference Dasgupta, Sinha and Kumar 6 ). The objective of our analysis was to assess the appropriateness of current MUAC cut-off values in identifying children with SAM in India in order to inform the future design and implementation of national and state programmes for the provision of care for children with SAM.
Methods
Our analysis concerns 6307 children with SAM admitted to nutrition rehabilitation centres (NRC) in the states of Jharkhand, Madhya Pradesh and Uttar Pradesh between 1 July 2009 and 31 December 2011.
The detection of children with SAM was ensured in the villages by frontline workers either as part of monthly growth monitoring and promotion sessions at the village anganwadi centre (passive case finding) or in the context of community drives for the identification of children with SAM (active case finding). All children aged 6–59 months with bilateral pitting oedema or weight-for-height Z-score (WHZ)<−3 or MUAC<115 mm were admitted to the NRC( 7 , 8 ).
Upon admission to the NRC, a medical doctor conducted a clinical examination of the children to detect the presence/absence of medical complications using the criteria for the Integrated Management of Neonatal and Childhood Illnesses( 9 ). At the NRC, children received therapeutic care following protocols based on the guidelines for management of SAM by the WHO and the Indian Academy of Pediatrics( 7 , 8 ). Children were discharged upon completion of a prescribed 14 d stay in the NRC, provided that they were active and alert, had no signs of oedema, fever or infection, had completed all age-appropriate immunizations and their primary caregiver had been informed about follow-up care.
Statistical analyses were carried out using the Stata software package release 12.
Results
Among the 6307 children admitted, 3279 (52·0 %) were girls, 4023 (63·8 %) were from scheduled caste or scheduled tribe families and 5023 (79·6 %) were in the age group 6–23 months. On admission, 231 (3·7 %) of the children had oedema, 1870 children (29·6 %) had severe wasting with medical complications (lethargy, loss of appetite, fever or hypothermia, severe pneumonia, severe dehydration, severe anaemia, malaria and/or tuberculosis) and 4206 children (66·7 %) had uncomplicated SAM (free of oedema and medical complications).
The following programme outcomes were recorded: forty-three children (0·7 %) died, 1442 children (22·9 %) left before discharge (defaulted) and 4822 (76·5 %) were discharged. The average length of stay among the discharged was 14·7 (sd 3·2) d and their average weight gain was 9·7 (sd 15·3) g/kg body weight per d. At the time of discharge, 1643 children (34·1 %) had gained ≥15 % of their initial weight (Table 1).
Table 1 Characteristics of 6–59-month-old children (n 6307) at admission and by programme outcomeFootnote *, Jharkhand, Madhya Pradesh and Uttar Pradesh, India, 2009–2011
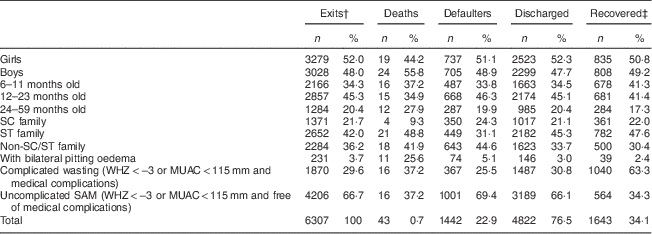
SC, scheduled caste; ST, scheduled tribe; WHZ, weight-for-height Z-score; MUAC, mid upper-arm circumference; SAM, severe acute malnutrition.
* Values are presented as absolute numbers (n) and as percentage of the total (%).
† Exits: deaths+defaulters+discharged.
‡ Recovered: children who, at discharge, had gained ≥15 % of their initial weight.
A total of 6076 children were severely wasted (non-oedematous SAM); among them, the proportion with WHZ<−3 was higher than the proportion with MUAC<115 mm (85·9 % v. 78·1 %, respectively). This difference was less pronounced among infants and young children aged 6–23 months (85·7 % v. 81·8 %) than among older children aged 24–59 months (86·4 % v. 63·0 %). The proportion of children with both WHZ<−3 and MUAC<115 mm was significantly higher among infants and young children aged 6–23 months than among older children aged 24–59 months (67·5 % v. 49·4 %; Table 2).
Table 2 Anthropometry and programme outcomes by age group among 6–59-month-old children who were free of oedema at admissionFootnote *, Jharkhand, Madhya Pradesh and Uttar Pradesh, India, 2009–2011
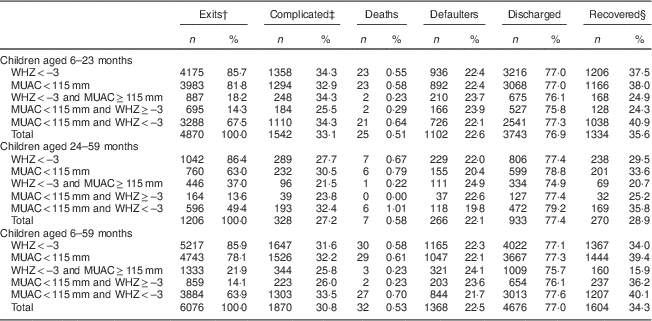
WHZ, weight-for-height Z-score; MUAC, mid upper-arm circumference.
* Values are presented as absolute numbers (n) and as percentage of the total (%).
† Exits: deaths+defaulters+discharged.
‡ Complicated: children who, at admission, had oedema or medical complications.
§ Recovered: children who, at discharge, had gained ≥15 % of their initial weight.
The proportion of children aged 6–59 months with WHZ<−3 and identified by MUAC<115 as being severely wasted was 74·4 %. This figure decreased with children’s age: 78·8 % among children aged 6–23 months v. 57·2 % among children aged 24–59 months. The proportion of children aged 6–59 months with MUAC<115 mm and whose WHZ was <−3 was 81·9 % and decreased with children’s age: 82·6 % among children aged 6–23 months v. 78·4 % among children aged 24–59 months.
Multivariable logistic regression analysis indicated that the presence of oedema was the most important predictor of death while in the NRC (adjusted OR=6·98; 95 % CI 2·07, 16·4). Eleven (25·6 %) of the forty-three children who died had oedema at admission. Death rates in children with oedema were nine times higher than those in oedema-free children. Among oedema-free children, the most important predictor of death was the presence of medical complications (adjusted OR=2·43; 95 % CI 1·28, 4·06). Death rates in oedema-free children with medical complications were 2·3 times higher than in children with uncomplicated severe wasting.
Among oedema-free children, the proportion of children with medical complications was similar in children with MUAC<115 mm and children with WHZ<−3 across all age groups (32·5 % v. 32·5 % in infants and young children aged 6–23 months; 32·2 % v. 31·6 % in children aged 24–59 months; and 26·0 % v. 25·8 % among children aged 6–59 months; data not presented). The proportion of deaths among oedema-free children with MUAC<115 mm was higher than among children with WHZ<−3 (0·61 % v. 0·58 %). Mortality rates among children with MUAC<110 mm were four times higher than among children with 110 mm≤MUAC<115 mm.
Discussion
Our findings must be interpreted keeping in mind two factors that limit their applicability to the general child population of India: (i) the data analysed apply to three samples of children that may not be representative of the general population of children in Jharkhand, Madhya Pradesh, Uttar Pradesh or India, as the villages where screening, referral and treatment took place were not randomly selected; and (ii) although in the villages covered by the NRC included in the analysis all efforts were made to screen, refer and treat all eligible children, the analysis presented here applies to the programme data collected on the children who were actually screened, referred and treated, whose characteristics at admission and response to treatment may not be representative of the child population at large.
However, on the basis of the results it seems legitimate to conclude that in populations in India similar to those included in our analysis, MUAC<115 mm appears to be at least as effective as (if not more effective than) WHZ<−3 to identify SAM children at a greater risk of medical complications and death, particularly among 6–23-month-old children, who represent the vast majority (~ 80 %) of the caseload of children with SAM.
In this population, MUAC<115 mm was effective in identifying children with SAM, particularly among young children aged 6–23 months. Among them, the proportion with WHZ<−3 was similar to that with MUAC<115 mm (85·7 % v. 81·8 %), with a significant overlap between both groups: the proportion of children aged 6–23 months with MUAC<115 mm whose WHZ was<−3 was 82·6 %; and the proportion of children aged 6–23 months with WHZ<−3 whose MUAC<115 mm was 78·8 %. Importantly, MUAC<115 mm was as effective as WHZ<−3 in identifying children with medical complications (32·2 % v. 31·6 %, respectively), the most important risk factor of death among oedema-free children. Furthermore, death rates in children with MUAC<115 mm were higher than in children with WHZ<−3 (0·61 % v. 0·58 %, respectively): 91 % of the deaths among oedema-free children were deaths of children with MUAC<115 mm. Death rates were four times higher among children with MUAC<110 mm than among children with 110 mm ≤ MUAC<115 mm; thus as many as 88 % of deaths among oedema-free children were deaths of children with MUAC<110 mm.
Two recent studies, one in India( Reference Dasgupta, Sinha and Kumar 6 ) and one in Cambodia( Reference Laillou, Prak and de Groot 10 ), have questioned the reliability of MUAC for the identification of children with SAM; they both focus on the ability of MUAC<115 mm to identify children with WHZ<−3, which is referred to as the ‘gold standard’ of case definition in both papers.
The differences between our findings and those in the study in India could be explained by methodological issues, as the latter short research letter reports findings on a purposive sample of children in a few villages in Madhya Pradesh with an extremely high prevalence of severe wasting and severe stunting( Reference Dasgupta, Sinha and Kumar 6 ).
The differences between our findings and those in the study in Cambodia could be explained by epidemiological differences. The latest national data available indicate that while the prevalence of moderate and severe stunting in children is very high (≥40 %) in both Cambodia and India, the prevalence of moderate and severe wasting is twice higher in India than in Cambodia (20 % v. 11 %, respectively)( 11 ). Furthermore, the prevalence of severe wasting in the Cambodia sample is 4·5 times lower than in India, indicating that the epidemiology of wasting (both extent and severity) in these two settings may be significantly different. The Cambodia paper does not provide any information on the prevalence of stunting or the mean height-for-age Z-score in children. Therefore comparisons in this respect are not possible.
An extensive literature documents the advantages of using MUAC for the screening and admission of children with SAM. Besides the simplicity of the use of MUAC and the ability to allow for high coverage, these reviews emphasize its superior effectiveness to identify children at high risk of death; they also indicate that there is no benefit in using WHZ<−3 in addition to MUAC<115 mm as MUAC identifies high-risk children better than WHZ<−3( Reference Collins, Dent and Binns 12 , Reference Briend, Maire and Fontaine 13 ). Moreover, the new global guidance by WHO indicating that MUAC≥125 mm (without oedema for at least two weeks) should be used as discharge criterion for children admitted with MUAC<115 mm can significantly simplify national protocols, the training and supervision of the staff involved in identifying and treating children, and the overall performance of programmes for the management of SAM( 14 ).
Conclusion
Our findings support the overall appropriateness of India’s Ministry of Health endorsement of the use of MUAC<115 mm as simple, affordable and evidence-based tool that community health workers can use as a screening and admission criterion in the management of children with SAM( 3 , 9 , Reference Laillou, Prak and de Groot 10 ). Active identification and early treatment of children with oedema and/or MUAC<115 mm should be the primary focus of programmes for the management of SAM in India, particularly among infants and young children 6–23 months old. Children with oedema and/or medical complications and/or MUAC<115 mm should be treated in a NRC as they are at a significantly higher risk of death, whereas children free of oedema and medical complications – at a much lower risk of death – should be appropriately managed in their communities to improve their nutritional status and avoid the onset of life-threatening conditions.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the commercial sector. UNICEF funded data analysis and paper writing. The opinions expressed on this paper are those of the authors and do not necessarily represent an official position of the organizations with which they are affiliated. Conflict of interest: None. Authorship: V.M.A. conceptualized the paper, led data analysis and wrote the manuscript. N.B. led data management. S.A. and K.S. contributed to data interpretation and manuscript finalization. All authors have read and approved the final manuscript. Ethics of human subject participation: Ethical approval was not sought as data entry and data analysis preserved children’s anonymity by using children’s unique identification number only. Therefore, we analysed anonymous data that could not be linked to individual children, caregivers or households.





