Fruits and vegetables promote healthy body weight and are protective against CVD and some cancers, yet are lacking in child and adult diets(Reference Kim, Moore and Galuska1, Reference Lock, Pomerleau and Causer2). Early childhood is of paramount importance for efforts to improve diet because food preferences are a primary driver of diet(Reference Birch3–Reference Russell and Worsley5) and early childhood is a sensitive period for the development of food preferences(Reference Ventura and Worobey6) that persist into later life(Reference Russell and Worsley5, Reference Mennella, Reiter and Daniels7, Reference Portella, Kajantie and Hovi8).
Breast-feeding, in addition to having direct benefits for maternal and child health(Reference Victora, Bahl and Barros9, Reference Rollins, Bhandari and Hajeebhoy10), may promote healthy diet in later childhood because it is a key factor in the development of flavour preferences. Infants are exposed to flavours from their mothers’ diets through breast milk and randomized trials have demonstrated that this exposure increases infants’ acceptance of those flavours during complementary feeding(Reference Mennella, Daniels and Reiter11, Reference Mennella, Jagnow and Beauchamp12). This mechanism may explain why, in observational studies of populations around the world, 2- to 13-year-old children breast-fed during infancy consume more fruits and vegetables than their formula-fed peers, even after accounting for key sociodemographic confounders (e.g. income, education)(Reference Scholtens, Brunekreef and Smit13–Reference de Wild, Jager and Olsen21). Alternatively, the observational nature and narrow focus of confounders included in these studies make it possible that other confounding factors can explain this association. Thus, further research on associations between breast-feeding and later child diet is needed that accounts for a wider array of likely confounders, such as the home food environment(Reference Blanchette and Brug22) and intention to breast-feed(Reference Amir and Donath23). Additionally, this research should evaluate whether associations between breast-feeding and child diet at later ages are consistent with the aforementioned biological mechanism underlying flavour learning.
To this end, we used data from the Infant Feeding Practices Study II (IFPS II), a 6-year cohort study of mother–child dyads. We hypothesized that, in accordance with the previously demonstrated effects of breast-feeding and maternal diet on infant food acceptance(Reference Mennella, Daniels and Reiter11, Reference Mennella, Jagnow and Beauchamp12), the combination of breast-feeding and higher maternal fruit and vegetable consumption during nursing would be associated with high child fruit and vegetable consumption at 12 months and 6 years, even after accounting for likely confounders not considered in previous studies.
Methods
Participants and recruitment
The IFPS II was a prospective cohort study from 2005 to 2012 with repeated measures of breast-feeding and child diet. Detailed information about participants and methods can be found elsewhere(Reference Fein, Labiner-Wolfe and Shealy24). Briefly, pregnant women ≥18 years old in their third trimester of pregnancy were recruited from a consumer opinion panel. Of the 4902 recruited, the only women invited to complete the study were those who responded to a birth screener telephone call (response rate = 82·9 %) and indicated they had singleton births at ≥35 weeks of gestation with infant birth weight ≥2·27 kg (≥5 lb). Infants with medical conditions that could affect feeding were excluded from the study. The cohort was sent questionnaires approximately monthly throughout the infants’ first year of life, with response rates ranging from 63 % to 83 %. While participants were from across the USA, the cohort was not nationally representative; compared with participants in a nationally representative sample of mothers, IFPS II participants were older, had higher educational attainment and income, and were more likely to be White, to breast-feed and to breast-feed longer(Reference Fein, Labiner-Wolfe and Shealy24).
The Year 6 Follow-Up Study (Y6FU) was conducted when the children were 6 years old. Detailed information about participants and methods can be found elsewhere(Reference Fein, Li and Chen25). The Y6FU was completed by 1542 of the 1624 mothers successfully contacted (response rate = 52 %, cooperation rate = 95 %). Compared with non-respondents, Y6FU respondents were older, more likely to be White and married, had higher educational attainment and income, and breast-fed their children longer(Reference Fein, Li and Chen25).
Breast-feeding duration
Each IFPS II questionnaire asked, ‘How old was your baby when you completely stopped breast-feeding and pumping milk?’ Mothers continuing to breast-feed at the time of the last IFPS II questionnaire they answered reported total breast-feeding duration in the Y6FU.
The first 16 weeks of life are a critical exposure period for flavour learning(Reference Mennella, Daniels and Reiter11, Reference Mennella, Jagnow and Beauchamp12); we therefore dichotomized breast-feeding duration as <16 weeks or ≥16 weeks. We fit models with two other categorizations of breast-feeding duration as sensitivity analyses. The first took into account the American Academy of Pediatrics’ recommendation to breast-feed for at least 1 year:(Reference Eidelman, Schanier and Johnston26) <16 weeks; 16 to <52 weeks (sufficient for flavour learning but less than recommended); and ≥52 weeks (meeting recommendations). The second considered the potential difference between exclusive formula-feeding and any breast-feeding: 0 weeks (exclusive formula-feeding); >0 to <16 weeks; and ≥16 weeks. In these sensitivity analyses, point estimates were similar, but due to smaller group sizes, se were larger than in the models fit with dichotomized breast-feeding duration. Only results with the dichotomized breast-feeding duration variable are presented here.
Maternal diet
Between the 3- and 4-month IFPS II questionnaires, 1463 mothers completed the National Cancer Institute’s diet history questionnaire (response rate = 82 %; Centers for Disease Control and Prevention, unpublished results). The questionnaire was modified to capture maternal diet in the past month, thus focusing on diet postpartum(Reference Fein, Labiner-Wolfe and Shealy24). A total of sixty-seven mothers were removed from the maternal diet sample due to implausible energy or carbohydrate intake (n 41) or missing postnatal data (n 26; Centers for Disease Control and Prevention, unpublished results).
Maternal daily fruit intake (servings/d) and daily vegetable intake (servings/d) were both centred at their means and included in models as continuous variables. Total energy intake was also included in all models, which energy-adjusts the fruit and vegetable intake measurements, thereby improving their validity(Reference Willett27).
Child diet
In each of the IFPS II questionnaires, mothers reported what they fed their infant in the past 7 d on a food frequency chart. Child fruit intake and vegetable intake at 12 months of age were used as the first outcomes in the present study. Both variables were dichotomized as <1·5 times/d v. ≥1·5 times/d, as was done by Rose et al. (Reference Rose, Savage and Birch28) to match the Child and Adult Care Food Program recommendations to serve fruits and vegetables three times daily.
During the Y6FU, mothers reported the frequency that their 6-year-old ate fruits (fresh, frozen or canned, not including juice) and vegetables(Reference Perrine, Galuska and Thompson15). Vegetable intake frequency was calculated as the sum of two variables: ‘Green leafy or lettuce salad, with or without other vegetables’ and ‘Other vegetables: fresh, frozen or canned (other than green leafy or lettuce salads, potatoes or cooked dried beans)’. Consistent with previous studies, both fruit and vegetable intake frequency at Y6FU were dichotomized by median split(Reference Perrine, Galuska and Thompson15). Henceforth, child fruit and vegetable intake at both ages is referred to as high consumption v. low consumption.
Covariates
To thoroughly address confounding, we adjusted for a wide range of covariates, including many not considered in previous studies. These variables were selected based on theory and previous research illustrating their associations with child diet as well as maternal diet or breast-feeding duration. Covariates included the following. (i) Maternal demographics: age; race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, non-Hispanic Asian/Pacific Islander, non-Hispanic Other); education (≤high school, 1–3 years of college, ≥4 years of college); household income as a percentage of the federal poverty line (<100, 100–185, 186–349, ≥350); marital status (married; divorced, widowed or separated; never married); parity (primiparous v. multiparous). (ii) Child characteristics: sex; preterm birth (<37 weeks); child age at time of questionnaire completion. (iii) Barriers and facilitators to breast-feeding: prenatal plans for breast-feeding duration (<6 months, 6–11 months, ≥12 months); maternal employment 3 months postpartum; childcare use 3 months postpartum. (iv) Child’s food environment: introduction to solid fruits or vegetables by the month 4 questionnaire; WIC (Special Supplemental Nutrition Program for Women, Infants, and Children) enrolment in the first year postpartum; SNAP (Supplemental Nutrition Assistance Program) enrolment at Y6FU; fruit and vegetable availability at home as a snack at Y6FU (rarely/sometimes, often, always); fast food for dinner less than once weekly at Y6FU. (v) Maternal health: pre-pregnancy BMI (underweight or normal weight, overweight, obese); smoking during pregnancy; depression (assessed in the month 2 questionnaire by the Edinburgh Postnatal Depression Scale and dichotomized per guidelines)(Reference Cox, Holden and Sagovsky29); gestational diabetes. (vi) Additional measures of maternal employment, childcare use and smoking: maternal employment at month 6, 9, 12 and any time by Y6FU; childcare use at month 6, 9 and 12; smoking during the first year postpartum and at Y6FU.
Statistical analysis
We had a final sample of 1396 mother–child dyads with complete data on breast-feeding duration and maternal diet for the current study. The level of missing data in the IFPS II is commensurate with other mother–child cohorts(Reference Boyd, Golding and Macleod30–Reference Heude, Forhan and Slama32). We imputed missing data for other variables using multiple imputation by chained equations(Reference Raghunathan, Lepkowski and Van Hoewyk33, Reference van Buuren34). The amount of missing data in all variables can be found in Table 1 and the online supplementary material, Supplemental Table S1. Continuous variables were imputed using predictive mean matching and categorical variables were imputed using the discriminant function. All reported results are based on fifty imputed data sets. Trace plots were checked for convergence and the imputation procedure achieved over 98 % efficiency for all parameter estimates. In addition to the variables included in our analytic models (both independent and dependent variables), imputation models included infant fruit and vegetable intake reported on the 6-, 7-, 9- and 10-month questionnaires, as these were strong predictors of infant fruit and vegetable intake at 12 months. Imputation models also included Y6FU measurements of maternal BMI and depression, as they were strong predictors of pre-pregnancy BMI and depression at 2 months postpartum, respectively. We had complete data on breast-feeding duration and maternal diet, so no imputation was required for the main effects or interaction of these key variables. To reduce bias in the estimated regression coefficients, interaction terms were included in the imputation models with the ‘just another variable’ approach(Reference White, Royston and Wood35). The imputation procedure was completed using PROC MI in the statistical software package SAS version 9.4. Full multiple imputation results can be found in Supplemental Tables S2 to S5; only results using the multiply imputed data are presented below. Complete case analysis results can be found in Supplemental Tables S6 and S7.
Table 1 Descriptive characteristics of the study participants stratified by duration of any breast-feeding; Infant Feeding Practices Study II (IFPS II) and Year 6 Follow-Up (Y6FU), USA, 2005–2012
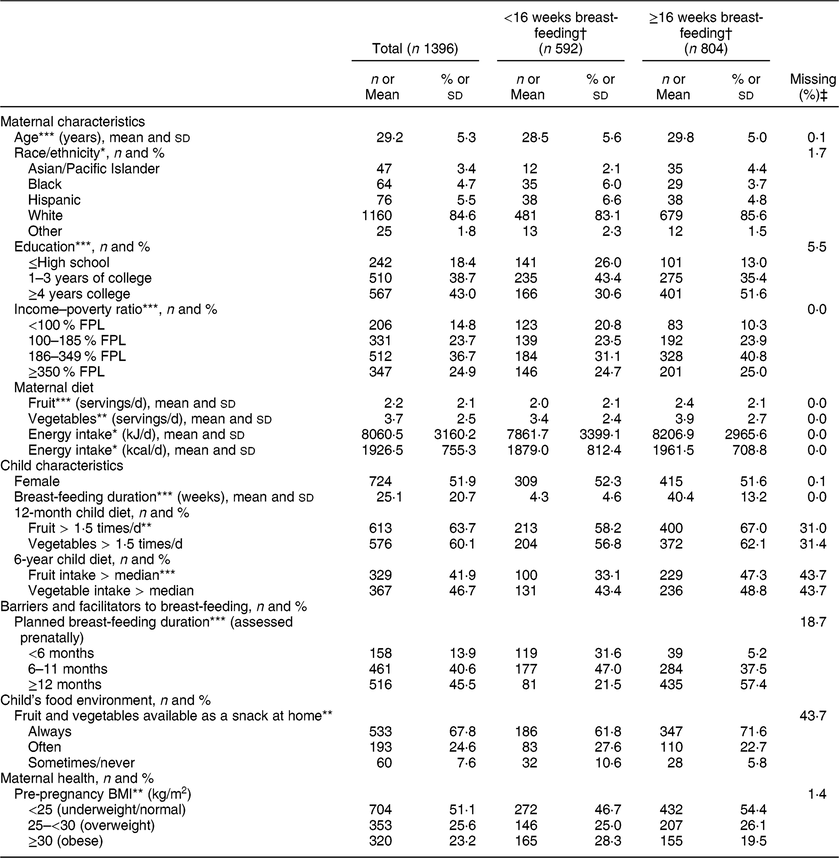
FPL, federal poverty level.
Differs by breast-feeding duration.
* P < 0·05, **P < 0·01, ***P < 0·001.
† Unadjusted statistical comparisons by breast-feeding duration were made with χ 2 or equal variance t tests.
‡ The percentage of the total sample (n 1396) with missing data for each variable.
Descriptive statistics were calculated for each independent variable. We tested for differences in these variables by breast-feeding duration using χ 2 tests and equal variance t tests. Our a priori hypotheses were that, consistent with experimental research demonstrating the combined effect of breast-feeding and maternal diet on infant acceptance of fruits and vegetables, we would observe a positive interaction between breast-feeding and maternal diet on later child diet. The outcomes in our multivariable analyses are high v. low child fruit or vegetable consumption at 12 months or 6 years of age. Logistic regression models were used to assess the association of these outcomes with breast-feeding duration, maternal diet and their interaction, adjusting for potential confounders. The impact of including each set of covariates on estimates of the main effects and interaction effect of maternal diet and breast-feeding duration are shown in Supplemental Tables S2 to S5. The type I error rate was set at 0·05. Because we considered these analyses exploratory, we did not make family-wise error rate or false discovery rate adjustments. All analyses were performed in SAS version 9.4.
Results
Children were breast-fed for an average of 25·1 weeks; 42·4 % of mothers breast-fed for less than 16 weeks even though 86·1 % intended to breast-feed for at least 24 weeks (Table 1). At 12 months, 64 % of children had high fruit consumption (≥1·5 times/d) and 60 % had high vegetable consumption (≥1·5 times/d; Table 1). At 6 years, the median fruit intake was once daily and the median vegetable intake was 1·14 times daily. At both 12 months and 6 years, significantly more children breast-fed for ≥16 weeks had high fruit consumption compared with those breast-fed for <16 weeks; there were no statistically significant differences in child vegetable consumption by breast-feeding duration (Table 1). Breast-feeding duration was also strongly associated with many demographic, maternal health and environmental variables including education, income, marital status, planned breast-feeding duration, maternal employment, childcare use, pre-pregnancy BMI, maternal smoking, and multiple measures of the child’s food environment (Table 1, Supplemental Table S1).
In fully adjusted analyses, there was a significant main effect of breast-feeding duration at 12 months; children breast-fed for ≥16 weeks had significantly higher odds of high fruit (adjusted OR = 1·42, 95 % CI 1·00, 2·01) and vegetable (adjusted OR = 1·44, 95 % CI 1·01, 2·04) consumption compared with children breast-fed for <16 weeks (Supplemental Tables S2 and S3). Neither the main effect of maternal diet during nursing nor the interaction between breast-feeding duration and maternal diet was statistically significant at 12 months (Fig. 1, Supplemental Tables S2 and S3).

Fig. 1 Multivariable-adjusted OR of high child fruit or vegetable consumption at (a) 12 months and (b) 6 years associated with a 1 serving/d increase in maternal fruit or vegetable consumption, respectively, by breast-feeding duration (•, <16 weeks (reference); ▪, ≥16 weeks), with 95 % CI represented by vertical bars; Infant Feeding Practices Study II (IFPS II) and Year 6 Follow-Up (Y6FU), USA, 2005–2012. (*)P for interaction < 0·10, *P for interaction < 0·10. All models were adjusted for maternal demographics (age, race/ethnicity, education, household income as a percentage of the federal poverty line, marital status, parity), child characteristics (sex, preterm birth, child age at time of questionnaire completion), barriers and facilitators to breast-feeding (prenatal plans for breast-feeding duration, maternal employment 3 months postpartum, childcare use 3 months postpartum), the child’s food environment (WIC enrolment in the first year postpartum, introduction to solid fruits or vegetables by the month 4 questionnaire), maternal health (pre-pregnancy BMI, smoking during pregnancy, depression, gestational diabetes), and additional measures of maternal employment, childcare use and smoking (maternal employment at month 6, 9 and 12; childcare use at month 6, 9 and 12; smoking during the first year postpartum). Year 6 models were additionally adjusted for the following variables measured only at Y6FU: fruit and vegetable availability at home as a snack, fast food for dinner less than once weekly, SNAP enrolment, maternal employment at Y6FU and maternal smoking at Y6FU. Full results can be found in the online supplementary material, Supplemental Tables S4 to S7, model 7 (WIC, Special Supplemental Nutrition Program for Women, Infants, and Children; SNAP, Supplemental Nutrition Assistance Program)
In fully adjusted analyses of child diet outcomes at 6 years, there were no significant main effects of breast-feeding duration or maternal diet, but there was a statistically significant interaction between breast-feeding duration and maternal vegetable consumption on child vegetable consumption (P for interaction = 0·037; Fig. 1, Supplemental Tables S4 and S5). Among children breast-fed for ≥16 weeks, each additional serving of vegetables in the mother’s diet during nursing was associated with a 22 % higher odds of high child vegetable consumption (adjusted OR = 1·22, 95 % CI 1·08, 1·37; Fig. 1 ). In contrast, among children breast-fed for <16 weeks, maternal vegetable consumption during nursing was not significantly associated with child vegetable consumption (adjusted OR = 1·04, 95 % CI 0·91, 1·19). The interaction between breast-feeding duration and maternal fruit consumption during nursing on child fruit consumption was not statistically significant (P = 0·096; Fig. 1).
Discussion
In fully adjusted analyses, we found that longer breast-feeding duration and higher maternal vegetable consumption while nursing had a synergistic association with child vegetable consumption at 6 years, supporting the hypothesis that exposures to the flavours of vegetables through breast milk during nursing may promote the child’s later vegetable consumption. We did not find a significant analogous interaction effect on child fruit consumption at 6 years, or on child fruit or vegetable consumption at 12 months. While previous research has demonstrated the influence of breast-feeding and maternal diet on infant food acceptance(Reference Mennella, Daniels and Reiter11, Reference Mennella, Jagnow and Beauchamp12) and that food preferences established early in life are carried forward into later life(Reference Russell and Worsley5, Reference Mennella, Reiter and Daniels7, Reference Portella, Kajantie and Hovi8), the present study is among the first to explore whether the combination of breast-feeding and maternal nursing diet is associated with diet in later childhood.
At 12 months of age, we found a significant main effect of breast-feeding duration on child fruit and vegetable consumption, even after controlling for a wide array of confounders. This result may reflect an effect of breast-feeding on food acceptance in early infancy. Many(Reference Hausner, Nicklaus and Issanchou36–Reference Forestell and Mennella38) but not all(Reference Schwartz, Chabanet and Lange39) previous studies have found that breast-fed infants are more accepting of novel fruits and vegetables than formula-fed infants. While acceptance of initially rejected foods can be improved through repeated exposure(Reference Anzman-Frasca, Ventura and Ehrenberg40), many parents do not continue serving previously rejected foods(Reference Goodell, Johnson and Antono41) perhaps because they find the recommendation to reintroduce rejected foods ten to fifteen times to promote acceptance confusing or difficult to implement(Reference Goodell, Johnson and Antono41, 42). As such, foods rejected early in infancy may not be reintroduced sufficiently to become a meaningful part of infants’ diets at 12 months of age. In the current study, infants breast-fed for <16 weeks may have been less accepting of fruits and vegetables, possibly explaining why they were significantly less likely to have high fruit or vegetable consumption at 12 months compared with children breast-fed longer.
At 6 years of age, we found a statistically significant interaction indicating that longer breast-feeding duration and higher maternal vegetable consumption had a synergistic association with high child vegetable consumption. Since vegetables are among children’s most disliked foods(Reference Fildes, van Jaarsveld and Wardle43) and child preferences are a key driver of the foods parents choose for their children(Reference Russell and Worsley5), early-life exposures that promote vegetable liking may be particularly important for promoting vegetable consumption. Although the observational nature of the current study limits causal interpretation about how early feeding experiences influence later diet, our findings are consistent with recommendations to expose infants to the flavours of healthy foods through breast milk to create a foundation for a healthy diet(Reference Anzman-Frasca, Ventura and Ehrenberg40).
Unlike child vegetable consumption, we did not find a statistically significant interaction between breast-feeding duration and maternal fruit consumption on child fruit consumption at 6 years of age. Young children may be more accepting of fruits because they have an inborn preference for sweet taste(Reference Fildes, Lopes and Moreira44–Reference Steiner46); familiarity with fruit flavours may therefore be less important for promoting child fruit consumption. However, it may be especially important to establish familiarity with vegetable flavours to help children overcome their inborn dislike for bitter tastes(Reference Fildes, Lopes and Moreira44–Reference Steiner46) and consume vegetables. Since children experience heightened neophobia, or the fear of new foods, starting at age 2 years(Reference Ventura and Worobey6), introducing vegetable flavours prior to the onset of neophobia, such as through the combination of breast-feeding and maternal vegetable consumption while nursing, may be an effective strategy for increasing their consumption(Reference Mennella, Daniels and Reiter11, Reference Mennella, Jagnow and Beauchamp12). In sum, inborn taste preferences and developmental patterns of neophobia may explain why, in the current study, we observed a statistically significant interaction between breast-feeding and maternal diet only for children who had entered their neophobic phase (i.e. only at 6 years of age) and only for vegetables.
Our study has many strengths, including the use of a prospective cohort of mother–child dyads to create models that match the biology of flavour learning during infancy and the social determinants of both breast-feeding and child diet. To model the mechanism of flavour learning through breast milk, we examined the interaction between maternal diet and breast-feeding, and we categorized breast-feeding duration according to infants’ critical developmental window for flavour learning(Reference Mennella, Daniels and Reiter11, Reference Mennella, Jagnow and Beauchamp12). Also, to isolate flavour learning through breast milk, we controlled for the timing of infants’ first exposure to solid fruits and vegetables because these exposures serve as opportunities for infants to learn the flavours of these foods and earlier exposures to vegetables tend to increase infant acceptance of novel vegetables(Reference Lange, Visalli and Jacob47). To reflect the social determinants of breast-feeding, we included common barriers like employment(Reference Thulier and Mercer48). We also adjusted for women’s planned breast-feeding duration; when mothers are asked during pregnancy how long they plan to breast-feed, they are able to factor in a broad array of barriers and facilitators to breast-feeding, which is why others have advocated for adjusting for this variable(Reference Amir and Donath23). Last, unlike previous studies, we adjusted for the child’s food environment, an important determinant of child fruit and vegetable consumption(Reference Blanchette and Brug22).
Despite these strengths, the observational nature of the present study limits the extent to which causal inference can be drawn from these results. Our results may be biased by uncontrolled or residual confounding, despite our adjustment for many likely confounders. Results may also be biased by measurement error; the FFQ used in the current study shows high validity(Reference Subar, Thompson and Kipnis49) but non-random and systematic biases in diet measurement have been reported with FFQ(Reference Kipnis, Midthune and Freedman50). Similarly, reporting error has been documented when parents complete FFQ about their children’s diets(Reference Burrows, Martin and Collins51). While others have found child vegetable intake to be strongly associated with child vegetable liking(Reference de Wild, Jager and Olsen21), child preferences were not measured in the current study and it is therefore not possible to say whether child preferences at 12 months or 6 years are associated with breast-feeding and/or maternal diet during nursing. Future studies should measure child preferences to test the hypothesis that exposures to vegetable flavours in breast milk during infancy can increase vegetable intake in later childhood by increasing child vegetable liking.
Another limitation to the current study is that there was considerable loss to follow-up over the 6-year study period. Unfortunately, for cohort studies of this duration, substantial dropout is the norm(Reference Boyd, Golding and Macleod30–Reference Heude, Forhan and Slama32). For example, of 2128 live births in another US cohort, 1279 mother–child dyads provided data for the mid-childhood visit (median 7·7 years)(Reference Oken, Baccarelli and Gold31). As such, studies on these cohorts should use rigorous missing data methods, as we have employed here.
While our use of multiple imputation was a strength that few previous studies on this topic have employed, the validity of our findings rests on the assumption of data missing at random. If data are not missing at random, the magnitude of the bias depends on the strength of the association between the missing data mechanism(s) and our variables of interest, independent of the observed population characteristics included in our multiple imputation approach. Because our multiple imputation step included many observed characteristics that have strong associations with our variables of interest, the independent association between unobserved characteristics and our variables of interest is unlikely to be strong. As such, the magnitude of bias is likely small. Nevertheless, our results must be interpreted cautiously and we encourage other investigators to repeat our analyses in different populations.
An additional direction for future studies is to carefully examine different breast-feeding durations. Using three, rather than two, breast-feeding duration categories in the current study produced similar results, but our ability to detect differences between three groups may have been limited by sample size. Of particular interest for future research is examining differences between infants exclusively formula-fed and infants breast-fed for at least 4 weeks, as there is experimental evidence that 4 weeks of exposure to a vegetable flavour through breast milk can affect infant acceptance of that flavour(Reference Mennella, Daniels and Reiter11). However, even with a larger sample, there is strong potential for confounding when comparing these groups, so conclusions must be drawn carefully.
Our findings, which suggest that the combination of breast-feeding and maternal vegetable consumption while nursing may promote later child vegetable consumption, have important implications for improving population health. However, when considering potential changes to practice and policy, it is critical to examine diet and breast-feeding in the larger biological and social contexts that shape the health behaviours of mothers and their children. First, breast-feeding is not the only opportunity for children to learn to like the flavours of healthy foods; flavour learning begins in utero, where fetuses are exposed to flavours from their mothers’ diets via the amniotic fluid(Reference Birch3, Reference Ventura and Worobey6). Flavour learning continues well after breast-feeding, with parental feeding practices and environmental factors playing a key role in children’s ultimate food preferences and diets(Reference Beckerman, Alike and Lovin52). Achieving improvements in child nutrition, and therefore population health, will likely require efforts across these stages of development. Second, within any given developmental stage, it is crucial to acknowledge the myriad factors that can facilitate or inhibit health-promoting practices. For example, the many well-established health benefits of breast-feeding(Reference Victora, Bahl and Barros9, Reference Eidelman, Schanier and Johnston26) are disproportionately enjoyed by mothers in the present and other studies(Reference Rollins, Bhandari and Hajeebhoy10, Reference Thulier and Mercer48) who have high income, high educational attainment, and who are not in paid employment. In the current study, 42·4 % of mothers stopped breast-feeding by 16 weeks even though 86·1 % intended to breast-feed for at least 24 weeks, highlighting the fact that desire to breast-feed is seldom the limiting factor. As such, population-level benefits of breast-feeding, including its potential role in promoting healthy child diet, may be best realized if clinicians, policy makers and others go beyond individual-level interventions directed at mothers and address broader contextual barriers to breast-feeding(Reference Beckerman, Alike and Lovin52). Similarly, the broader context must be considered to effectively promote healthy maternal diet during pregnancy and nursing.
Conclusion
In conclusion, we found that the combination of longer breast-feeding duration and higher maternal vegetable consumption during nursing is associated with child vegetable consumption at 6 years of age. These results align with results from randomized trials, which have demonstrated that exposing infants to vegetable flavours through breast milk can increase infants’ acceptance of vegetable flavours in solid foods(Reference Mennella, Daniels and Reiter11, Reference Mennella, Jagnow and Beauchamp12). Our results also align with observational studies of older children, which have found that breast-feeding in infancy is associated with greater vegetable intake in childhood(Reference Scholtens, Brunekreef and Smit13–Reference Okubo, Miyake and Sasaki17, Reference Grieger, Scott and Cobiac19–Reference de Wild, Jager and Olsen21). As such, our study adds to the evidence that breast-feeding, and specifically exposure to vegetable flavours through breast milk, may help to lay a foundation for acceptance of a healthy diet later in childhood.
Acknowledgements
Acknowledgements: The authors would like to thank Dr Julie Mennella for her feedback on the manuscript. Financial support: This work was supported by a National Institutes of Health (NIH) Training Grant in Academic Nutrition (J.P.B., grant number T32DK0077). The NIH had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: J.P.B. conceptualized and designed the study, performed the statistical analyses, and drafted, reviewed and revised the manuscript. E.S. oversaw the analysis and reviewed and revised the manuscript. A.K.V. conceptualized and designed the study, oversaw the analysis, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. Ethics of human subject participation: As a secondary analysis of de-identified data, the present study was deemed exempt from approval by the Harvard University and California Polytechnic State University Institutional Review Boards. Initial data collection procedures were approved by the Research Involving Human Subjects Committee of the US Food and Drug Administration and the US Office of Management and Budget.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980019001782




