Fe requirements in girls increase dramatically during adolescence, from a pre-adolescent requirement of approximately 0·8 mg/d to as much as 2·2 mg/d, due to increases in blood volume and lean body mass, and the onset of menses(Reference Beard1). Pregnancy increases Fe requirements even further, to 7·5 mg/d in the third trimester(Reference Milman2). Adolescent girls are at increased risk of adverse pregnancy outcomes associated with anaemia(Reference Mahavarkar, Madhu and Mule3, Reference Briggs, Hopman and Jamieson4). Where Fe-deficiency anaemia is highly prevalent and childbearing at young ages is common, there is an urgent public health need to prevent anaemia among adolescent girls(Reference Kurz and Galloway5, Reference Gupta and Jain6).
The prevalence of anaemia among girls aged 15–19 years in India is 56 %(7). Girls in this age group account for 17 % of total fertility in India; 3 % of girls aged 15 years and 36 % of girls aged 19 years will have begun childbearing(7). The provision of Fe and folic acid supplements to pregnant women is an integral part of the Reproductive and Child Health Programme in India. The programme recommends that women consume 100 daily doses of Fe (100 mg) and folic acid (400 μg) during pregnancy. In practice, although 65 % of mothers receive Fe and folic acid supplements, only 23 % of women consume these supplements for at least 90 d(7). This low level of adherence may be partly due to the frequent gastrointestinal side-effects of Fe supplements(Reference Reveiz, Gyte and Cuervo8).
In the context of low-income countries, there is a need to develop low-cost, locally produced micronutrient supplements to ensure the sustainability of anaemia prevention campaigns. Such supplements must be palatable and culturally acceptable. In the present study we investigated the effectiveness of leaf concentrate for treating anaemia in adolescent girls in urban India. Leaf concentrate was discovered in France in the 18th century and developed as a foodstuff in England between 1940 and 1970(Reference Pirie9). It has since been promoted by several non-governmental organisations, including Find Your Feet in the UK, Leaf for Life in the USA and the Association pour la Promotion des Extraits Foliaires en Nutrition (APEF) in France, as a sustainable form of protein and micronutrient supplementation in low-income communities(Reference Kennedy10, 11). In the present study we compared daily supplements of leaf concentrate with daily supplements of Fe and folic acid for their effects on Hb, serum ferritin and other anaemia-related blood parameters.
Methods
Study population
Our target population was anaemic adolescent girls aged 14–18 years living in a low-income area of Jaipur. Girls were contacted by investigators working door-to-door with the help of local community workers. A rapport was established with the girls at meetings held on three or four occasions at a central location in the community. At these meetings the study’s objectives were explained by the investigators. Girls who were willing to participate, were unmarried and free of any chronic ailment were eligible to have their Hb measured to determine whether they were anaemic. We aimed to achieve a sample size of fifty girls in each arm of the trial, corresponding to 90 % power to detect a 10 % difference (between and within groups) in mean Hb (from 10·5 to 11·5 g/dl, sd 1·5 g/dl for both measurements) at the 5 % level of significance.
Study design
Hb was measured for 163 adolescent girls, of whom 102 (62·6 %) were anaemic (Hb < 12 g/dl)(Reference Blanc, Finch and Hallberg12). These 102 girls were randomized into two groups by selecting alternately from a list ordered by Hb level. The numbers allocated were fifty-four girls to the Fe and folic acid (IFA) group and forty-eight girls to the leaf concentrate (LC) group. The IFA group received a single daily tablet of Fe (60 mg as FeSO4) and folic acid (500 μg; Rajasthan Drugs and Pharmaceutical Ltd, Jaipur, India). The LC group received 10 g of dry lucerne leaf concentrate powder (France-Luzerne Agricultural Co-operative, Aulnay-aux-Planches, France) containing 5 mg Fe and 13 μg folic acid (Table 1). The leaf concentrate was given in a pouch which could be taken orally and drunk with plain water, lemon water or buttermilk.
Table 1 Composition of lucerne leaf concentrate

DRI, Dietary Reference Intake.
*Retinol activity equivalents (RAE).
†Corresponding to ascorbic acid added during manufacturing to prevent oxidation.
Each participant was given a calendar and asked to put a tick on each date when she consumed the supplement and a cross whenever she forgot to consume it or did not consume it. Daily monitoring visits were made to the home of each participant with the help of local community workers. Intestinal parasitic infestation is known to be a major cause of anaemia; hence each participant was given a single albendazole tablet (Zentel®, containing 400 mg albendazole) before the start of the trial.
Dietary intakes were measured at baseline using a 24 h dietary recall method and standardized utensils. These measurements were converted into daily energy, protein and Fe intakes(Reference Gopalan, Ramasastri and Balasubramanian13).
Biochemical analyses
Blood parameter measurements for each trial participant were made at baseline and at the end of the trial (135 d). Venous blood samples were collected at home visits and immediately prepared for biological measurements. Hb was measured by the cyanmethaemoglobin method, and anaemia was defined as Hb < 12 g/dl. Microcytosis, a sign of Fe deficiency, was detected by measuring mean red cell volume (MCV) using an electronic counter (Adonis 19 Plus; Axon Instruments, Sunnyvale, CA, USA) in blood samples collected in sterile Vacutainers containing K3EDTA to prevent coagulation (Becton, Dickinson & Co., Franklin Lakes, NJ, USA). Fe status was determined by measuring serum Fe and total Fe-binding capacity with a commercial reagent kit (Raichem; Hemagen Diagnostics, Columbia, MD, USA), and by measuring ferritin in serum samples frozen at −29°C using a chemiluminescence kit (Immulite; Diagnostic Products Corporation, Deerfield, IL, USA). Percentage of transferrin saturation was calculated manually (100 × serum Fe/total Fe-binding capacity). Fe deficiency was defined as ferritin <12 μg/ml, noting that total Fe-binding capacity may also detect Fe deficiency in the presence of inflammation, tending to maintain serum ferritin at a higher level than 12 μg/ml(Reference Konijn14).
Data analyses
Data were entered into a computer using Microsoft® Excel (Redmond, WA, USA) and were analysed using the STATA statistical software package release 10 (StataCorp., College Station, TX, USA). Differences in mean blood levels were tested using Student’s t test and differences between proportions were tested using the χ 2 test or Fisher’s exact test. Serum Fe and serum ferritin levels were log-transformed. Linear regression models were fitted for each blood parameter, in which the dependent variable was the final value and the independent variables were the baseline value and the intervention (LC v. IFA). These models estimated any difference in effectiveness of LC v. IFA adjusted for baseline value.
Ethical approval
The study was approved by the ethical committee of the Department of Home Science, University of Rajasthan, Jaipur. Permission to carry out the study at ICDS Anganwadi centres was obtained from the Department of Women and Child Development, Government of Rajasthan. Written informed consent was obtained from the participants and their parent or guardian.
Results
Characteristics of trial participants
The study was conducted in an area of low socio-economic status (mean per capita monthly income approximately $US 24). The majority of the girls (71 %) lived with their families (average six members) in pucca (brick and stone construction) or semi-pucca (brick and mud construction) houses. Only 17 % of the girls had graduated from high school and 9 % had received no education. The mean age of the subjects was 16·8 years. The age at menarche ranged from 12·5 to 15·6 years (mean 13 years).
The mean height of the subjects was 151 (sd 4) cm and their mean weight was 45·7 (sd 5·4) kg; mean BMI was 19·8 (sd 2·3) kg/m2. All participants were within the WHO reference range for BMI. The mean daily energy intake of the subjects was below the recommended daily allowance (RDA; 8619 kJ/d (2060 kcal/d)) in both groups: 7452 (sd 879) kJ/d (1781 (sd 210) kcal/d) in the IFA group; 7159 (sd 1732) kJ/d (1711 (sd 414) kcal/d) in the LC group. Protein intake was also below the RDA (50 g/d): 42 (sd 10) g/d in the IFA group; 43 (sd 8) g/d in the LC group. The Fe content of the diet was also poor: 16·7 (sd 14·6) mg/d in the IFA group; 17·5 (sd 5·1) mg/d in the LC group (RDA = 30 mg/d).
There was no statistical evidence (for means, Student’s t test, P > 0·05; for proportions, χ 2 test, P > 0·05) that any of the above characteristics (age, age at menarche, education, household characteristics, BMI, dietary intakes) differed between the two trial groups.
Loss to follow-up and adherence
Fourteen of the fifty-four participants in the IFA group and two of the forty-eight participants in the LC group were excluded from the analysis because they had no final blood measurements: eleven of the fourteen lost from the IFA group withdrew from the trial due to apparent side-effects of the supplement (nausea, vomiting, diarrhoea); the three other girls in the IFA group were lost to follow-up because they moved out of the study area. One girl in the LC group withdrew from the trial because she did not like the taste of the supplement and the other moved out of the study area. Hence the proportions of girls in the IFA and LC groups who withdrew due to side-effects were 20·4 % (11/54) and 2·1 % (1/48), respectively (Fisher’s exact test, P = 0·005). Adherence for participants who completed the trial (the number of days on which the participant recorded that they had taken the supplement) was slightly better in the IFA than in the LC group: 126 (sd 7) d in the IFA group v. 120 (sd 11) d in the LC group (Student’s t test, P = 0·004). Mean baseline Hb for the sixteen participants lost to follow-up did not differ from the mean for those who completed the trial (Student’s t test, P = 0·5).
Baseline and post-intervention blood measurements
At baseline (among the eighty-six trial participants who were not lost to follow-up), four girls (4·7 %) were severely anaemic (Hb < 7 g/dl), twenty-two (25·6 %) were moderately anaemic (Hb ≥ 7 g/dl, <10 g/dl) and sixty (69·8 %) were mildly anaemic (Hb ≥ 10 g/dl, <12 g/dl). Although the LC group had higher proportions of severely anaemic (6·5 % (3/46) v. 2·5 % (1/40)) and moderately anaemic (28·3 % (13/46) v. 22·5 % (9/40)) girls, the overall proportions by severity of anaemia were statistically equivalent (χ 2 test, P = 0·6). None of the mean baseline values of blood parameters differed between the two groups with the exception of transferrin saturation, which was lower in the IFA group (Student’s t test, P = 0·006), and serum ferritin, which was lower in the LC group (Student’s t test, P = 0·01; Table 2). There was a strong correlation at baseline between Hb and ferritin (r = 0·49, P < 0·001) and between MCV and ferritin (r = 0·36, P < 0·001).
Table 2 Baseline and final blood parameters: adolescent girls (n 86) aged 14–18 years, Jaipur, India
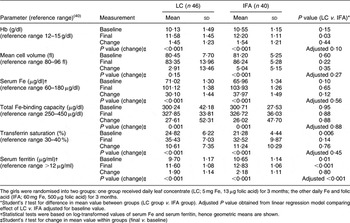
The girls were randomised into two groups: one group received daily leaf concentrate (LC; 5 mg Fe, 13 μg folic acid) for 3 months; the other daily Fe and folic acid (IFA; 60 mg Fe, 500 μg folic acid) for 3 months.
*Student’s t test for difference in mean value between groups (LC group v. IFA group). Adjusted P value obtained from linear regression model comparing effect of LC v. IFA adjusted for baseline value.
†Statistical tests were based on log-transformed values of serum Fe and serum ferritin, hence geometric means are shown.
‡Student’s t test for change in mean value within groups (final v. baseline)
At the end of the trial, none of the eighty-six participants were severely anaemic (Hb < 7 g/dl), nine (10·5 %) were moderately anaemic (Hb ≥ 7 g/dl, <10 g/dl) and twenty-six (30·2 %) were mildly anaemic (Hb ≥ 10 g/dl, <12 g/dl); fifty-one (59·3 %) had normal Hb levels (≥12 g/dl). The LC group had a higher proportion of girls with moderate anaemia (17·4 % (8/46) v. 2·5 % (1/40)) and a correspondingly lower proportion of girls with normal Hb (52·2 % (24/46) v. 67·5 % (27/40)), but statistical evidence of an overall difference in these proportions was weak (χ 2 test, P = 0·07).
At the end of the trial there was strong evidence (Student’s t test, P < 0·001) of improvements in all of the blood parameters within both groups, with the exception of MCV in the LC group (Student’s t test, P = 0·2). Post-intervention means of serum Fe, total Fe-binding capacity, transferrin saturation and MCV were the same in both groups, but the mean values of Hb and serum ferritin were higher in the IFA group (Table 2). However, the differences between final and baseline mean blood parameter values were the same in both groups for all blood parameters (Student’s t test, P > 0·05; Table 2).
Linear regression models were fitted for each blood parameter, in which the dependent variable was the final value and the independent variables were the baseline value and the intervention (LC v. IFA). These models showed that, after adjustment for baseline value, LC was neither more nor less effective than IFA, except for a smaller increase in serum ferritin (LC less effective by 1·08 (95 % CI 1·05, 1·11) μg/ml, P < 0·001). These models also showed that, with the exception of serum Fe and total Fe-binding capacity, the mean increase in each blood parameter was inversely proportional to its baseline value, i.e. participants with the lowest baseline values showed the biggest improvement.
Discussion
The present study has demonstrated that daily servings of leaf concentrate, containing 5 mg Fe and 13 μg folic acid, are as effective as daily supplements containing 60 mg Fe and 500 μg folic acid for treating anaemia in adolescent girls. Similar improvements in the blood parameters of the participants were seen in both arms of the trial, suggesting that the lower Fe content of leaf concentrate may be offset by better bioavailability of Fe in leaf concentrate and/or synergistic effects of other components of leaf concentrate. The correlations at baseline between Hb and ferritin (r = 0.49, P < 0·001) and between MCV and ferritin (r = 0·36, P < 0·001) indicate that Fe deficiency contributed significantly to the physiopathology of anaemia and microcytosis among the girls in our study. The initial and final values of the blood parameters were consistent with successful treatment of Fe-deficiency anaemia, and it is conceivable that a longer period of supplementation would have brought about further improvements in all blood parameters. We also found that fewer girls withdrew from the leaf concentrate arm of the trial due to side-effects.
Our study is the first randomised controlled trial of leaf concentrate as an alternative to Fe and folic acid supplements. It provides the strongest evidence to date for the effectiveness of leaf concentrate as a food-based approach to combating micronutrient deficiencies, and is a first step towards substantiating the predominantly anecdotal evidence that has been reported over the past few years by advocates of leaf concentrate(11). Our findings are of particular importance to public health campaigns which aim to combat anaemia, given the need to find supplements which are palatable and without side-effects, and which have the potential for local production(Reference Kennedy10). Leaf concentrate, which is obtained after near-total exclusion of fibres and phytates, is likely to be a particularly acceptable food-based supplement in our study population, given that >90 % of people consume dark green leafy vegetables at least once weekly(7).
In addition to Fe and folic acid, lucerne leaf concentrate contains nutritionally beneficial amounts of β-carotene (54 % of RDA), vitamin E (66 % of RDA), Ca (26 % of RDA) and Cu (8 % of RDA; Table 1). Evidence for synergistic effects of these micronutrients on Fe and folic acid supplementation for the treatment of anaemia is inconsistent(Reference Gera, Sachdev and Nestel15–Reference Kolsteren, Rahman, Hilderbrand and Diniz19), although it is known that Cu has a role in haematopoiesis(Reference Hart, Steenbock, Waddell and Elvehjem20) and vitamin A and β-carotene have been shown to improve Fe absorption, possibly by preventing the inhibitory effects of phytates and polyphenols(Reference Garcia-Casal, Layrisse, Solano, Baron, Arguello, Llovera, Ramírez, Leets and Tropper21, Reference Gargari, Razavieh, Mahboob, Niknafs and Kooshavar22). Regardless of these possible synergies, it is indisputable that girls approaching childbearing age would benefit from the correction of other prevalent nutritional deficiencies, particularly of vitamin A(Reference Ahmed, Khan, Banu, Qazi and Akhtaruzzaman23–Reference Thankachan, Muthayya, Walczyk, Kurpad and Hurrell27) and Ca(Reference Kumar, Devi, Batra, Singh and Shukla28).
The acceptability and sustainability of leaf concentrate as an alternative to pharmaceutical supplements are key issues. It was reported from a similar setting that girls were aware of the symptoms of anaemia and knew that these could be remedied by consumption of green leafy vegetables and by Fe tablets and syrups(Reference Kanani29). The absence of side-effects from consumption of leaf concentrate may address the problem of low adherence to anaemia prevention campaigns based on Fe tablets(Reference Deshmukh, Garg and Bharambe30). This advantage may derive from better bioavailability of Fe within leaf concentrate, as suggested by trials of other food-based supplements(Reference Mani, Iyer, Dhruv, Mani and Sharma31, Reference Mohankumar and Bhavani32). Reliable production of leaf concentrate has been demonstrated successfully on industrial and artisanal scales: the former at an ex-works cost of 1000€/t, hence <4€ per annum for the supplement given in the present study; the latter has potential benefits at a community level, including income generation and cultivation of nitrogen-fixing crops(Reference Kennedy10, Reference Zanin33).
The main limitation of our study was the unmeasured effect of inflammation(Reference Mburu, Thurnham, Mwaniki, Muniu, Alumasa and de Wagt34), which could have confounded our results if unequally distributed between the two groups or biased our results if resolved by anthelmintic treatment. When inflammation is present, total Fe-binding capacity tends to decrease and ferritin tends to increase(Reference Konijn14). The mean baseline values of total Fe-binding capacity were not elevated in our study groups (Table 2), suggesting that chronic inflammation was present in the population. Although both groups had low mean serum ferritin levels and cell volumes, consistent with the presence of Fe-deficiency anaemia, the increases in serum ferritin were modest in both groups. While it is possible that consumption of tea and phytates reduced Fe absorption(Reference Zijp, Korver and Tijburg35), improvements in inflammatory status due to anthelmintic treatment could have suppressed the increases in ferritin which would be expected as a result of Fe supplementation. Anthelmintic treatment has been associated with increases in Hb(Reference Gulani, Nagpal, Osmond and Sachdev36), and we cannot discount the possible effect of anthelmintic treatment on our results. However, evidence suggests that anthelmintic drugs are most effective when combined with Fe supplementation(Reference Gulani, Nagpal, Osmond and Sachdev36, Reference Nga, Winichagoon, Dijkhuizen, Khan, Wasantwisut, Furr and Wieringa37), and the emerging consensus is that an integrated approach to anaemia treatment and prevention is required(Reference Hall38). Our results show that such an approach would be equally effective whether based on leaf concentrate or Fe and folic acid supplements.
The slightly unequal numbers in each group at baseline may have been due to a failure to conceal allocation, hence there is a risk of some selection bias. It is unlikely that the higher losses to follow-up in the IFA group would have substantially affected our findings, since the characteristics of the girls who were lost to follow-up did not differ from those who completed follow-up. Also, the difference in acceptability of the two supplements, as indicated by losses to follow-up due to side-effects of supplementation, was an important finding of our study. That the LC group showed a smaller increase in serum ferritin could be due to a lower level of serum ferritin at baseline in the LC group. The increases in total Fe-binding capacity, serum Fe and transferrin saturation did not differ between the two groups, suggesting that the amount of Fe available for Hb production was similar.
In summary, we have demonstrated in a small trial that leaf concentrate is a viable, and more palatable, alternative to Fe and folic acid supplements for treating anaemia in adolescent girls from communities in which anaemia is highly prevalent. It remains to replicate our findings in larger randomised controlled trials (including markers of inflammation) and among diverse target populations, and to study further the economical aspects of various types of leaf concentrate production.
Acknowledgements
Source of funding: The study was funded by APEF, a French non-profit organisation (registration no. 93/2617, Paris, June 1993), which was established to promote leaf concentrate as a nutritional supplement to combat malnutrition in low-income countries. Conflicts of interest: None of the authors has any conflicts of interest to declare. Authors’ contributions: S.V. was the field researcher; B.M. conceived of and supervised the study; E.B. and G.J.D. provided technical support and guidance; S.M.C. analysed the data. All authors were involved in drafting and revising the manuscript.




