Breast cancer is the most common cancer among women worldwide with the incidence and mortality varying from countries and regions(Reference Torre, Bray and Siegel1). Environmental factors, especially diet, may play important roles in its tissue growth and tumour progression(Reference De Cicco, Catani and Gasperi2).
Fe, obtained almost from the diets(Reference Hurrell and Egli3) and necessary for body growth and metabolism, has strong pro-oxidant properties(Reference Emerit, Beaumont and Trivin4) and thus, Fe has a pivotal role in oxidative stress, inducing DNA damage and lipid peroxidation(Reference Toyokuni5). Furthermore, the interaction between Fe and estrogen in oxidative stress and other pathways has been suggested to implicate breast cancer development(Reference Torti and Torti6,Reference Liehr and Jones7) . As such, Fe has been hypothesised to have the risk of breast cancer(Reference Huang8,Reference Kabat and Rohan9) .
Epidemiological studies concerning the association between dietary Fe intake and breast cancer have reported negative association(Reference Negri, La Vecchia and Franceschi10–Reference Cade, Thomas and Vail12), positive association(Reference Moore, Shannon and Chen13–Reference Diallo, Deschasaux and Partula15) and no association(Reference Levi, Pasche and Lucchini16–Reference Chang, Cotterchio and Bondy25). Of the studies(Reference Farvid, Cho and Chen11,Reference Ferrucci, Cross and Graubard14,Reference Kabat, Miller and Jain20,Reference Kabat, Cross and Park22,Reference Farvid, Cho and Chen24–Reference Inoue-Choi, Sinha and Gierach26) examining the relationships between different forms of Fe intake and breast cancer risk, only one study(Reference Inoue-Choi, Sinha and Gierach26) found positive association of heme Fe while other studies(Reference Farvid, Cho and Chen11,Reference Ferrucci, Cross and Graubard14,Reference Kabat, Miller and Jain20,Reference Kabat, Cross and Park22,Reference Farvid, Cho and Chen24,Reference Chang, Cotterchio and Bondy25) reported null association, and two studies(Reference Kabat, Miller and Jain20,Reference Chang, Cotterchio and Bondy25) assessing non-heme Fe reported no association, but a recent meta-analysis reported a modest but statistically significant positive association between heme Fe intake, serum Fe levels and breast cancer risk(Reference Chang, Cotterchio and Khoo27). Some studies examined the association between different food sources of Fe intake and breast cancer risk and found no significant association between Fe from plants(Reference Kallianpur, Lee and Gao21), Fe from meat(Reference Ferrucci, Cross and Graubard14,Reference Kabat, Miller and Jain20,Reference Kabat, Cross and Park22) , Fe from red meat(Reference Diallo, Deschasaux and Partula15,Reference Kabat, Miller and Jain20,Reference Kabat, Cross and Park22) and breast cancer risk, with the exception of a case–control study(Reference Kallianpur, Lee and Gao21) claiming that Fe from animal food sources was positively associated with breast cancer risk. To our knowledge, no study has reported the association between Fe from white meat and breast cancer risk.
Most of the studies have been performed in Western countries, where lifestyle and dietary habits differ from China. To date, only two studies have been conducted in Shanghai, China, which reported positive associations between dietary Fe(Reference Moore, Shannon and Chen13), animal-derived Fe(Reference Kallianpur, Lee and Gao21) and breast cancer risk. The prior studies generally had limited adjustment for dietary factors and other subtypes of Fe variables in the model. Moreover, most of the previous studies did not evaluate the linear or non-linear dose–response relation between dietary Fe and breast cancer risk. Therefore, the objective of the current study was to evaluate the associations between total dietary Fe, different forms and sources of Fe intake and breast cancer risk in Southern Chinese women. Given that Fe is a pro-oxidant, our hypothesis was that dietary Fe intake was positively associated with breast cancer risk.
Materials and methods
Study population
The details of this ongoing two-stage case–control study have been reported previously(Reference Zhang, Ho and Chen28,Reference Zhang, Pan and Li29) . Briefly, potential case subjects were recruited in the first stage during June 2007 to August 2008 and the second stage from September 2011 to March 2019 from patients admitted to three major teaching and general hospitals of the study areas in Guangzhou. The inclusion criteria included females aged 25–70 years, natives in Guangdong or having lived in Guangdong for at least 5 years, with incident, primary, histologically confirmed breast cancer diagnosed no more than 3 months before the interview. Patients were excluded if they could not understand or speak Mandarin/Cantonese or had a prior cancer history. Overall, a total of 1778 eligible cases were identified and 1600 were successfully interviewed, with a response rate of 90·0 %. Additionally, subjects with an energy intake <2510 or >14 644 kJ/d (<600 or >3500 kcal/d)(Reference Nimptsch, Zhang and Cassidy30) were excluded from the analysis (n 9). Ultimately, 1591 eligible cases were included in the current study.
Control subjects were admitted to the same hospitals during the same time period as cases, and frequency matched to the cases by 5-year age interval. The controls were females aged 25–70 years old who have never been diagnosed with any cancer and otherwise shared the same eligibility criteria as the cases. They were selected from the Departments of Ophthalmology, Plastic and Reconstructive Surgery, Vascular Surgery, Ear-Nose-Throat, and Orthopedics and Microsurgery. In total, 1622 out of 1786 eligible controls participated in the current study, yielding a response rate of 90·8 %.
Data collection
The recruited patients were interviewed face-to-face by trained interviewers using a structured questionnaire, which was used to collect information on socio-demographic characteristics, anthropometry factors, lifestyle factors, menstrual and reproductive history, history of benign breast disease and family history of cancers. Relevant medical information, medical diagnosis, histological findings, and estrogen receptor (ER) and progesterone receptor (PR) status were obtained from hospital medical records.
Measurement of dietary exposure
Study subjects reported their usual dietary consumption for the past year via a validated eight-one-item FFQ(Reference Zhang and Ho31). The daily consumption of each FFQ food item was collected and portion size was quantified with the help of photographs of commonly consumed foods. For the current analysis, plants included cereal, legumes, vegetables and fruits. Meats were grouped into red meat and white meat. Red meat consisted of pork, beef, lamb, organ meat and processed meat. White meat primarily constituted poultry (chickens, ducks and geese) and fish (freshwater and saltwater fish, crab, shrimp and shellfish). Energy and nutrients in foods were computed by using the 2002 Chinese Food Composition Table(Reference Yang, Wang and Pan32).
In the current study, total dietary Fe contained only Fe from diet but not from supplements. Heme Fe intake was calculated based on meat-specific heme Fe proportions such as 65 % for beef, 39 % for pork and pork products, 26 % for chicken and fish, and 21 % for liver(Reference Balder, Vogel and Jansen33). Dietary non-heme Fe intake was determined by subtracting dietary heme Fe from total dietary Fe intake. Fe from plants, Fe from meat, Fe from red meat and Fe from white meat were derived as the sum of dietary Fe for all of the plant foods, all of the meat, all of the red meat and all of the white meat intake, respectively.
Statistical analysis
Characteristics between cases and controls were compared by using t test or Wilcoxon rank-sum test for continuous variables and χ 2 test for categorical variables. The dietary intake data were adjusted for total energy intake using the residual method(Reference Willett, Howe and Kushi34). Dietary Fe intake was categorised into quartiles (Q1-Q4) based on the distribution among the controls. Multivariable logistic regression models were used to estimate the OR and 95 % CI for the associations, with the lowest quartile as the reference group. Tests for trend were performed by entering the categorical variables as continuous variables in the regression models.
Covariates in the multivariable models were selected by a significant level of P < 0·05 in univariable analysis, changing the OR by 10 % for the main variables of interest or based on known risk factors for breast cancer. The following variables were included in the multivariable models: age, age at menarche, educational level, income, occupational activity, first-degree relative with cancer, history of benign breast disease, regular smoking, passive smoking, regular drinking, ever used an oral contraceptive and BMI. Models were adjusted for covariates in a stepwise procedure. The values of model 1 were shown as crude OR and 95 % CI. Model 2 was adjusted for above-mentioned non-dietary factors. Model 3 was further adjusted for dietary intake of fat, fibre, vitamin A, C and E which were reported having influence on Fe metabolism(Reference Kallianpur, Lee and Gao21,Reference Hallberg and Hulthen35) . Additional adjustment for Ca, Se, Mg or flavonoids was also conducted. Mutual adjustment was performed for dietary heme Fe and non-heme Fe, Fe from plants and Fe from meat. Fe from red meat and Fe from white meat were adjusted for each other, and simultaneously adjusted for Fe from plants.
Restricted cubic spline (RCS) model was used to reveal the potential non-linear associations in model 3. Based on Akaike information criteria, three knots (at 10th, 50th and 90th percentiles of total dietary Fe, non-heme Fe and Fe from white meat intake) or four knots (at 5th, 35th, 65th, and 95th percentiles of heme Fe, Fe from plants, Fe from meat and Fe from red meat intake) were suggested to fit the models better(Reference Harrell36). The lowest dietary Fe intake was used as the reference value. Owing to the effect of sparse data on the RCS curve, the subjects with dietary intake > 99 % were excluded in cases and controls. P non-linearity was calculated by using a Wald test.
Stratified analysis by menopausal status assessed the interactions by adding the multiplicative interaction terms (dietary Fe intake × menopausal status) to the multivariable models as indicator variables. Subgroup analysis by sex hormone receptor status (ER+, ER− v. controls and PR+, PR− v. controls) was performed using polytomous logistic regression. Possible heterogeneity was examined in a case-only analysis where ER/PR status was used as the dependent variable (outcome) and dietary Fe (categorical) as independent variable in the logistic regression model. Sensitivity analysis was performed by excluding nutrient supplement users or restricting to invasive breast cancer cases to see whether the results remained consistent. All data analyses were conducted using SPSS 25.0 and Stata 15.1. All P values were two sided, and P values < 0·05 were considered as statistically significant.
Results
Baseline characteristics and dietary factors compared between cases and controls are shown in Tables 1 and 2, respectively. Overall, breast cancer cases had younger age at menarche, less education, lower income, more occupational activities and higher BMI and were more likely to drink and smoke regularly, be exposed to second-hand smoke, have a first-degree relative with cancer, suffer from benign breast disease and use oral contraceptive. Compared with the controls, cases had a lower intake of total dietary Fe, non-heme Fe, Fe from plants and Fe from white meat, while red meat intake was significantly higher in the cases than the controls.
Table 1 Socio-demographic and selected characteristics of breast cancer in the studied population*

ER, estrogen receptor; PR, progesterone receptor.
* Continuous variables were evaluated using t test and categorical variables were evaluated using χ 2 test.
Table 2 Intakes of total dietary Fe and different types of Fe and selected dietary variables among cases and controls*,†

* The consumption was adjusted for total energy intake by the residual method.
† Wilcoxon rank-sum test was used to compare the median consumption levels between cases and controls.
As shown in Table 3, higher intake of total dietary Fe was associated with a lower risk of breast cancer in model 2 (OR = 0·54, 95 % CI 0·44, 0·66, P trend < 0·001). However, this inverse association became null after adjusting for dietary factors in model 3, with an adjusted OR of 1·06 (95 % CI 0·79, 1·43) comparing the highest with the lowest quartile (P trend = 0·995).
Table 3 OR and 95 % CI of breast cancer according to quartiles (Q) of different types of Fe
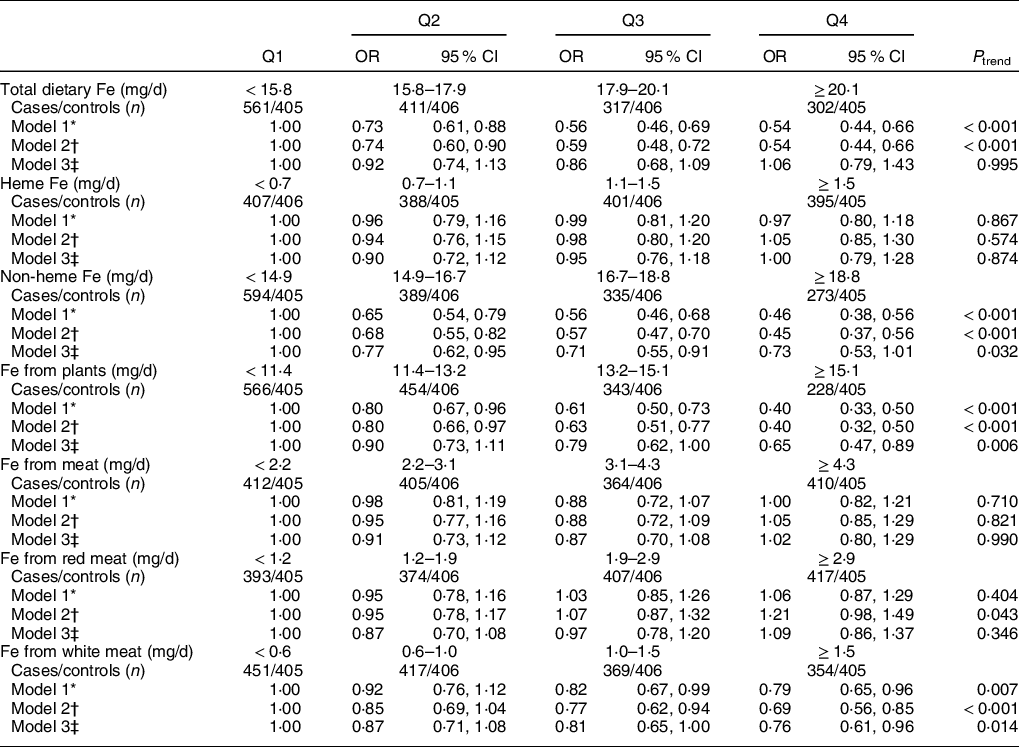
* Values in model 1 were showed as crude OR and 95 % CI.
† Model 2 was adjusted for age, age at menarche, educational level, income, occupational activity, first-degree relative with cancer, history of benign breast disease, ever used an oral contraceptive, regular smoking, passive smoking, regular drinking and BMI.
‡ Model 3 was adjusted for confounders from model 2 plus intakes of dietary fat, fibre, vitamin A, C and E. Mutual adjustment was performed for dietary heme Fe and non-heme Fe, Fe from plants and Fe from meat. Fe from red meat and Fe from white meat were adjusted for each other and simultaneously adjusted for Fe from plants.
Heme Fe and non-heme Fe intakes were not significantly associated with breast cancer risk in quartile analyses (Table 3). Comparing the highest with the lowest quartile, the multivariable OR in model 3 was 1·00 (95 % CI 0·79, 1·28, P trend = 0·874) for heme Fe and 0·73 (95 % CI 0·53, 1·01, P trend = 0·032) for non-heme Fe, respectively.
Intakes of Fe from meat and Fe from red meat were not significantly related to breast cancer risk, whereas Fe from plants and Fe from white meat were inversely associated with breast cancer risk (Table 3). These associations were consistent in all models. The adjusted OR in model 3 was 1·02 (95 % CI 0·80, 1·29, P trend = 0·990) for Fe from meat, 1·09 (95 % CI 0·86, 1·37, P trend = 0·346) for Fe from red meat, 0·65 (95 % CI 0·47, 0·89, P trend = 0·006) for Fe from plants and 0·76 (95 % CI 0·61, 0·96, P trend = 0·014) for Fe from white meat, respectively. Additional adjustment for Ca, Se, Mg or flavonoids did not change the results significantly (data not shown).
As shown in Fig. 1, RCS models showed no significant non-linear associations between Fe from plants, Fe from white meat and breast cancer risk (P non-linearity > 0·05). However, a significant J-shaped relationship was observed between total dietary Fe and breast cancer risk, of which with increasing total dietary Fe intake, the risk of breast cancer first decreased and reached the lowest risk at around 17·96 mg/d, and then increased rapidly afterwards. The trend for non-heme Fe was similar with total dietary Fe. A lower intake of non-heme Fe was associated with decreased breast cancer risk, whereas higher non-heme Fe intake (>17·84 mg/d) was associated with increased risk of breast cancer. A reverse L-shaped curve with threshold effect for heme Fe intake and breast cancer risk was found. The breast cancer risk was relatively flat until 1·45 mg/d of heme Fe intake and significantly increased beyond 2·20 mg/d. Similar trends were observed for Fe from meat and Fe from red meat of which breast cancer risk increased significantly when Fe from meat exceeded 6·45 mg/d or Fe from red meat exceeded 4·24 mg/d.
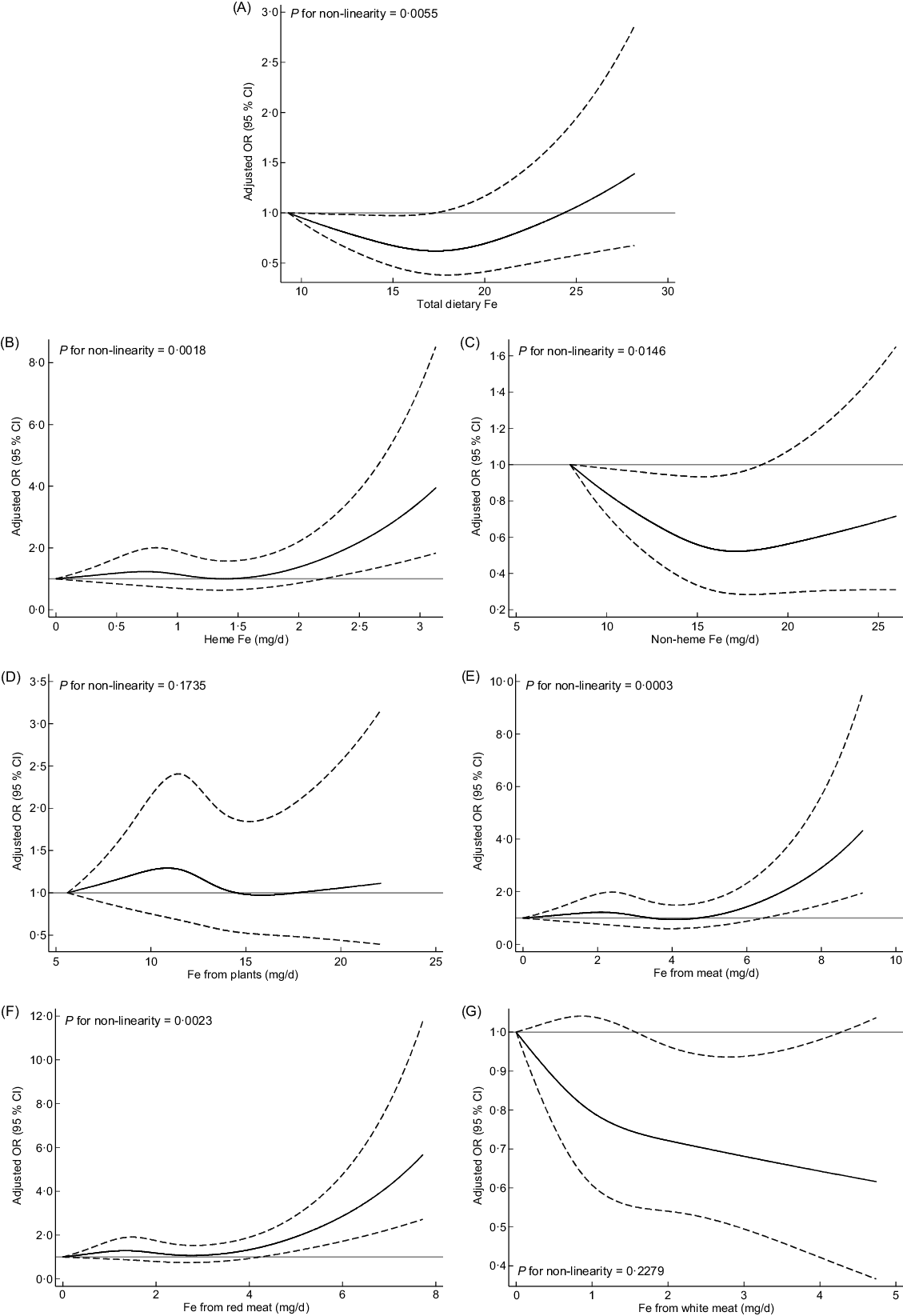
Fig. 1 Multivariable-adjusted OR (black solid lines) and 95 % CI (dashed lines) for breast cancer risk according to dietary intake of total dietary Fe (A), different forms of Fe (B and C) and different sources of Fe (D–G) in model 3. The lowest intakes were set as references (gray solid lines) (OR = 1·00)
Stratified analyses by menopausal status (2034 premenopausal and 1179 postmenopausal) showed that inverse relationships between non-heme Fe, Fe from plants and Fe from white meat intake and breast cancer risk were restricted to premenopausal women (Table 4). However, there were no statistically significant interactions (P interaction > 0·05). No significant association was found between total dietary Fe, heme Fe, Fe form meat and Fe from red meat intake and breast cancer risk in pre- and postmenopausal women.
Table 4 OR and 95 % CI of breast cancer according to quartiles (Q) of different types of Fe by menopausal status
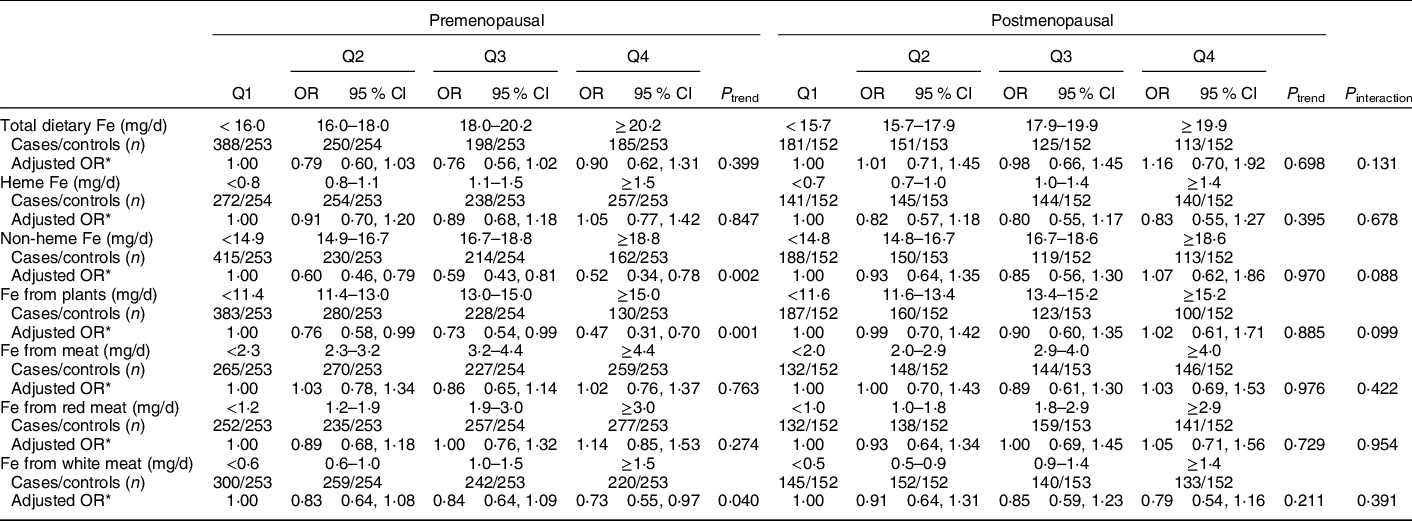
* OR was adjusted for age, age at menarche, educational level, income, occupational activity, first-degree relative with cancer, history of benign breast disease, ever used an oral contraceptive, regular smoking, passive smoking, regular drinking, BMI and intakes of dietary fat, fibre, vitamin A, C and E. Mutual adjustment was performed for dietary heme Fe and non-heme Fe, Fe from plants and Fe from meat. Fe from red meat and Fe from white meat were adjusted for each other and simultaneously adjusted for Fe from plants.
Among 1332 (83·7 %) cases with information on hormone receptor status, the cases with ER+, ER−, PR+ and PR− accounted for 1049 (78·8 %), 282 (21·2 %), 1061 (79·7 %) and 269 (20·2 %), respectively. As shown in Table 5, there was no evidence of heterogeneity (P heterogeneity > 0·05).
Table 5 OR and 95 % CI of breast cancer according to quartiles (Q) of different types of Fe by sex hormone receptor status
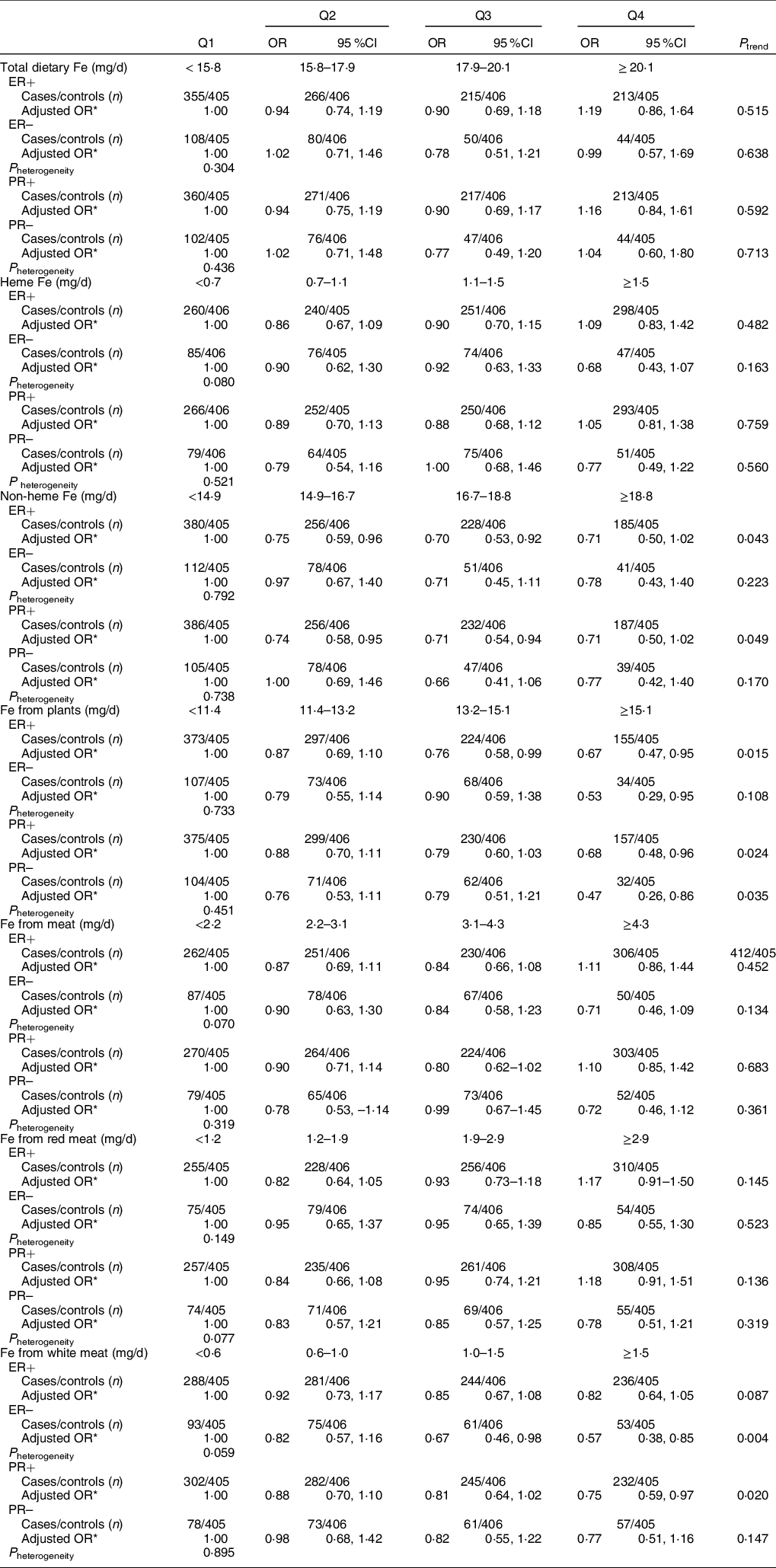
ER, estrogen receptor; PR, progesterone receptor
* OR was adjusted for age, age at menarche, educational level, income, occupational activity, first-degree relative with cancer, history of benign breast disease, ever used an oral contraceptive, regular smoking, passive smoking, regular drinking, BMI and intakes of dietary fat, fibre, vitamin A, C and E. Mutual adjustment was performed for dietary heme Fe and non-heme Fe, Fe from plants and Fe from meat. Fe from red meat and Fe from white meat were adjusted for each other and simultaneously adjusted for Fe from plants.
The prevalence of nutritional supplement users was not significantly different among breast cancer cases and controls (27·3 % in cases v. 24·7 % in controls, P = 0·099). Sensitivity analysis by excluding all nutrient supplement users did not materially change the results (data not shown). Analysis restricted to invasive breast cancer cases did not materially change the results (data not shown).
Discussion
The quartile analyses of the current study showed that the intakes of Fe from plants and Fe from white meat were inversely associated with breast cancer risk. Furthermore, RCS analysis showed significant non-linear J-shaped associations between total dietary Fe, non-heme Fe and breast cancer risk, and reverse L-shaped associations between heme Fe, Fe from meat and Fe from red meat and breast cancer risk.
The association between total dietary Fe intake and breast cancer risk has been examined in some epidemiological studies, but the results were inconsistent. In line with the present study, three prospective studies(Reference Kabat, Miller and Jain20,Reference Kabat, Cross and Park22,Reference Farvid, Cho and Chen24) and ten case–control studies(Reference Negri, La Vecchia and Franceschi10,Reference Cade, Thomas and Vail12,Reference Moore, Shannon and Chen13,Reference Levi, Pasche and Lucchini16–Reference Hong, Ambrosone and Ahn19,Reference Kallianpur, Lee and Gao21,Reference Bradshaw, Khankari and Teitelbaum23,Reference Chang, Cotterchio and Bondy25) did not support a significant association between total dietary Fe intake and breast cancer risk. However, two prospective studies conducted in the USA(Reference Ferrucci, Cross and Graubard14) and France(Reference Diallo, Deschasaux and Partula15), respectively, found a positive association between dietary Fe intake and breast cancer risk by means of quartile analyses. In the RCS model, the present study showed a significant J-shaped association between total dietary Fe intake and breast cancer risk. Consistent with our result, a meta-analysis showed a significant J-shaped relationship between serum Fe level and breast cancer risk(Reference Chang, Cotterchio and Khoo27). In contrast, a case-control study in Canada did not support a non-linear relationship between dietary Fe intake and breast cancer risk(Reference Chang, Cotterchio and Bondy25).
Some possible biological explanations might account for this J-shaped non-linear association between total dietary Fe and breast cancer risk. First, Fe is a necessary micronutrient in human body and it is a component of various metabolic substances, such as Hb, cytochromes and tissue enzymes(Reference Carpenter and Mahoney37). An appropriate amount of Fe in vivo plays an essential role in transport of oxygen and carbon dioxide, energy production and immune system functioning(Reference Carpenter and Mahoney37). Therefore, an appropriate consumption of dietary Fe would help body metabolism and growth development. Second, due to its redox potential, Fe might cause oxidative stress through productions of free radicals and peroxide by catalysing the Fenton and Haber-Weiss reactions(Reference Papanikolaou and Pantopoulos38). Excess Fe could be harmful to DNA, protein and lipid(Reference Emerit, Beaumont and Trivin4). Animal experimental studies have shown that low-Fe diet could suppress 1-methyl-1-nitrosourea-induced mammary carcinogenesis, whereas high intakes might promote tumour occurrence(Reference Thompson, Kennedy and Witt39,Reference Singh, Lu and Briggs40) . This could partially explain the lower risk of breast cancer at relatively lower intake of total dietary Fe and the higher risk of breast cancer at relatively higher intake of total dietary Fe in our study.
In the present study, the quartile analysis did not show significant association between heme Fe intake and breast cancer risk. However, a reverse L-shaped association was found between heme Fe intake and breast cancer risk. Higher heme Fe intake (> 2·20 mg/d) was significantly associated with an increased risk of breast cancer. So far, most epidemiological studies(Reference Farvid, Cho and Chen11,Reference Ferrucci, Cross and Graubard14,Reference Diallo, Deschasaux and Partula15,Reference Kabat, Miller and Jain20,Reference Kabat, Cross and Park22,Reference Farvid, Cho and Chen24,Reference Chang, Cotterchio and Bondy25) have reported a null association between heme Fe intake and breast cancer risk, but the National Institutes of Health-American Association of Retired Persons Diet and Health Study(Reference Inoue-Choi, Sinha and Gierach26) and a recent meta-analysis(Reference Chang, Cotterchio and Khoo27) reported a positive association in quartile analyses. A threshold effect in non-linear dose–response analysis was also found in the above-mentioned meta-analysis(Reference Chang, Cotterchio and Khoo27). However, another population-based case–control study in Canada did not find a non-linear dose-response relationship between heme Fe intake and breast cancer risk(Reference Chang, Cotterchio and Bondy25).
The non-linear association between heme Fe intake and breast cancer risk could be explained by some plausible reasons. Heme Fe is a principal contributor to body Fe stores due to its easier absorption than non-heme Fe(Reference Kallianpur, Lee and Gao21). Red meat, the main source of heme Fe, has been reported to be linked with increased breast cancer risk(Reference Farvid, Cho and Chen11). Heme Fe in red meat can coordinate with biological components like nitrogen or oxygen of amino acids and then be transported to every organ and tissue(Reference Tappel41). Excess intracellular heme could accelerate the expression of hemeoxygenase, thus increasing the excess heme breakdown rate(Reference Kumar and Bandyopadhyay42). This reaction could lead to the intracellular accumulation of free heme and labile Fe. An animal study indicated that mice fed with high heme diet showed acute oxidative stress(Reference IJssennagger, Rijnierse and de Wit43). Due to its involvement in endogenous N-nitroso compound formation, lipid peroxidation and cellular oxidative damage, heme Fe was considered to have a positive association with cancer risk(Reference Tappel41,Reference Cross, Pollock and Bingham44) .
So far, only two studies conducted in Canada(Reference Kabat, Miller and Jain20,Reference Chang, Cotterchio and Bondy25) evaluated the association between non-heme Fe intake and breast cancer risk. These studies demonstrated a null association, which was consistent with our results in quartile analysis. However, a significant J-shaped association was observed in the present study. Relatively, low non-heme Fe intake was associated with decreased risk and higher non-heme Fe intake (>17·84 mg/d) was associated with increased risk of breast cancer. In our population, approximately 80 % non-heme Fe intake derived from plant foods such as cereal products, legumes, vegetables and fruits. These food sources contained many beneficial substances such as antioxidant vitamins(Reference Zhang, Hunter and Forman45) and phytochemicals(Reference Kapinova, Kubatka and Golubnitschaja46). On the other hand, non-heme Fe was found to be associated with serum ferritin in Chinese females with a predominantly plant-based diet(Reference He, Shen and Fang47). High level of serum ferritin was found to be associated with an elevated risk of breast cancer(Reference Ulbrich, Lebrecht and Schneider48).
Consistent with our results of quartile analyses, the null associations between Fe from meat, Fe from red meat and breast cancer risk were also reported in several cohort studies(Reference Ferrucci, Cross and Graubard14,Reference Diallo, Deschasaux and Partula15,Reference Kabat, Miller and Jain20,Reference Kabat, Cross and Park22) . In RCS models, the present study showed that higher intake of Fe from meat (>6·45 mg/d) and Fe from red meat (>4·24 mg/d) was significantly associated with increased risk of breast cancer. Meat, including red meat, contains essential amino acids and micronutrients, but the consumption of red meat was classified as ‘probably carcinogenic to humans’(Reference Gamage, Dissabandara and Lam49). The mechanism of the potential harmful effects of higher intake of Fe from meat and Fe from red meat could be partially explained by heme Fe. Other carcinogenic mediators found in red meat, such as N-nitroso compounds, heterocyclic amines and polycyclic aromatic hydrocarbons, might also contribute to these associations(Reference Inoue-Choi, Sinha and Gierach26).
Contrary to our hypothesis, the present study found a significant inverse association between Fe from white meat and breast cancer risk. It is known that white meat contained less heme Fe, probably 26 %, than red meat(Reference Balder, Vogel and Jansen33). Higher intake of white meat generally indicated a healthier dietary pattern(Reference Daniel, Cross and Graubard50). A pooled analysis in the USA showed that white meat intake was inversely associated with breast cancer risk(Reference Kim, Lundgreen and Wolff51). Our observation of an inverse association between Fe from white meat and breast cancer risk persisted after further adjusting for vitamin C, vitamin E, vitamin A, fat, fibre, Ca, Se or Mg. There might be some other components of white meat attributable to the protective effect of Fe from white meat.
So far, only one population-based case–control study has reported the association between plant-derived Fe intake and breast cancer risk and found a null association(Reference Kallianpur, Lee and Gao21). This is in contrast to the inverse association between plant-derived Fe intake and breast cancer risk observed in the present study. The inverse association persisted after further adjustment for other substances contained in plant foods such as flavonoids, fibre or Ca. There might be some other reasons to explain or contribute to this inverse association. Further studies are needed to clarify this issue.
There was no significant interaction between Fe intake and menopausal status in the current study. Similarly, some studies(Reference Farvid, Cho and Chen11,Reference Kabat, Miller and Jain20,Reference Kallianpur, Lee and Gao21,Reference Farvid, Cho and Chen24) also found the associations between total dietary Fe(Reference Farvid, Cho and Chen11,Reference Kabat, Miller and Jain20,Reference Kallianpur, Lee and Gao21,Reference Farvid, Cho and Chen24) , heme Fe(Reference Farvid, Cho and Chen11,Reference Kabat, Miller and Jain20,Reference Farvid, Cho and Chen24) , meat Fe(Reference Kabat, Miller and Jain20), animal sources of Fe(Reference Kallianpur, Lee and Gao21), plant sources of Fe(Reference Kallianpur, Lee and Gao21) and breast cancer risk were not modified by menopausal status. On the other hand, no significant heterogeneity across sex hormone receptor status was observed. Two cohort studies(Reference Kabat, Cross and Park22,Reference Inoue-Choi, Sinha and Gierach26) and a case–control study(Reference Chang, Cotterchio and Bondy25) have also reported null associations between dietary Fe intake and hormone receptor-defined breast cancer risk. Given the limited literature, further studies are warranted to clarify this issue.
To our knowledge, this is the first study to examine the association between two forms and four sources of Fe intake and breast cancer risk in Southern Chinese women. Strengths included the large sample size, the nutrient intakes reflecting the local level, a wide range of potential confounders and potential non-linear associations assessed by RCS models.
Some limitations also need to be considered. First, selection bias and recall bias are difficult to rule out in hospital-based case–control studies. However, cases were consecutively recruited from three major hospitals in Guangzhou and the high participation rate (90·0 % for cases and 90·8 % for controls) helped to reduce selection bias in our results. Second, Fe from dietary supplements was not calculated as part of the exposure, but sensitivity analysis excluding nutritional supplement users did not materially change the results. Third, some potential residual confounding may still exist. Fourth, due to multiple comparisons, chance findings may exist and therefore the current results should be interpreted with caution. Fifth, measurement errors and misclassification were inevitable due to the use of FFQ, but the potential misclassification was likely to be non-differential and tended to attenuate the association to null. Moreover, although the present study did not evaluate the relationship between dietary Fe intake estimated by FFQ and body Fe status, some studies have shown that dietary Fe intake was related to body Fe status(Reference De Carli, Dias and Morimoto52,Reference Waldmann, Koschizke and Leitzmann53) .
In conclusion, Fe from plants and Fe from white meat were inversely associated with breast cancer risk in quartile analyses, whereas total dietary Fe, heme Fe, non-heme Fe, Fe from meat and Fe from red meat intake were non-linearly associated with breast cancer risk, showing J-shaped associations between total dietary Fe, non-heme Fe and breast cancer risk, and reverse L-shaped associations between heme Fe, Fe from meat and Fe from red meat and breast cancer risk.
Acknowledgements
Acknowledgements: The authors thank the contribution of the study participants, without them the study would not have been possible. Financial support: This work was supported by the National Natural Science Foundation of China (no. 81102188). The funders had no role in the design of the study, analysis of the data or writing of this manuscript. Conflict of interest: None. Authorship: K.-Y.L. conducted the data collection, analysed the data and writing of this paper. X.-L.F., X.Z., C.-Y.H., A.A. and L.L. participated in the data collection and data entry. X.-F.M. and F.-Y.L. were responsible for connecting and coordinating the field work. C.-X.Z. constructed the project design, supervised and contributed to the manuscript writing. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the Ethical Committee of School of Public Health, Sun Yat-sen University. Written informed consent was obtained from all subjects.









