Breast-feeding is one of the most important factors that can influence child health in low- and middle-income countries( Reference Black, Victora and Walker 1 , Reference Bhutta, Das and Rizvi 2 ). During lactation, women have increased requirements for energy and micronutrients( 3 , 4 ). An inadequate maternal intake of certain nutrients during this period may have consequences for both the mother’s health and nutritional status, as well as those of the breast-fed infant( Reference Allen 5 – Reference Dawodu and Tsang 7 ). The breast-milk levels of some micronutrients vary with the mother’s own stores and dietary intake; the nutrients most affected by low maternal intake and stores are thiamin, riboflavin, vitamins A, D, B6, B12, Se and iodine( Reference Allen 5 – Reference Butte, Lopez-Alarcon and Garza 8 ).
Women in low- and middle-income countries are at high risk of inadequate intake of several micronutrients( Reference Jiang, Christian and Khatry 9 , Reference Allen 10 ). Despite the particular importance of good maternal nutrition during the lactation period, there is a dearth of information on lactating mothers’ micronutrient intakes( Reference Torheim, Ferguson and Penrose 11 ) and a great need for more studies documenting the adequacy of nutrient intakes during this critical period.
Urbanization is a major trend in most low- and middle-income countries and is likely influencing dietary intake in many ways. However, few studies have assessed the adequacy of micronutrient intake among urban populations( Reference Becquey and Martin-Prevel 12 , Reference Smith 13 ). The objectives of the present paper were to assess the adequacy level of the micronutrient intakes of lactating women in a peri-urban area (Bhaktapur) in Nepal and to describe relationships between micronutrient intake adequacy, dietary diversity and sociodemographic variables.
Methods
Study design and population
A cross-sectional survey was carried out among 500 randomly selected healthy lactating women (17–44 years old) from the Bhaktapur municipality, Nepal. Bhaktapur is an urban area located 15 km east of the capital of Kathmandu and was chosen because of the socio-economic diversity of this population, which offers a unique opportunity to explore dietary variation. It has a total population of approximately 75 000, made up predominantly of the Newari ethnic group, with mostly farmers, semi-skilled or unskilled labourers and daily wage earners.
The sample size of our study was chosen based on the assumption that the prevalence of dietary deficiency of key micronutrients including Zn, Fe and vitamin B12 would be 25 %, with a desired absolute precision of 4 %. Assuming incomplete sampling from approximately 10 % of these women we calculated a final desired sample size of 500 women. For several years nutrition-related research has been undertaken in this area and demographic information has been collected and used to estimate population density in the different geographic areas (toles). In the first stage of sampling we used a probability-proportional-to-size method to select sixty-six of 160 toles. In the second stage we obtained census lists of all women who had given birth over the last 12 months in the sixty-six toles and who were still breast-feeding, and selected randomly from these lists. We had to approach 582 women in order to enrol 500 women in the study. A total of 500 lactating women (both exclusively breast-feeding and partially breast-feeding) were enrolled in the study and completed the first 24 h dietary recall. Due to loss to follow-up, the sample sizes for the second and third 24 h recalls were 487 and 477, respectively. Eleven women were excluded due to incomplete 24 h recalls; therefore the final sample size consisted of 466 lactating women who had completed three 24 h recalls.
The women participating in the study came to the hospital to receive physical examinations, dietary interviews and blood draws. The first woman was enrolled in January of 2008 and the last in February of 2009. The inclusion criteria were that the women were lactating, had no self-reported ongoing infections and were able to provide household information. Women with anaemia (Hb<12·3 g/dl) were offered free treatment with Fe supplements according to the national guidelines. All women provided written informed consent before the start of the study, which was approved by the ethical review board of the Institute of Medicine at Tribhuvan University in Nepal.
Dietary data collection
Each woman participated in three 24 h recalls( Reference Gibson and Ferguson 14 ) which were conducted by Nepali- and Newari-speaking fieldworkers. The fieldworkers were trained in interview techniques, the use of electronic scales, estimation of the volume of different foods, collection of recipes and the use of food codes by a dietitian over a 2-month period prior to the study. This included a pilot study in which each fieldworker practised the 24 h recall interview on five women. To minimize interviewer bias and to help ensure that the recalls reflected typical dietary consumption, recalls for each woman were obtained on three different days (including weekends) with 2–11 d separating the recall period, by three different fieldworkers. Data collection took place over one year in order to reflect seasonal variations in their diets.
At the hospital, a technique of multiple-pass 24 h recalls was used( Reference Gibson and Ferguson 14 ). During each 24 h recall the women were asked to name all foods and drinks consumed during the preceding day, including anything consumed outside the home, as well as the time of consumption. They were also asked to describe the ingredients and cooking methods used for any recipe prepared during the recall period. The amounts of the foods and dishes were estimated using an electronic scale (Philips scales, England) with a precision of 1 g and a maximum capacity of 5 kg, which was calibrated daily. Cooked rice was used to estimate the volume of rice, vegetable curry (tarkari) and pickles. Water was used to estimate the volume of lentil soup (dal), and fresh vegetables were used to measure the size and quantity of the vegetables in the recipes. Utensils commonly used in the households were purchased and used at the hospital for estimating amounts. Clay models were used to estimate the portion sizes of meat and fish, wooden models were used for bread, and pictures were used to estimate the size of the fruits that were consumed. Finally, the women were asked to recall snacks (foods consumed between meals) during the previous 24 h from a list of snacks prepared for the study. Additionally, recipes for items prepared at home were collected and used. For items commonly consumed or purchased outside the house, including tea, spices (masala), lentil soup, bread, vegetable curry and pickles, a set of standard recipes were developed and used based on the pilot study in which twelve recipes from each dish were collected and the averages of ingredients were taken. Information on the consumption of fortified foods was not collected. Most of the fortified foods available in the area were designed for infants and pre-school children, and were not commonly consumed by adults.
Dietary data analysis
Because there is no standard food composition table available for Nepal, a food composition table was compiled for the present study which drew upon nutrient values for foods from WorldFood 2( 15 ), the Nutritive Value of Indian Foods ( Reference Gopalan, Rama Sastri and Balasubramanian 16 ) and, where necessary, from the Thai food composition table( 17 ) or the US food composition table( 18 ). The three 24 h dietary recalls and the compiled food composition table were entered into nutrient analysis software designed for the study where it was possible to enter both dietary intake and personal recipes. The 24 h recalls were then converted into daily nutrient intakes. Retinol equivalents (RE) were calculated as the sum of retinol and β-carotene, using the following conversions: 1 µg retinol=1 µg RE and 1 µg β-carotene=0·167 µg RE( 19 ).
The prevalence of adequate nutrient intakes was assessed with the three 24 h recalls and the Institute of Medicine’s probability approach( 20 ), using the statistical software package STATA 12·0. The nutrient intakes, which were skewed, were transformed using a Box–Cox transformation. Then, using the transformed intake variables, we calculated within- and between-person variances and the best linear unbiased predictor of the usual intake for each nutrient for each woman. The best linear unbiased predictors were also used to calculate the probability of adequacy for each nutrient( Reference Joseph and Carriquiry 21 ). The usual macronutrient intake distributions were calculated by the Multiple Source Method( Reference Haubrock, Nothlings and Volatier 22 , Reference Harttig, Haubrock and Knuppel 23 ), which is characterized by a two-part shrinkage technique applied to the residuals of two regression models, one for the positive daily intake data and one for the event of consumption.
The adequacy of the micronutrient intake was assessed using the WHO/FAO (2004) Estimated Average Requirements (EAR) for vitamins A, C, B6 and B12, folate, thiamin, riboflavin, niacin and Fe( 4 ). For Ca, the Institute of Medicine EAR from 2011 was used( 24 ), and for Zn we used the International Zinc Nutrition Consultative Group EAR for a mixed or refined diet, assuming 34 % bioavailability( Reference Brown and Rivera 25 ). The estimation of the dietary Fe availability in this population was calculated according to Murphy et al.( Reference Murphy, Beaton and Calloway 26 ) and Bhargava et al.( Reference Bhargava, Bouis and Scrimshaw 27 ), and gave average bioavailabilities of 4·5 % and 1·8 %, respectively. The WHO recommends the use of a calculation of 5 or 10 % Fe absorption in developing countries, depending on the diet( 19 ). Therefore, we used the 5 % bioavailability assumption for the evaluation of Fe intake in the present study.
The sd of the requirements was calculated using the CV of the requirements and the EAR; the CV was 12·5 % for Zn( Reference Brown and Rivera 25 ), 20 % for vitamin A, 15 % for niacin, 10 % for vitamins C, B6 and B12, thiamin, riboflavin, niacin, folate( 28 ) and Ca( 24 ), and 30 % for Fe( 20 ). The probability of adequacy (PA) was calculated for each nutrient, ranging from 0 to 1, and an overall mean PA (MPA) was calculated by averaging the PA values across the eleven nutrients for each individual. When the PA for a nutrient is averaged across individuals, it is equivalent to the population prevalence of adequacy expressed as a percentage( 20 ).
The use of Black’s adaptation of the Goldberg approach to identify under- and over-reporters was investigated for the sample( Reference Black 29 ). In this approach, the estimated energy intake is divided by the BMR and the resulting BMR factor is compared with lower and upper cut-offs of the physical activity levels acceptable for the population. The usual estimated energy intake was calculated by the Multiple Source Method described above and the BMR was estimated by the FAO/WHO equation, while adding extra energy expenditures due to lactation (2092 kJ/d (500 kcal/d) for women with children below 6 months and 1674 kJ/d (400 kcal/d) for women with children above 6 months of age)( 3 ). When comparing the resulting BMR factor with the lower and upper 95 % CI for the 3 d dietary records and sedentary lifestyle according to Black( Reference Black 29 ), forty-six women were classified as under-reporters. We decided not to exclude these women since we could not rule out the possibility that they were actually low consumers with a negative energy balance and because there was no material difference to the findings in a sensitivity analysis excluding them.
Dietary diversity scores (DDS) were calculated using eight food groups, similar to the FAO methodology, with the exception of organ meat being included with meat and fish( Reference Kennedy, Ballard and Dop 30 ). The eight food groups included: (i) starchy staples; (ii) dark green leafy vegetables; (iii) other vitamin A-rich fruits and vegetables; (iv) other fruits and vegetables; (v) meat and fish; (vi) eggs; (vii) legumes, nuts and seeds; and (viii) milk and milk products. We used a restrictive minimum amount consumed of 15 g/d in order for the women to be counted as having consumed a food group( Reference Arimond, Wiesmann and Becquey 31 ). The DDS was calculated by summing the number of food groups consumed on each day and averaging it over the 3 d( Reference Arsenault, Yakes and Islam 32 ). The mean DDS for the 3 d is denoted as MDDS.
Anthropometric measurements
The measurements of the weights of the women were conducted by using a UNICEF weighing scale (Salter; SECA, Germany) and the heights were measured with a locally made board in the clinic that was calibrated weekly. The maternal BMI was calculated as weight/height2 (kg/m2); BMI<18·5 kg/m2 was considered to be underweight, while 18·5≤BMI<25·0 kg/m2 was considered a normal weight and BMI≥25·0 kg/m2 was overweight.
Statistical analysis
The data were analysed using the statistical software packages SPSS version 17 and STATA version 12. Continuous data were presented as mean and standard deviation, and, for data that were not normally distributed, as the median. Pearson correlation coefficients were calculated to describe the relationships between energy intake, MPA and MDDS.
Prior to including variables in regression models, all continuous variables were log or square-root transformed to obtain normality. Variables were categorized as follows: educational level was used as a continuous variable; parity was categorized into 1 and ≥2 children; women’s occupation was categorized into ‘no paid employment’ and ‘paid employment’; husbands’ occupation was categorized into ‘no paid employment/agriculture/carpet corker’, ‘daily wage earner’ and ‘self-employed/services/working abroad’. Seasons were defined as pre-monsoon (March–May), monsoon (June-August), post-monsoon (September–November) and winter (December–February). All covariates showing a linear association (P<0·20) in the crude regression model with MPA as outcome were included in a preliminary multiple regression model. Excluded variables were reintroduced, and those that were still significantly associated in this model (P<0·20) were retained in the final model( Reference Hosmer and Lemeshow 33 ).
We identified predictors for MPA in multiple linear regression models and present three separate models. A theory-based approach was used for selection of candidate variables for inclusion in the models. Model 1 included intake variables that would directly influence MPA, such as dietary diversity and energy intake which reflects amounts consumed. Model 2 included external factors which were hypothesized to influence dietary intake and thus micronutrient intake adequacy. An initial crude model 2 included infant’s age and sex, women’s age, parity and BMI, women’s and husband’s educational levels and occupation, socio-economic status indicated by house ownership (rent or own) and seasonality. A third model included predictors from both model 1 and 2. The intakes of the food groups were included in a separate regression model together with energy intake to examine which food groups were main determinants for the MPA. We analysed the residuals of these models to examine their fit. An adjustment for clustering within the toles was done using the ‘SVY’ group of commands for complex survey data in STATA.
Results
The mean age of the lactating women was 25·7 (sd 4·1) years (Table 1), the mean age of the breast-feeding children was 6·8 (sd 3·0) months and the mean duration of exclusive breast-feeding was 0·9 (sd 1·4) months. Forty-one per cent of the women had one child, 42 % had two children and 18 % had three or more children. The mean BMI was 22·4 (sd 3·1) kg/m2; and 78·7 % had a normal BMI (between 18·5 and 25·0 kg/m2), 16·6 % were classified as overweight and 4·7 % as underweight. Additionally, 29·7 % had completed school up to the 10th grade and 18·5 % of the women were illiterate. Most of the women (65·6 %) had no paid work, while 15·5 % were daily wage earners and 7·1 % were self-employed. Thirty-seven per cent of the women’s families rented a house, while 63 % were home owners.
Table 1 Descriptive personal and household characteristicsFootnote * of lactating women (n 466) aged 17–44 years, Bhaktapur municipality, Nepal, January 2008–February 2009

* Values are means and standard deviations or percentages.
† Intermediate, School Leaving Certificate (SLC) or bachelor’s degree.
Macro- and micronutrient intakes
The mean energy intake was 8464 (sd 1305) kJ/d (2023 (sd 312) kcal/d; Table 2). On average, the percentage of energy derived from protein was 11 %, from fat was 13 % and from carbohydrates was 76 %. The mean or median usual micronutrient intakes were below the EAR for all micronutrients except vitamin C and Zn (Table 3). The women’s mean or median prevalence of adequacy was ≤1 % for folate, riboflavin and vitamin B12; between 2 % and 25 % for thiamin, vitamin A and Ca; between 25 % and 50 % for niacin, vitamin B6, vitamin C and Fe; and 66 % for zinc; and the MPA across eleven micronutrients was 0·19 (sd 0·16). Including the twenty-three women with incomplete number of recalls (twelve women with one recall and eleven women with two recalls) resulted in only minor changes in PA while MPA was unchanged.
Table 2 Usual energy and macronutrient intakesFootnote *, and recommended intakes, of lactating women (n 466) aged 17–44 years, Bhaktapur municipality, Nepal, January 2008–February 2009
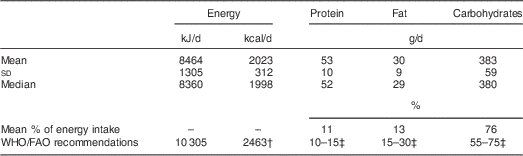
* Usual energy and macronutrient intakes according to the Multiple Source Method( Reference Haubrock, Nothlings and Volatier 22 , Reference Harttig, Haubrock and Knuppel 23 ).
† Estimated energy requirement for an ‘average’ woman aged 18–29 years weighing 50 kg and with light physical activity (8205 kJ/d (1961 kcal/d)), plus extra energy for lactation (2100 kJ/d (502 kcal/d))( 3 ).
‡ WHO/FAO recommendations for macronutrient intakes are expressed as percentages of energy( 3 ).
Table 3 Micronutrient requirements, usual micronutrient intakesFootnote *, prevalence of adequacy and MPA in lactating women (n 466) aged 17–44 years, Bhaktapur municipality, Nepal, January 2008–February 2009
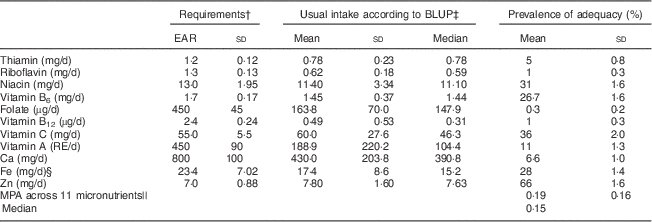
MPA, mean probability of adequacy; EAR, Estimated Average Requirement; RE, retinol equivalent.
* The mean is the overall mean of each individual’s usual intake based on 3 d recall.
† The EAR are for lactating women; the EAR for vitamins A, C, B6 and B12, folate, thiamin, riboflavin, niacin and Fe were from WHO/FAO 2004( 4 ). For Ca, the Institute of Medicine EAR from 2011 was used( 24 ). For Zn, the International Zinc Nutrition Consultative Group EAR for a mixed or refined diet was used assuming 44 % bioavailability( Reference Brown and Rivera 25 ). The sd of requirements was calculated using the CV of requirements and the EAR; the CV were 12·5 % for Zn( Reference Brown and Rivera 25 ), 20 % for vitamin A, 15 % for niacin, 10 % for vitamins C, B6, B12, thiamin, riboflavin, folate( 28 ) and Ca( 24 ), and 30 % for Fe( 20 ).
‡ Best linear unbiased predictor( Reference Joseph and Carriquiry 21 ).
§ Assuming 5 % bioavailability.
|| The mean probability of adequacy is the average of the probability of adequacy of the eleven micronutrients.
Dietary pattern and food intake
The typical dietary pattern consisted of three main meals: breakfast (with morning tea at about 07.00 hours and rice with side dishes at about 08.00 hours), lunch and dinner (Table 4). The daily diet was characterized by the consumption of large amounts of rice with side dishes (typically vegetable curry, lentil soup and pickles). The vegetable curry was usually made of potatoes, green leaves, some vegetables and masala spices (garlic, chilli, ginger, cumin, salt, oil and turmeric). These dishes were commonly consumed for both breakfast and dinner. For lunch, vegetable curry, beaten rice (flattened rice), unleavened bread (roti) and tea were most commonly consumed. Snacking was relatively frequent (by 76 %), and the most common snacks were buns, doughnuts, samosa and biscuits. Alcohol, mainly rice beer, was consumed by 23 % of the women and the mean intake was 125 g/d. When looking at the use of ready-made foods (purchased food, made outside the household), ready-made vegetable curry and ready-made pickles were the most commonly consumed (by 30 % and 25 %, respectively).
Table 4 Percentage who consumed main types of dishes for breakfast, lunch and dinner, and the dishes’ contribution to energy intake (based on three 24 h recalls), among lactating women (n 466) aged 17–44 years, Bhaktapur municipality, Nepal, January 2008–February 2009
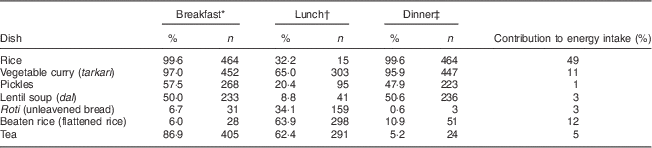
* Breakfast includes foods and drinks consumed from 06.00 hours in the morning to 12.00 hours.
† Lunch includes foods and drinks consumed from 12.00 to 18.00 hours.
‡ Dinner includes foods and drinks consumed from 18.00 to 0.00 hours.
The main contributors to the women’s total energy intake were white cooked rice, beaten rice and vegetable curry, contributing 49 %, 12 % and 11 % of the energy, respectively (Table 4). Both roti and dal contributed 3 % of the total energy intake.
The MDDS ranged from 1·3 to 6·7, with a mean of 3·9 (sd 1·0). Starchy staples were consumed by all the women, whereas dark green leafy vegetables, legumes, nuts and seeds, and other fruits and vegetables were consumed by 79–83 %. The meat and fish food group was consumed by 62 %, with meat representing 98 % of the intake from this group. Milk (separately or with tea) was consumed by 68 %, including curd, which was consumed by 13 %. The meat and milk were mainly from buffalo, with mean daily intakes of 47 g and 94 g, respectively; while the eggs (consumed by 25 %) were mainly from chickens (mean daily intake was 23 g).
Determinants of mean probability of adequacy
The MPA was highly correlated with the energy intake (r=0·71, P<0·001) and it was also significantly correlated with the MDDS (r=0·30, P<0·001). In the multivariate analysis controlling for energy, the association between the MPA and MDDS was attenuated but still significant (Table 5).
Table 5 Summary of multiple linear regression models for variables predicting MPAFootnote * of micronutrient intakes among lactating women (n 466) aged 17–44 years, Bhaktapur municipality, Nepal, January 2008–February 2009

MPA, mean probability of adequacy.
* Square-root transformed.
† Linear regression model with MPA as the dependent variable and the independent variables that remained significant at the P<0·20 level after initial testing. Variables which were tested but not found significant were infant’s age and sex, parity, women’s occupation and husband’s occupation.
‡ Natural-log transformed.
§ Usual energy intake according to the Multiple Source Method( Reference Haubrock, Nothlings and Volatier 22 , Reference Harttig, Haubrock and Knuppel 23 ).
|| Mean dietary diversity score.
¶ Treated as continuous variable from 1 (no schooling) to 6 (higher education).
In the second multivariate model, the MPA among the women was positively associated with the women’s age, educational level, ownership of the house they were living in and the winter season. When combining all covariates in a joint regression model, the women’s education, house ownership, and winter or pre-monsoon season had a significant positive association with MPA, along with energy intake and MDDS. The final model explained 56 % of the variance in the MPA (Table 5).
Multivariate regression analyses were conducted to determine which of the eight food groups were predictive of the MPA (data not shown). In a regression model unadjusted for energy intake, the intake of all food groups was significantly positively associated with the MPA. When adjusting for energy intake, the intake of staples was significantly negatively associated with the MPA, whereas the intakes of dark green leafy vegetables, vitamin A-rich and other fruits and vegetables, as well as meat and fish, remained significant positive predictors of the MPA. Adjusting for sociodemographic factors did not change these findings.
Discussion
The adequacy of the overall micronutrient intake among lactating women was low (19 %) in this peri-urban area of Nepal, and the adequacy was particularly low for some micronutrients (riboflavin, vitamin B12, folate, thiamin, Ca and vitamin A). The high micronutrient requirements for lactating women combined with a low intake of micronutrient-rich foods are the main explanatory factors for the low MPA.
The lactating women in the present study had an energy intake below their estimated requirements; protein intake was within the acceptable range of 10–15 % of the energy contribution, whereas the fat intake was in the lower range and the carbohydrate intake was correspondingly high, compared with the WHO/FAO recommendations( 3 ). These findings are in line with other studies evaluating the diet of Nepalese women( Reference Reynolds, Moser and Acharya 34 – Reference Parajuli, Umezaki and Watanabe 36 ). Rice contributed about 61 % of the women’s total energy intake: rice with vegetable curry and, to a certain extent, lentil soup was widely consumed by the women, as described in other studies from Nepal( Reference Ohno, Hirai and Sato 35 , Reference Parajuli, Umezaki and Watanabe 36 ).
The low adequacy of the intake of folate, vitamin B12 and riboflavin in the present study coincides with the findings of studies among women of reproductive age from Burkina Faso( Reference Becquey and Martin-Prevel 12 ), Mali( Reference Arimond, Wiesmann and Becquey 31 ) and Bangladesh( Reference Arsenault, Yakes and Islam 32 ). Additionally, vitamin B12 deficiency is quite common and is mainly explained by a diet low in animal products( Reference Allen 37 ). Two previous studies of pregnant women in Nepal documented that vitamin B12 deficiency (serum level<150 pmol/l) was common; 49 % in Kathmandu( Reference Bondevik, Schneede and Refsum 38 ) and 28 % in the Sarlahi District( Reference Jiang, Christian and Khatry 9 ). In our study, the main sources of vitamin B12 were meat, dairy products and eggs, which were consumed in small amounts by 62 %, 68 % and 25 % of the women, respectively.
Two prior studies of pregnant Nepali women each found a low prevalence of folate deficiency: 7 %<4·5 nmol/l( Reference Bondevik, Schneede and Refsum 38 ) and 11 %<6·7 nmol/l( Reference Jiang, Christian and Khatry 9 ). A study on folate status in Nepali infants found that folate deficiency was non-existent( Reference Ulak, Chandyo and Adhikari 39 ). It is therefore surprising that the probability of adequate folate intake was close to zero for the women in our study. This low folate intake could be due to an underestimation of the intake of folate-containing foods. Although folate-containing green leafy vegetables and lentils were consumed by more than 80 % of the women in the study and were the main sources of folate (41 % and 15 %, respectively), the reported quantities consumed were also small (65 g/d and 35 g/d, respectively). The low folate intake might also be due to an underestimation of the folate content of the foods in the food composition table, which is a recognized problem( 40 ).
Only one-third of the women in our study had a diet which covered the EAR for Fe. In another analysis of the same population, we found a low prevalence of anaemia and Fe deficiency, which may be explained by the widespread use of Fe/folic acid supplements during pregnancy( Reference Henjum, Manger and Skeie 41 ). Because the lifespan of erythrocytes is >90 d, the intake of prenatal Fe supplements is likely to have had a positive effect on Hb concentration for the first few postnatal months. It is unlikely that these women cover their needs for Fe through their diet, based on the low intakes of Fe-rich foods. Our estimates of Zn intake are in line with other studies of Nepalese women of reproductive age( Reference Parajuli, Umezaki and Watanabe 36 , Reference Chandyo, Strand and Mathisen 42 ). A study from Bhaktapur showed that more than three-quarters of the women were Zn deficient as defined by low plasma Zn concentrations, despite a relatively high intake of Zn (mean intake of 8·6 mg/d)( Reference Chandyo, Strand and Mathisen 42 ). One explanation could be that the typically high intake of phytic acid in the Nepali diet inhibits absorption of Zn or that the food composition table might not be reflecting the actual content of Zn. Another explanation could be that Zn intake is a poor marker of Zn status. In fact, Chandyo et al. demonstrated that phytic acid, but not Zn intake, predicted plasma Zn concentration and that phytic acid was negatively associated with plasma Zn levels( Reference Chandyo, Strand and Mathisen 42 ).
The women’s diet was characterized by a low DDS and similar to those previously reported in women in Bangladesh using the same definition of dietary diversity( Reference Arsenault, Yakes and Islam 32 ). The correlation between dietary diversity and overall micronutrient intake adequacy was also similar to what was found among the Bangladeshi women( Reference Arsenault, Yakes and Islam 32 ), but not as strong as found among women from five resource-poor settings in five developing countries( Reference Arimond, Wiesmann and Becquey 31 ). The MPA was mainly determined by the energy intake and dietary diversity.
One-third of the women had bought ready-made foods, but these foods covered only 1 % of their energy intake. Another urban food practice was snacking (consumption between meals), which was practised by 76 % of the women and contributed to 4 % of the energy intake. The most frequent snacks were ready-made samosa, doughnuts and different types of local baked goods (sel, bom, puri, momo and nimki), most of which had a high content of fat and sugar( Reference Steyn, Mchiza and Hill 43 ).
The lactating women in the present study represented a relatively well-off Nepali peri-urban population; nevertheless, their level of micronutrient inadequacy was high and this was partly due to a low dietary diversity. Strategies to increase dietary diversity, and in particular the intakes of vitamin-A rich fruits and vegetables, eggs, dairy products, meat and fish, are warranted in this population. Other strategies such as micronutrient supplementation and fortification should also be considered( Reference Mason, Saldanha and Ramakrishnan 44 ). The intake of processed foods was relatively low at the time of the study; however, changes and ‘Westernization’ of the diet, and the potential consequences, should be monitored( Reference Smith 13 , Reference Turin, Shahana and Wangchuk 45 ).
Our study had a number of strengths. For example, we had a representative sample of lactating women, with a relatively large sample compared with most other dietary studies. We knew the population size of each cluster (tole) from previous studies in this area and the probability of selecting a cluster was proportional to its population size. A list of eligible women from all clusters was generated and the women were randomly selected from the list. Based on this procedure all the women had approximately similar probability of being selected. We therefore believe that that our sampling approach yielded a representative sample of the population living in and around Bhaktapur municipality. There is, of course, a risk that some of our population estimates were inaccurate or that the population had changed since our last census. However, we do not think that this would have had any meaningful impact on our estimates, which is also supported by the fact that the design effect (i.e. the effect of clustering) was negligible. We had three interactive 24 h recalls with personal recipes adapted to local foods which are more likely to represent typical diet than a single recall. Similarly the fact that interviews were done throughout the year enabled us to capture seasonal variations in food. However, a weakness of our study was the reliance on external food composition tables, which may not reflect the true nutrient composition of local Nepali foods.
Conclusions
The lactating women in this peri-urban area had an inadequate intake of several micronutrients, which may compromise their own health as well as that of their infants. The main causes of the low MPA were low dietary diversity and low quantities of micronutrient-rich foods. Investigations into the consequences of maternal and infant micronutrient status are warranted, as is the implementation of policies and programmes to improve the nutritional situation of women and children in Nepal.
Acknowledgements
Financial support: This work was supported by a grant from the Norwegian Council of Universities’ Committee for Development Research and Education (project number NUFUSM-2007/10177); the Research Council of Norway (project number 172226); and a grant from the GCRieber Funds and Feed the Future Food Security Innovation Lab: Collaborative Research on Nutrition, which is funded by the US Agency for International Development and the National Institutes of Health (training grant number T32 #DK 007703). The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: T.A.S., R.C., S.H., W.W.F. and P.S.S. formulated the research questions and designed the study; R.C. and S.H. conducted the data collection; S.H. and L.E.T. analysed the data; S.H., L.E.T. and A.L.T-L. wrote the paper; S.H., L.E.T. and T.A.S. had primary responsibility for the final content; all authors read and approved the final manuscript. Ethics of human subject participation: Ethical clearance was given by the Institute of Medicine at Tribhuvan University in Nepal.








