Newborn birth weight <2500 g, regardless of gestational age, is defined as low birth weight (LBW)(1), a condition that compromises infant growth(Reference Xiong, Wightkin and Magnus2) and cognitive development(Reference Fan, Portuguez and Nunes3) and is strongly linked to infant mortality, morbidity(Reference Chidiebere, Ekwochi and Ndu Ikenna4) and chronic diseases later in life(Reference Knop, Geng and Gorny5). Globally, it is estimated that 15–20 % of all births are LBW, corresponding to more than 20 million births per year(1). One of the WHO global nutrition targets is to reduce the number of LBW babies by 30 % between 2012 and 2025(1). At the population level, the proportion of LBW infants represents a multifarious public health problem. Known risk factors for LBW include preterm birth(Reference Tshotetsi, Dzikiti and Hajison6), intrauterine growth restriction(Reference Qian, Chou and Gimenez7), maternal factors such as young age(Reference Restrepo-Méndez, Lawlor and Horta8), multiple pregnancies(Reference Ooki9), poor nutrition(Reference Tran, Nguyen and Berde10), unfavourable work conditions(Reference Mahmoodi, Karimlou and Sajjadi11), chronic disease(Reference Graham, Zhang and Schwalberg12), alcohol abuse(Reference Chen13), inadequate prenatal care(Reference Zhou, Wang and Huang14) and environmental factors such as smoking(Reference Zheng, Suzuki and Tanaka15), lead exposure(Reference Zhang, Xia and Li16) and air pollution(Reference Liu, Xu and Chen17).
Maternal nutrition during pregnancy is a key factor influencing birth outcomes. Pregnant women are at increased risk of various micronutrient deficiencies, particularly in developing countries(Reference Gernand, Schulze and Stewart18,Reference Darnton-Hill and Mkparu19) . Besides, most LBW infants in these countries are full-term newborns with intrauterine growth restriction due to maternal malnutrition and poor gestational weight gain(Reference Salam, Das and Ali20,Reference Hasan, Khan and Ahmed21) . Evidence also indicates that maternal malnutrition contributes to detrimental pregnancy outcomes(Reference Abu-Saad and Fraser22,Reference Black, Allen and Bhutta23) , reduced newborn survival(Reference Black, Allen and Bhutta23,Reference Herring, Bazer and Johnson24) and an increased risk of chronic diseases(Reference Darnton-Hill and Mkparu19,Reference Christian and Stewart25,Reference Lee, Collins and Gordon26) and mental and cognitive impairment in later life(Reference Veena, Gale and Krishnaveni27,Reference Borge, Aase and Brantsæter28) . Consuming invariant and monotonous diets can cause micronutrient deficiencies, which subsequently impact fetal growth and, hence, increase the odds of LBW(Reference Henjum, Torheim and Thorne-Lyman29,Reference Yeneabat, Adugna and Asmamaw30) .
Dietary diversity is a qualitative measure of food intake that, in a snapshot form, addresses individual access to different types of foods(Reference Kennedy, Ballard and Dop31). It is an indicator of nutrient adequacy of an individual’s diet and is a proxy for multiple macro- and/or micronutrient sufficiency of the diet(Reference Kennedy, Ballard and Dop31). Different indicators are used for the assessment of dietary diversity. The Minimum Dietary Diversity for Women (MDD-W) and Women’s Dietary Diversity Score (WDDS) are most used tools adopted by the FAO(32). The tools advocated for use in women aged 15–49 years and validated against micronutrient adequacy assessed by multiple 24-h recalls(32). Based on food items consumed in the past 24 h, individuals are allocated the number of food groups they consumed, ranging from 0 to 9 (for WDDS) or 0 to10 (for MDD-W).
Numerous investigations have evaluated the association between maternal dietary diversity during pregnancy and LBW risk in offspring(Reference Abubakari and Jahn33–Reference Zerfu, Umeta and Baye41). However, there is still no comprehensive research summarising all of these reports. The research question was as follows: Is maternal dietary diversity associated with risk of LBW in newborns? Therefore, this systematic review was designed to systematically evaluate and summarise the literature in order to determine whether is there a relationship between maternal dietary diversity during pregnancy and the risk of LBW in newborns?
Methods
This systematic review was conducted following the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines (2015 Statement)(Reference Moher, Shamseer and Clarke42).
Search strategy
A literature search was conducted in the PubMed, Google Scholar and Scopus databases, as well as Google, until June 2020, with no date restrictions. The following keywords were employed in the search: ‘dietary diversity’ OR ‘diet diversity’ OR ‘food diversity’ OR ‘dietary variety’ OR ‘diet variety’ OR ‘food variety’ in title-abstract-keywords AND ‘birth weight’ in title-abstract-keywords. Limits were English language and original articles. Details on the search strategy used for PubMed, Google Scholar and Scopus databases are included in Table 1. A manual search of the reference lists of the included articles was done to find further studies.
Table 1 Association between maternal dietary diversity and risk of low birth weight: method of the database search strategy using PubMed and SCOPUS
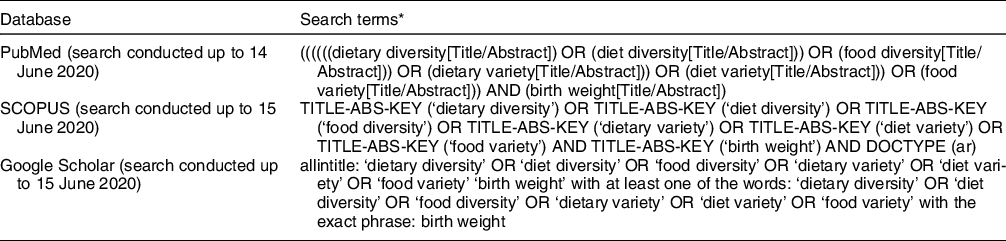
* Searches were limited to observational studies, original articles and studies published in the English language using the appropriate filters and/or search terms depending on the database.
Eligibility criteria
Original articles published in English were included. There was no restriction regarding geographic region or economic condition of countries. Cohort, cross-sectional and case-control studies addressing the relationship between maternal dietary diversity and the risk of LWB in newborns were included. Studies referring to emergency conditions or natural disasters such as cyclones were excluded. Studies that assessed the relationship of dietary diversity with other health issues such as anaemia, diabetes or hypertension were excluded. Moreover, studies that investigated the effect of dietary patterns (e.g., Western, traditional, healthy and unhealthy patterns) during pregnancy on LBW were also excluded.
Selection of the studies
The extracted investigations were transferred to an Endnote file and arranged to remove duplicate articles. The titles and abstracts of the remaining articles were screened by two independent reviewers to identify articles potentially eligible for this review. The full texts of the screened studies were then critically reviewed separately for eligibility and data extraction. Any discrepancy in evaluation between the two reviewers was resolved through discussion.
Data extraction
The extracted data were as follows: first author and year of publication; country and study design; number of study population; maternal age and time of data collection; study location (urban or rural) and data collection location; method of dietary diversity assessment; duration of food intake information; number of food groups considered and cut-off point for inadequate dietary diversity; total number of LBW infants and number of LBW infants in low DDS group; covariates adjusted; findings accompanied by OR, CI or other indicators of correlation, P value, if available.
Quality assessment
Selected studies were assessed for methodologic quality by two independent reviewers. The Newcastle-Ottawa Quality Assessment tool for observational cohort and case-control studies(Reference Wells, Shea and O’Connell43) was used to evaluate the quality and risk of bias of the included studies based on three domains: the selection of exposed and non-exposed groups, ascertainment of exposure; the comparability of groups on the basis of the design or analysis controlled for confounders; and the outcome regarding assessment and follow-up time. A star system was applied to classify the articles as good, fair or poor quality. Studies with a total score of 6 or higher were classified as high quality.
Results
Selection of studies
As shown in Figure 1, ninety-four studies were first retrieved by the search strategy and four studies by manual searching. Duplicates were removed and sixty-four studies remained. Of those, twenty-three publications met the topic and scope of the study during the screening phase. During critical review, eight studies were excluded because they did not meet the eligibility criteria or were conducted under emergency conditions. Finally, fifteen articles were included in the review (Fig. 1).
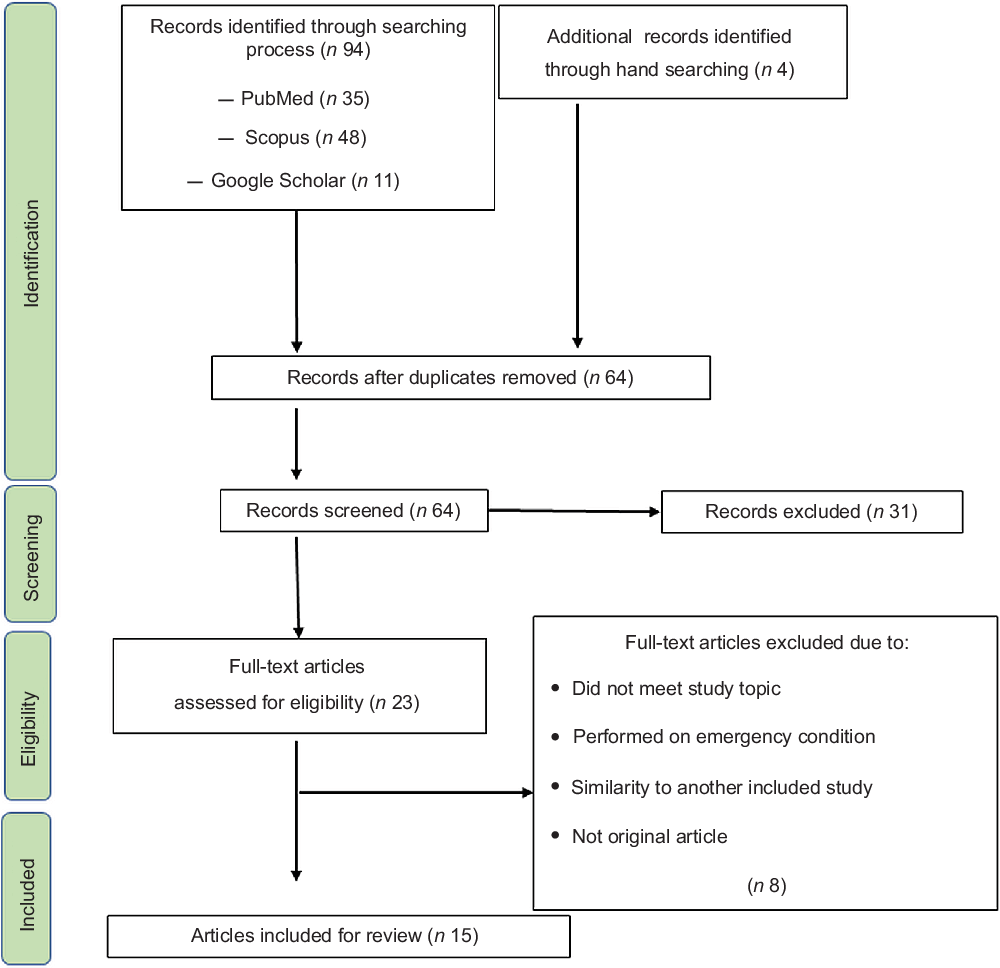
Fig. 1 Flow diagram of the literature search and screening process for a systematic review assessing the relationship of dietary diversity and risk of low birth weight
Characteristics of the included studies
As shown in Table 2, all included studies were from low- and middle-income countries and were published between 2013 and 2020. All studies applied a cohort, cross-sectional or case-control design. In ten of the studies, dietary intake information was collected only over the prior 24 h. All studies were conducted in hospitals or health facilities. All but two studies(Reference Rammohan, Goli and Singh37,Reference Jamalzehi, Javadi and Dashipour44) used FAO adopted instrument for the assessment of DDS(32). The time of data collection was after delivery in four of the included studies(Reference Ahmed, Hassen and Wakayo34,Reference Quansah and Boateng36–Reference Rashid, Park and Macneal38) and for the other studies it was in second and/or third trimesters(Reference Abubakari and Jahn33,Reference Manerkar and Gokhale35,Reference Tela, Bezabih and Adhanu40,Reference Zerfu, Umeta and Baye41,Reference Jamalzehi, Javadi and Dashipour44,Reference Madlala45,Reference Vanié, Gbogouri and Edjème-Aké46) or between 34 and 36 weeks(Reference Saaka39), first 8 weeks(Reference Alemu and Gashu47) and 9–15 weeks(Reference Nsereko, Uwase and Mukabutera48) of gestation. Several studies were conducted solely in urban areas (n 6). One of the studies was online Master’s theses(Reference Madlala45). Various criteria or cut-off points were used to identify low DDS across the included studies. Low DDS ranged from <3 to ≤7, with most used cut-off of ≤3 or <5, across the studies.
Table 2 Summary and characteristics of the fifteen selected observational studies assessing the relationship of maternal dietary diversity and risk of low birth weight
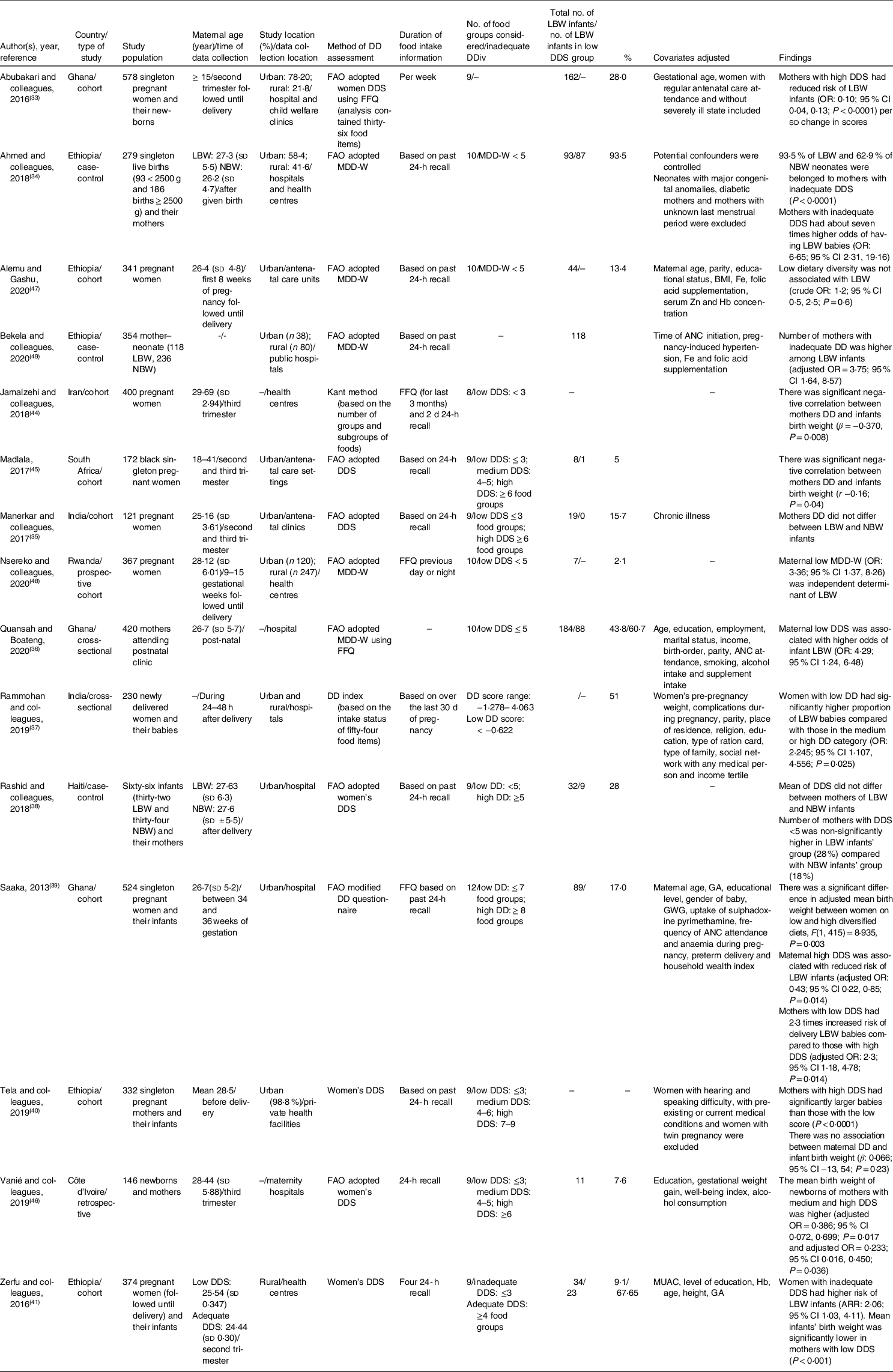
ARR, adjusted relative risk; DDiv, dietary diversity; DDS, dietary diversity score; FFQ, food frequency questionnaire; LBW, low birth weight; NBW, normal birth weight; ANC, antenatal clinic (care); GA, gestational age; GWG, gestational weight gain; OR, odds ratio; FAO, Food and Agriculture Organisation; MDD-W, Minimum Dietary Diversity for Women.
Quality of articles
All included studies were rated as good quality (online supplementary material, Supplemental Tables 3 and 4). Quality scores for cohort studies ranged from six to eight (out of nine representing the lowest degree of bias) (online supplementary material, Supplemental Table 3). Main concern was comparability of exposed and unexposed participants based on design or analysis. The key potential confounding factors including age of mothers and infants’ gender were not taken into account in the analysis of seven studies (out of ten cohort studies). Quality scores for case-control studies ranged from six to seven (out of nine) (online supplementary material, Supplemental Table 4). Main concerns were comparability of cases and controls on the basis of the design or analysis and non-response rate of the groups. None of the five case-control studies controlled the role of gender in the analysis and indicated dropout rate.
Dietary diversity and low birth weight
Eighty percentage of the studies (twelve of fifteen) indicated that a low maternal DDS during pregnancy is associated with an increased risk of LBW in infants. In a study on 578 singleton pregnant women, Abubakari et al. (Reference Abubakari and Jahn33) showed that mothers with LBW infants had significantly lower DDS. Ahmed et al. (Reference Ahmed, Hassen and Wakayo34), studying 279 singleton live births, found that mothers with low DDS had about seven times higher odds of having LBW babies. A significant negative correlation between mothers’ dietary diversity and infant birth weight has also been reported by Madlala(Reference Madlala45). In a study involving 420 mothers, Quansah(Reference Quansah and Boateng36) observed that among mothers with low DDS, the number of LBW infants was about two-fold that of normal birth weight (NBW) infants and the risk of LBW infants was four times higher in the low DDS group compared with the high DDS group. Rammohan et al. (Reference Rammohan, Goli and Singh37), investigating 230 newly delivered women, indicated that women with low dietary diversity had a significantly higher proportion of LBW babies compared with those in the medium or high dietary diversity category. Saaka(Reference Saaka39), in a study on 524 singleton pregnant women, showed that the mean birth weight of infants was significantly lower among women with low DDS compared with women who consumed diversified diets. The author reported that a high maternal DDS was significantly associated with a reduced risk of LBW and women with low DDS were 2·3 times more likely to deliver LBW babies than those with high DDS. Tela et al. (Reference Tela, Bezabih and Adhanu40), in a study on singleton pregnant mothers, showed that mothers’ DDS was significantly associated with mean birth weight of infants and mothers with high DDS had significantly larger infants than those with low DDS. Zerfu et al. (Reference Zerfu, Umeta and Baye41), studying 374 pregnant women, found that women with inadequate DDS had a significantly higher risk of LBW and the infants’ mean birth weight was significantly lower in the inadequate group. Bekela et al. (Reference Bekela, Shimbre and Gebabo49) in a study on 354 mother–neonate indicated that number of mothers with inadequate dietary diversity was higher among LBW infants. Nsereko et al. (Reference Nsereko, Uwase and Mukabutera48), studying 367 pregnant women, showed that maternal low dietary diversity was independent determinant of LBW. Vanié et al. (Reference Vanié, Gbogouri and Edjème-Aké46) and Jamalzehi et al. (Reference Jamalzehi, Javadi and Dashipour44) also reported that the mean birth weight of newborns of mothers with low dietary diversity was significantly lower.
However, three of the included studies did not find any relationship between maternal DDS and newborn LBW. Manerkar et al. (Reference Manerkar and Gokhale35), studying 121 pregnant women (nineteen LBW, 102 NBW), reported no difference in maternal DDS between the LBW and NBW groups. Rashid et al. (Reference Rashid, Park and Macneal38), in a study on sixty-six infants (thirty-two LBW and thirty-four NBW), showed that mean DDS did not differ between mothers giving birth to LBW and NBW infants. However, the number of mothers with low DDS was non-significantly higher in the LBW group (28 %) compared with the NBW group (18 %). Alemu and Gashu(Reference Alemu and Gashu47) also reported that maternal low dietary diversity is not associated with LBW.
Discussion
The current study identified fifteen observational studies that assessed the relationship of maternal dietary diversity during pregnancy and LBW risk in offspring. The results of the reviewed articles indicate that maternal gestational DDS is negatively associated with the risk of LBW in infants. However, Manerkar et al. (Reference Ahmed, Hassen and Wakayo34), Rashid et al. (Reference Rammohan, Goli and Singh37) and Alemu and Gashu(Reference Alemu and Gashu47) did not observe such association. The small number of LBW infants and neglection of potential confounding factors on crosstalk between maternal dietary diversity and occurrence of LBW might have affected the finding.
Several maternal factors including age, smoking, chronic diseases, seasonality of food availability and socio-economic factors have been shown to be associated with maternal DDS. Gitagia et al. (Reference Gitagia, Ramkat and Mituki50), in a cross-sectional study, reported age as an important determinant of dietary diversity among women of reproductive age. Alkerwi et al. (Reference Alkerwi, Baydarlioglu and Sauvageot51) demonstrated an inverse association between the intensity of smoking and overall diet quality and reported that heavy smokers exhibited less dietary diversity in their food choices. The prospective cohort study of Conklin et al. (Reference Conklin, Monsivais and Khaw52) showed that higher diet diversity was correlated with a 30 % lower risk of developing type 2 diabetes in the United Kingdom. Besides, these maternal factors during pregnancy have been reported to act as risk factors for having LBW infants. The results of a systematic review showed that excessive gestational weight gain is associated with increased infant weight and that insufficient gestational weight gain is a risk factor for LBW infants(Reference Siega-Riz, Viswanathan and Moos53). In a cohort study, Restrepo-Méndez(Reference Restrepo-Méndez, Lawlor and Horta8) demonstrated that very young (< 16 years) or advanced maternal age (≥ 35 years) was associated with enhanced odds of LBW infants. Maternal smoking, heavy alcohol drinking and chronic diseases such as hypertension and diabetes have also been reported as risk factors for having LBW infants(Reference Graham, Zhang and Schwalberg12,Reference Chen13,Reference Zheng, Suzuki and Tanaka15) . Moreover, seasonality of food availability may act as a confounder in dietary diversity surveys in developing countries. It has been reported that food availability and access are strongly affected by seasonality and are associated with both maternal and child nutritional status(Reference Abizari, Azupogo and Nagasu54,Reference Poole, Amiri and Amiri55) .
Socio-economic status of the household is another factor that may influence dietary diversity of both mother and child. Mayén et al. (Reference Mayén, Marques-Vidal and Paccaud56) in a systematic review study found that high socio-economic status was associated with higher diet quality and diversity, in low- and middle-income countries. Kiboi et al. (Reference Kiboi, Kimiywe and Chege57) in a cross-sectional study on 254 pregnant women showed socio-economic factors including education level, employment status, monthly income, household assets and land ownership as effective factors on dietary diversity of the women. Rammohan et al. (Reference Rammohan, Goli and Singh37), in a study on 230 newly delivered women, reported that low maternal education and economic status were significantly associated with poor dietary diversity of the women.
Evidence from several studies indicated that nutrition supplements (such as Fe and folic acid) intake may be inversely or positively correlated with LBW(Reference Adu-Afarwuah, Lartey and Okronipa58–Reference da Silva Lopes, Ota and Shakya60). Intake of nutrient supplements may promote maternal resistance to infections during pregnancy, ameliorate nutritional status and improve birth consequences(Reference Keats, Haider and Tam59). An overview of twenty-three systematic reviews of randomised controlled trials focusing on nutritional interventions before or during gestation showed that multiple micronutrients supplementation and improving maternal nutritional status positively influence LBW(Reference da Silva Lopes, Ota and Shakya60).
Taken together, the above-mentioned factors can be interpreted as having a confounding effect on the relationship between DDS and LBW. However, this concern has not been addressed in several studies reviewed.
Apparently, dietary diversity during pregnancy prevents neonate LBW by affecting maternal gestational weight gain. It has been reported that high maternal dietary diversity during pregnancy positively contributes to her gestational weight gain(Reference Ali, Thaver and Khan61). Evidence indicates that mothers with higher gestational weight gain tend to deliver heavier babies(Reference Tela, Bezabih and Adhanu40,Reference Lima, Batista and Ribeiro62) . Besides, dietary diversity during pregnancy minimises the occurrence of nutritional deficiencies, in particular the development of anaemia in mothers which, in turn, leads to the improvement of fetal growth. The results of systematic reviews and meta-analysis studies demonstrated a significant association between maternal anaemia and infant LBW(Reference Rahmati, Delpishe and Azami63,Reference Figueiredo, Gomes-Filho and Silva64) . Zerfu et al. (Reference Zerfu, Umeta and Baye41) suggested that maternal dietary diversity during pregnancy is linked to a decreased risk of anaemia in the mother. In a cohort study involving 1675 pregnant women, Ghosh et al. (Reference Ghosh, Trevino and Davis65) reported that dietary diversity is positively associated with serum Hb. However, other studies found no such association(Reference Adokiya, Aryeetey and Yost66).
Various criteria or cut-off points were used to identify inadequate dietary diversity across the studies which may influence the accuracy of the results. None of the studies indicated accuracy of the criteria or cut-off points in predicting newborns’ LBW risk. Thus, it was needed to perform appropriate statistical methods such as receiver operating characteristic analysis to determine optimal threshold and to calculate the sensitivity and specificity of the different criteria or cut-off points in predicting risk of LBW.
Dietary diversity scoring was based on the number of food groups consumed by individuals, in the reviewed articles. Around 86·6 % of the reviewed articles used FAO adopted tools of WDDS (53·33 %) or MDD-W (33·33 %) for the assessment of dietary diversity. The differences between the two indicators are in the number of food groups, with nine groups in WDDS and ten in MDD-W(32). The food groups of WDDS include starchy foods, dark green leafy vegetables, meat and fish, other vegetables and fruits, vegetables and fruits rich in vitamin A,organ meats, milk and dairy products, eggs, legumes, nuts and seeds. The food groups of MDD-W are composed of grains, white roots and tubers, and plantains; pulses (beans, peas and lentils); nuts and seeds; dairy; meat, poultry and fish; eggs; dark green leafy vegetables; other vitamin A-rich fruits and vegetables; other vegetables and other fruits(32). In MDD-W, vegetables and fruits combined in one group but have separated in MDD-W. Therefore, according to the similarity of food groups between the two indicators, it seems that the method of dietary diversity scoring or the type of tool used for dietary diversity assessment are not factors that bias the findings.
Limitations of the review
Most studies conducted in urban areas, while rural areas have been neglected. In several studies, the dietary diversity data were collected after giving birth, a time that might not reflect the infant birth weight. Food intake information was collected retrospectively in most of the studies, a fact that may increase reporting bias(Reference Ventura, Loken and Mitchell67). Using methods which employ health workers/proficient enumerators who are able to bring a cultural knowledge of local foods may help to avoid such biases(Reference Hanley-Cook, Tung and Sattamini68). In addition, the period of data collection only comprised the previous 24 h, which might not be indicative of individual habitual intake. The number of LBW infants was too small in several studies, which makes the results controversial. Furthermore, there are many potential variables that can influence maternal DDS and the risk of LBW, including maternal age, gestational weight gain, gestational age, chronic diseases, smoking, alcohol use, gender of baby, infant congenital anomalies, nutritional supplements intake, seasonality of food availability and socio-economic factors and twin pregnancy. However, the confounding effects of these variables have not been addressed in several studies or the number of factors taken into account was too small.
This review is restricted by lack of study from developed countries. The scoring of low dietary diversity varied across the studies which make it difficult to compare results across studies. Lack of interventional study was another limitation. Further evidence from interventional studies is needed to confirm findings from observational studies.
Implication of the findings
The findings of the current study imply an interaction between DDS and LBW. Thus, it is suggested that implementation of nutrition education and counselling in pregnancy(Reference Demilew, Getu Degu Alene and Belachew69,70) and peri-conceptional(70) care processes are helpful to improve birth outcomes. In addition, improving maternal nutritional status through multiple micronutrients supplementation during pregnancy might be an effective strategy to reduce the risk of LBW in newborns(Reference da Silva Lopes, Ota and Shakya60,Reference Ramakrishnan71,Reference Oh, Keats and Bhutta72,Reference Zerfu and Ayele73) . Such interventions must be incorporated in health promotion strategies targeting the peri-conceptional and pregnant mothers in order to improve their dietary diversity. However, there is a need to further identify environmental factors contributing to poor DDS, in particular among low- and middle-income communities. Furthermore, pregnant mothers with poor dietary diversity must be regularly screened and properly identified and the importance of a healthy and diverse diet during pregnancy must be emphasised.
Conclusion
The results of the present study suggest that high maternal dietary diversity during pregnancy may be associated with the risk of LBW infants in developing countries.
Acknowledgements
Acknowledgements: None. Financial support: The current study was supported by Tabriz University of Medical Sciences. Conflict of interest: There are no conflicts of interest. Authorship: Both of the authors were involved in the searching and selection of the articles, data extraction and participated in manuscript writing. Both of the authors read and approved the final manuscript. Ethics of human subject participation: The protocol of the study was registered and approved in the Research Vice Chancellor of Tabriz University of Medical Sciences (IR.TBZMED.REC.1399.150) (http://ethics.research.ac.ir/IR.TBZMED.REC.1399.150) grant number of the article as follows: Grant No: 64878.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980021000276






