Universal salt iodization (USI) is a mass fortification approach that is intended to cover the iodine requirements of all individuals in the population; it is estimated that 128 countries have established iodized salt programmes(Reference Timmer1). As USI programmes mature in many countries, greater emphasis is being placed on ensuring that USI meets the increased needs of pregnant women because of the risk of irreversible fetal brain damage due to iodine deficiency(Reference Bernal2, Reference Zoeller and Rovet3). Another focus of mature programmes is the need for careful monitoring to avoid not only iodine deficiency but also iodine excess(Reference Timmer1). Median urinary iodine concentration (UIC) in school-aged children (SAC) and household access to adequately iodized salt based on national standards are routinely used as the primary indicator of the impact of USI programmes. However, the iodine requirement of SAC is disproportionately lower than that of pregnant women, which leaves only a narrow intake range to meet the needs of pregnant women without leading to more-than-adequate intake in children according to the current UIC cut-off of 200μg/l(4). The WHO/UNICEF/International Council for the Control of Iodine Deficiency Disorders (ICCIDD) have estimated that the iodine requirement during pregnancy is increased by >50% compared with non-pregnancy in order to compensate for the increased need for thyroid hormones in the mother and fetus(4). The WHO/UNICEF/ICCIDD have also stated that an established USI programme with adequate salt iodine levels and good population coverage can meet the high iodine requirement of pregnant women. However, there are concerns that this may be possible only at the expense of more-than-adequate and excessive intakes, reflected by UIC in the range of 200–299 μg/l and >300 μg/l, respectively, in SAC.
There are several reasons for this concern. The daily requirement for iodine during pregnancy is estimated to be 250 μg, more than twice the 120 μg requirement, for instance, in SAC(4). In populations consuming iodized salt, iodine intake during pregnancy increases due to higher energy intake, but does not fully compensate for the higher demand for iodine. The daily energy requirement of a pregnant woman is 10 460 kJ/d (2500 kcal/d) in the second and third trimester (+1255 kJ/d (+300 kcal/d) compared with a non-pregnant woman), as compared with the daily energy requirement of 8368–10 460 kJ/d (2000–2500 kcal) for SAC(5). Thus, the difference in energy requirement in pregnant women is only 0–25% compared with SAC, while the difference in iodine requirement is >100%. The relatively small increase in food intake during pregnancy is therefore unlikely per se to provide the additional iodine requirement unless iodine-rich foods are preferentially selected.
Adequately iodized salt at the household level is defined by WHO as salt containing 15–40 mg iodine/kg(4, 6), and many USI programme managers, aware of the irreversible effects of iodine deficiency on fetal development, feel justified in setting salt iodization levels near the upper end of the recommended range in order to ensure adequate iodine intake during pregnancy. However, only a limited number of countries have completed UIC surveys in pregnant women and women of reproductive age on the national or sub-national level, and thus there are insufficient data to directly estimate the regional or global prevalence of low iodine intake in these important target groups(Reference Caldwell, Makhmudov and Ely7). Indian salt legislation stipulates that the iodine content in salt at consumption level should be at least 15 ppm(Reference Tiwari8) and USI remains the single most important source of dietary iodine for the Indian population(Reference Longvah, Toteja and Upadhyay9).
There has been a remarkable improvement in the consumption of adequately iodized salt in India, with the national coverage reaching 51% in 2005–2006 and 71% in 2009(10, 11). Access to iodized salt was higher in urban (83·2%) than rural households (66·1%)(12); however, nearly 20% of households were found to be consuming inadequately iodized salt and 9% were using salt that was not iodized(11). A recent review recommended the mandatory use of adequately iodized salt in the mid-day meal programme for SAC in order to reach the last 30% of households that are likely to be least accessible and most socio-economically disadvantaged(Reference Rah, Anas and Chakrabarty13).
Studies have assessed concurrent iodine intakes in SAC and pregnant women, and reported that USI provides adequate iodine to pregnant women only when iodine intakes for children are more than adequate (median UIC >200 μg/l)(Reference Kusić, Jukić and Rogan14, Reference Wong, Sullivan and Perrine15). However, these studies did not assess pregnant women and children from the same household sharing all meals together, which limits comparisons between the groups. Therefore, the aim of the present study was to assess iodine intake (based on UIC), and potential determinants of intake, in Indian pregnant women and their children who are sharing all meals. Our hypotheses were: (i) effective USI can ensure adequate iodine intake in pregnant women; but (ii) this may lead to more-than-adequate or excessive iodine intake in their children.
Methods
The present study was carried out in southern India in Bangalore, one of the districts in the Indian state of Karnataka. Bangalore is India’s third most populous city with 9·6 million inhabitants and the fifth-most populous urban agglomeration(16, 17). The average literacy rate in Karnataka is 75%, being 69% and 88% in rural and urban Karnataka, respectively(18). The average literacy rate of Bangalore district is 88%(16) and the city is among the top ten preferred entrepreneurial locations in the world(Reference Jayadevan19). The study site was the antenatal clinic (morning and evening) of the Obstetrics and Gynecology Department of St Martha’s Hospital, which serves Bangalore’s less-affluent population. The morning antenatal clinic operates between 08.30 and 12:30 hours and the evening antenatal clinic operates between 16.00 and 18.00 hours. We aimed to recruit 200 pairs of pregnant women and their children who were sharing all meals in the same household. Our inclusion criteria were healthy pregnant women across all trimesters, aged 18–35 years, who had healthy children, aged 3–15 years. It is a common practice in India for a working mother to leave her children at their grandparents’ home during the day. In such a scenario, sharing of meals from a common household basket does not hold. We thus excluded from the study such mother–child pairs. In addition, the Government of India runs a Mid-Day Meal Programme, one of the largest school lunch programmes in the world(20). This flagship programme for achievement of universalization of elementary education is being implemented in partnership with the State Government(21). The programme sources double-fortified salt from the Tamil Nadu Salt Corporation. The double-fortified salt premix produces a salt with 50 ppm iodine (as KI) and 1000 ppm Fe at production level(22). For the purpose of the current study, we ensured that the children we enrolled were not participating in this programme. Thus, the schoolchildren in the present study were those who were carrying food from home, which is a common practice in Indian settings, and thus sharing all meals including lunch with their mothers. We excluded women with a multiple pregnancy and those who were still breast-feeding. Data were collected from May 2008 to September 2011.
Information was obtained by a structured questionnaire and included: (i) age of the mother and her child; (ii) sex of the child; (iii) parity as per the antenatal record; (iv) number of members (adults and children) in the household; (v) education and occupation of the pregnant woman and her husband; (vi) monthly income of the household; (vii) household usage of iodized or non-iodized salt including brand and type of salt; (viii) cooking practices using salt; (ix) average household consumption of salt; (x) use and frequency of seafood consumption; and (xi) knowledge of iodine and opinion on salt type preferred with its reason, this information was collected on a subset of the sample. Data on iodine intake from supplements and multi-micronutrient powders available in the market were collected from a subset of pregnant women and their children. This subset was a convenience sample. Crude average per capita consumption of powdered and crystal salt was estimated by dividing the reported monthly consumption of salt (using single recall) by the number of members in the household.
Pregnant women were classified into three trimesters according to their gestational age. This was based on their reported first day of the last menstrual period but, where available, first-trimester ultrasound scan was used. Body weight and height were recorded using standard anthropometric techniques(23). Weight was recorded to the nearest 0·1 kg using a digital weighing scale (Salters 9016, Tonbridge, UK). Height was recorded to the nearest 0·1 cm using a locally made stadiometer (Biorad, Chennai, India).
A single spot urine sample, obtained throughout the day, was collected from all mothers and children within a week of each other. Samples were transported on ice, divided into aliquots and stored at −20ºC until analysis. UIC was determined using the Pino modification of the Sandell–Kolthoff reaction(Reference Pino, Fang and Braverman24) with external reference standards (C. Zeder, ETH Zurich, Switzerland). The intra-assay CV at a mean UIC of 74, 162 and 282 μg/l (n 21 each) was 11, 9 and 13%, respectively. The iodine nutrition status in pregnant women and their children was evaluated according to the recommended WHO/UNICEF/ICCIDD criteria(4). Salt samples of all the types used by the household were collected in clean plastic containers and stored at −80°C until analysis. Composite samples from each recognized commercial brand were analysed for iodine content by using iodometric titration; those from an unknown source were analysed only qualitatively (iodine presence: yes/no) using a test kit (MBI Chemicals, Chennai, India). Twenty-two duplicate titrations of brand-specific composite samples (made by mixing 10 g of salt from five different households and sampling 2×10 g of this homogenized mixture for analysis) were done. It would have required fifty-seven more duplicate titrations to also analyse the samples from unknown brands individually, which was not possible within the resources available for this project.
Thyroid gland volume of the pregnant women was measured using an Aloka SSD-500 Echocamera (Aloka, Mure, Japan) with a 7·5 MHz linear transducer and ultrasound coupling gel. The thyroid volume of each lobe was calculated using the formula for a prolate ellipsoid(4), where Thyroid volume (ml)=0·479×length (cm)×breadth (cm)×depth (cm). The thyroid volume was calculated as the sum of the volumes of both lobes and did not include the isthmus.
Statistical analyses were performed using the statistical software package SPSS version 14·0 and Microsoft® Excel (MS Office 2003). Weight-for-age Z-scores (WAZ), height-for-age Z-scores (HAZ) and BMI-for-age Z-scores (BAZ) were computed from data on height, weight, age and gender using the WHO Anthro and AnthroPlus software. Data are presented as mean and standard deviation when normally distributed. For non-normal distributions, data are presented as median (range) or median (25th, 75th percentile). Mann–Whitney and Kruskal–Wallis tests were used for comparisons and P values <0·05 were considered significant. Proportions were compared using χ 2 tests. Spearman correlations were performed for associations.
Results
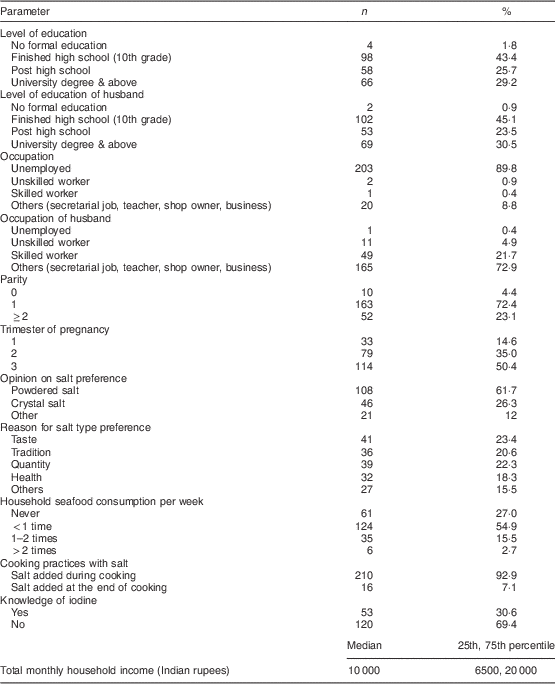
We recruited 226 pregnant women and their children for the study, and 194 complete sets of data for mother–child pairs were obtained. Maternal indicators are shown in Table 1; 78·3% (n 177) of the study women attended the outpatient clinic in the morning while 21·7% (n 49) attended the evening clinic. Although the UIC of pregnant women from the morning clinic was higher than for pregnant women from the evening clinic (187 v. 128 µg/l), this difference was not statistically significant. Data on intake of iodine-containing supplements and multi-micronutrient powders were collected from a subset of pregnant women and their children (n 105). Of these, 50·5% (n 53) of the pregnant women and 46·7% (n 49) of the children were consuming iodine-containing multi-micronutrient powders, averaging 18·5 and 15·9 µg/d, respectively. There was no significant difference in median UIC between mothers or children consuming iodine-containing multi-micronutrient powders as compared with those not consuming them (192 v. 163 µg/l in mothers and 228 v. 218 µg/l in children, respectively).
Table 1 Maternal indicators among the pregnant women aged 18–35 years, Bangalore, India, May 2008 to September 2011

We collected samples of all available salt types from the study households. Some households were using both powdered and crystal salt. In total, 275 salt samples were obtained, and 86% of the powdered salt samples (n 164) and 71% of the crystal salt samples (n 106) were from a known brand; 79% (n 216) of the salt samples were analysed for their iodine content by titration. The median (range) iodine concentration of household powdered and crystal salt was 55·9 (17·2–65·9) ppm and 18·9 (2·2–68·2) ppm, respectively. All of the remaining powdered salt samples (n 23) tested positive for iodine content by the kit method, whereas 75% of the remaining crystal salt samples (n 31) were iodized as determined by the kit method. Overall, inadequately iodized crystal salt (<15 ppm) was found in 3·1% (n 8) of the households, but in each case except one with crystal salt iodine content of 12·7 ppm and no powdered salt use, the households had adequately iodized powdered salt. In the households that used both types of salts, crystal salt contributed 57% to the monthly salt usage. Regarding salt preference, 62% (n 108) of the pregnant women preferred powdered salt, 26% (n 46) preferred crystal salt, and 12% (n 21) preferred both types of salts. There was a significant difference in the median UIC of pregnant women and children among these three groups of preferred salt use: powdered salt v. crystal salt v. both types of salt (P=0·006; 188 v. 133 v. 96 µg/l in mothers and 248 v. 151 v. 218 µg/l in children, respectively). Figure 1 shows the UIC of pregnant mothers and their children by usage of powdered and crystal salt and the salt iodine content (<45 ppm and >45 ppm). There was no statistically significant difference in the median UIC of pregnant mothers and their children between the salt iodine content categories among the salt type groups. The estimated average daily consumption of salt per capita based on dietary recall was 13 (sd 6·8) g/d; calculated from the median iodine content in the salt samples, the estimated median (range) per capita iodine intake from salt was ≈301 (0–2283) µg/d.

Fig. 1 Median urinary iodine concentration (UIC) in matched pairs of pregnant women aged 18–35 years (![]() ) and their children aged 3–15 years (
) and their children aged 3–15 years (![]() ) by household salt type and its iodine content, Bangalore, India, May 2008 to September 2011
) by household salt type and its iodine content, Bangalore, India, May 2008 to September 2011
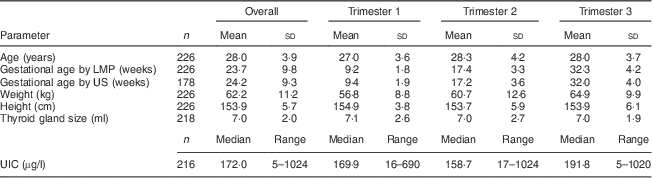
Maternal gestational age, anthropometrics, thyroid volume and UIC by trimester are shown in Table 2. Gestational age calculated from the date of the last menstrual period correlated highly with that from the uterine ultrasound scan (r=0·99, P=0·001). Mean thyroid volume and median UIC did not differ significantly among trimesters. There was no correlation between the median UIC and thyroid volume in these pregnant women. The median (range) UIC in the pregnant women was 172 (5–1024) µg/l and indicates iodine sufficiency; the distribution of UIC is shown in Fig. 2(a). Overall, the estimated median (range) daily iodine intake as calculated from UIC in pregnant women was ≈278 (0–1670) µg/d assuming a daily urine volume of 1·5 litres and 92% dietary iodine bioavailability(5).

Fig. 2 Distributions of urinary iodine concentration (UIC) among (a) pregnant women aged 18–35 years and (b) their children aged 3–15 years, Bangalore, India, May 2008 to September 2011
Table 2 Characteristics of the pregnant women aged 18–35 years, Bangalore, India, May 2008 to September 2011

LMP, last menstrual period; US, ultrasound; UIC, urinary iodine concentration.
Age, anthropometrics and UIC of the children (pre-schoolers and SAC) are shown in Table 3; 45·2% of them were boys. The median (range) UIC in the children was 220 (10–782) µg/l, significantly higher than the median UIC in their mothers (P=0·008) and indicating more-than-adequate iodine intake in this group. The distribution of UIC among children is shown in Fig. 2(b). Using the formula of the US Institute of Medicine(5) to estimate iodine intakes from UIC and body weight in children, a median UIC of 220 µg/l in 6–15-year-old children (n 52) with a mean age of 7·8 years and a body weight of 24·2 kg would correspond to a median iodine intake of ≈140 (range 9–413) µg/d. As shown in Fig. 3, there was a weak but significant positive correlation between the UIC of the children and that of their pregnant mothers (r=0·160; P=0·03). By trimester, this correlation was significant only in the first trimester (r=0·423; P=0·035), not in the second (r=0·162; P=0·21) or the third (r=0·063; P=0·54).

Fig. 3 (colour online) Median urinary iodine concentration (UIC) in matched pairs of pregnant women aged 18–35 years and their children aged 3–15 years by trimester of pregnancy: (a) trimester 1 (y = 0·305x + 133·6; R 2 = 0·114), (b) trimester 2 (y = 0·314x + 154·9; R 2 = 0·065) and (c) trimester 3 (y = 0·107x + 202·6; R 2 = 0·011); Bangalore, India, May 2008 to September 2011. Lines – – – – – represent the cut-offs for iodine adequacy for the two groups
Table 3 Characteristics of the children aged 3–15 years, Bangalore, India, May 2008 to September 2011

WAZ, weight-for-age Z-score; HAZ, height-for-age Z-score; BAZ, BMI-for-age Z-score; UIC, urinary iodine concentration.
Discussion
The current cross-sectional study is the first to report iodine intakes in pregnant women and their children sharing a common household food basket and all meals. Based on median UIC, pregnant women were iodine sufficient but children had more-than-adequate iodine intake. A recent review concluded that when the median UIC in SAC or non-pregnant women indicated iodine intake was adequate or above requirements in a region, about half the time pregnant women in that same region had inadequate iodine intake(Reference Wong, Sullivan and Perrine15). Multiple studies have reported that when SAC have adequate iodine status (median UIC=100–199 µg/l), pregnant women are deficient (median UIC <150 µg/l), and that iodine sufficiency in pregnancy is attained only when the SAC have more-than-adequate (median UIC=200–299 µg/l) iodine status(Reference Kusić, Jukić and Rogan14, Reference Wong, Sullivan and Perrine15, Reference Gowachirapant, Winichagoon and Wyss25–28). In an earlier Thai study using similar methodology(Reference Gowachirapant, Winichagoon and Wyss25) but in families where not all meals were consumed within the household, despite a median UIC of 200 μg/l in SAC, iodine intakes in pregnant women were inadequate, with a median UIC of only 108 μg/l. In Tasmania, iodine status of pregnant women before and after iodine fortification of bread was compared with concurrent data for schoolchildren; the data showed pregnant women to be iodine deficient after supplementation, while children were sufficient(Reference Burgess, Seal and Stilwell29). Recent studies from Belgium(Reference Vandevijvere, Mourri and Amsalkhir30) and China(Reference Burgess, Seal and Stilwell31) also suggest that median UIC in SAC may not be a good proxy of iodine status in the entire population. In our data, there was a modest positive correlation between UIC in pregnant mothers and their children, and this correlation was strongest in the first trimester, consistent with an earlier study from Thailand(Reference Gowachirapant, Winichagoon and Wyss25). In a survey in Kyrgyzstan where children and pregnant women did not live together but were from the same settlements with access to the same salt supplies, their median UIC values were also positively correlated (r=0·63; P<0·001)(Reference Sultanalieva, Mamutova and van der Haar26).
Our findings form a valuable contribution to the discussion on whether salt iodization programmes can adequately cover the iodine requirements of all population groups including that of pregnant women. In our study population, the salt iodization programme was adequately covering the iodine requirement of pregnant women. A systematic review of iodine nutrition status in Indian pregnant women found that median UIC were in the range of 95–178 µg/l with 60–95% of pregnant women consuming adequately iodized salt(Reference Yadav, Srivastava and Badhal32). A recent study in rural and urban settings in the Maharashtra region of India reported median UIC of 203 and 211 µg/l at 17 and 34 weeks of pregnancy, suggesting adequate dietary provision at both gestational stages studied and implying that iodine deficiency was unlikely to be a frequent problem in that population(Reference Lean, Lean and Yajnik33). However, studies from rural India, especially in tribal populations, report significant iodine deficiency in pregnant women and areas where only 19% of salt is adequately iodized(Reference Menon, Skeaff and Thomson34, Reference Singh, Fotedar and Lakshminarayana35). Thus, our results should not be generalized to less-affluent, rural areas of India where adequately iodized salt is not available and where pregnant women are likely to still have inadequate iodine intakes. Because there are wide variations in iodized salt quality and coverage across India, additional population-based studies in other regions of India are needed to confirm these findings.
There are some previous data from the Bangalore region on iodine intake. The WHO Global database on iodine deficiency 2007 reported that the median UIC in 11–18-year-old children in the Bangalore urban district was 185 µg/l(36). The National Family Health Survey (NFHS-3) for the state of Karnataka, India (2005–2006) found 66·5 and 27·6% of households in urban and rural areas, respectively, were using salt iodized at >15 ppm(10). The 2009 Coverage Evaluation Survey conducted by UNICEF reported that the use of non-iodized salt seemed more common in the state of Karnataka compared with other states, with 40·1, 23·9 and 35·9% of the households using non-iodized salt (0 ppm), inadequately iodized salt (<15 ppm) and adequately iodized salt (≥15 ppm), respectively(12). The Iodized Salt Coverage Study in 2010 found only a marginal improvement from NFHS-3 in the consumption of adequately iodized salt in rural Karnataka (35·4%) and attributed this to the continued preference for crystal salt that tends to be less well iodized(37), which is confirmed by our study. Currently, in Karnataka, iodized crystal salt is distributed through the public distribution system at 3·00 Indian rupees/kg(Reference Kapil38) and 64·2% of the pregnant women in our study preferred powdered to crystal salt; this likely explains why non-iodized crystal salt was present in only 4% of households (but in each case, adequately iodized powdered salt was present) and the median UIC in pregnant women indicated sufficient intake. However, two-thirds of households (64·3%) were using both types of salt and in these households crystal salt contributed 57% of the monthly salt usage. Therefore, it is important that crystal salt, along with powdered salt, continues to be adequately iodized.
A better understanding of the iodine sources, planned and un-planned, is crucial for the design and monitoring of national iodine nutrition programmes(Reference Timmer1). Accounting for iodized salt intake obtained from processed foods is becoming increasingly important, as well as iodine-containing products such as iodine supplements, multi-micronutrient supplements, home fortification products such as micronutrient powders, ready-to-use supplementary foods and, in some specific cases, iodine in the natural environment(Reference Timmer1). In our study, the contribution of iodine-containing supplements and multi-micronutrient powders to iodine intakes was negligible; the only significant source of iodine was household salt, and the mean per capita consumption of household salt was estimated to be high, at 13 (sd 6·8) g/d. This was a crude estimate derived by dividing the monthly consumption of salt by the number of members in the household that included both adults and children. Although our data provide only a rough estimate of actual salt consumption, it is in general agreement with the mean per capita salt intake of 11·3 (sd 5·1) g/d calculated using 3 d weighed food records in a recent study in Bangalore(Reference Andersson, Thankachan and Muthayya39). The estimated daily iodine intake in pregnant women and 6–15-year-old children (n 52) in the present study was ≈278 and 140 µg/d, respectively(5). While we did not collect dietary data for iodine intakes in the present study, recent 24 h dietary recall intake data from a comparable group of pregnant women in this area found that these women consume a traditional South Indian meal with dairy products but no bread and a minimum amount of processed foods (N Jaiswal, unpublished data). Thus, iodized salt is most likely the most important source of iodine and a secondary source could be dairy products in these households. However, it is important that native dietary sources of iodine and processed foods be identified and accounted for iodine intake in populations. The findings of the current study can likely be generalized to other households in Bangalore. The lunch meal the children shared with their pregnant mothers in the present study would be similar to that provided in the noon meal programme in Karnataka. The suggested menu for the meals in this programme are meals of rice and lentils, semolina with vegetables and rice, and lentils with vegetables, all cooked with double-fortified salt containing Fe and 50 ppm iodine(40). The median iodine concentration of powdered salt in our study was 55 ppm.
Although iodine intake in our study appeared mainly due to iodized salt, in other countries, both dietary intake of foods with high native iodine content and iodine supplements contribute to iodine intakes during pregnancy. For example, many Japanese eat seaweed and make soup stock from kelp on a daily basis; and in fact it is widely believed in Japanese society that seaweed intake is good for pregnancy(Reference Orito, Oku and Kubota41). In the US diet, the common sources of iodine are iodized salt, dairy products, breads and seafood(42). To achieve a total of 250 μg iodine ingestion daily in North America, the American Thyroid Association recommends that pregnant women should supplement their diet with a daily oral supplement that contains 150 μg iodine(Reference Stagnaro-Green, Abalovich and Alexander43). However, iodine intake in the USA continues to fall and pregnant women are iodine deficient; despite recommendations for iodine supplementation by experts, iodine supplements are used by only 22% of US pregnant women(44). The American Thyroid Association also recommends that in areas of the world outside North America, strategies for ensuring adequate iodine intake during pregnancy will vary according to regional dietary patterns and availability of iodized salt(Reference Stagnaro-Green, Abalovich and Alexander43). In the UK, a recent study called for iodine deficiency in pregnant women to be treated as an important public health issue, particularly considering there is no national salt iodization programme and dietary guidelines from the UK government are outdated(Reference Bath, Steer and Golding45). In Australia, mandatory use of iodized salt (25–65 mg/kg) by bread manufacturers and a daily supplement intake of 150 μg iodine by pregnant women are recommended by the National Health and Medical Research Council as the two strategies to achieve optimal iodine intakes in this group(Reference Charlton, Yeatman and Brock46). Using predictive modelling, pregnant women in New Zealand were expected to achieve adequate but not excessive iodine intakes when 150 μg of supplemental iodine was taken daily, taking into account the contribution of iodized salt in bread(Reference Schiess, Cressey and Thomson47). However, disparities in supplement use by New Zealand pregnant women highlight the need for further efforts towards USI, such as the mandatory fortification of additional processed foods with iodized salt(Reference Mallard and Houghton48).
A limitation of our study is the use of a single spot urine sample collected from pregnant mothers and their children within a week of each other. Considering that UIC in spot samples varies substantially between days and seasons(Reference Als, Haldimann and Burgi49–Reference Rasmussen, Ovesen and Christiansen51), as a consequence of a circadian rhythm of iodine excretion(Reference Als, Helbling and Peter52) and due to differences in fluid intake(Reference Remer, Fonteyn and Alexy53), it would have been preferable to obtain samples at the same time. Although we did not attempt to estimate the proportion of our population with high and low intakes of iodine, a sub-sample collection would have removed the within-person variance thus permitting a correction to the population distribution. In a population with a median UIC in the sufficient range, this correction of the tails would lead to a decrease in the proportion at either extreme of the distribution(Reference Mackerras, Singh and Eastman54). However in the present study, we found a good significant correlation (r=0·432; P=0·035) between the UIC values of the children and their pregnant mothers in their first trimester.
We are aware of no published data that show iodine intakes by SAC in the more-than-adequate range indicated by a median UIC of 200–299 µg/l have adverse effects. On the contrary, a recent study including data from twelve countries and more than 2500 children concluded that chronic iodine intakes from iodized salt resulting in more-than-adequate UIC values do not cause thyroid dysfunction in 6–12-year-old children(Reference Zimmermann, Aeberli and Andersson55). That study recommended that the more-than-adequate iodine intake range (200–299 µg/l) should be reconsidered and merged with the adequate iodine intake range resulting in a widened range of 100–299 µg/l indicating sufficient intake in children(Reference Zimmermann, Aeberli and Andersson55). Earlier, it had been shown that UIC up to 500 µg/l is not associated with thyroid volume in a large international sample of 6–12-year-old children with iodine intakes ranging from adequate to excessive(Reference Zimmermann, Ito and Hess56). Most people who are iodine sufficient are remarkably tolerant to high dietary intakes of iodine, and intakes up to 1100 µg/d are tolerated well by healthy adults(5). There are concerns that more-than-adequate iodine intake could increase thyroid autoimmunity in adults(Reference Teng, Shan and Chen57) but findings are equivocal. Therefore, considering the available evidence, we feel the iodine intake level of the children in the current study is very likely to be safe.
It could be argued that the current Indian salt legislation with a cut-off value for adequacy at 15 ppm needs a revision in the current scenario to incorporate a range with an upper limit for salt iodization, as is done in many national programme standards. According to WHO/UNICEF/ICCIDD, an adequate iodine level in household salt is defined as salt containing 15–40 mg iodine/kg(4, 6). We found a median (range) iodine concentration of 55·0 (17·2–65·9) µg/g in powdered salt and 18·9 (2·2–68·2) µg/g in crystal salt in the present study. Studies from Rajasthan, India and China showed that optimal iodine status of both children and pregnant women was attained when the salt iodine content was approximately 30 mg/kg(Reference Ategbo, Sankar and Schultink58, Reference Wang, Zhang and Ge59). In the Rajasthan study, it was proposed that the iodization of household salt should be increased from the current level in order to provide optimal iodine intakes in the population(Reference Ategbo, Sankar and Schultink58). In a recent cross-sectional study from Shanghai, China where the median salt iodine concentration was 29·5 mg/kg and 91·5% of households were using adequately iodized salt, pregnant women were still iodine deficient while the general population had adequate iodine nutrition(Reference Zou, Wu and Guo31). In Kyrgyzstan, pregnant women had adequate iodine intakes only in those households where salt iodine content was ≥25 mg/kg(Reference Sultanalieva, Mamutova and van der Haar26). In these studies, salt was the main source of iodine intake in the diet. In populations where a substantial proportion of the total iodine intake comes from milk, UIC in SAC may overestimate the iodine status of the general adult population because milk consumption usually is higher in children(Reference Caldwell, Makhmudov and Ely7). However, in most countries where salt is the primary source of iodine in the diet, the differences between children and adults is smaller and the median UIC in SAC may be used to represent iodine status of the population at large(Reference Andersson, Karumbunathan and Zimmermann60).
Conclusion
In conclusion, our data indicate that in this selected urban population of southern India, salt iodization (including iodization of crystal salt) ensures adequate iodine intake in pregnant women, although iodine intake in their children is in the more-than-adequate range according to current cut-off criteria. Although the study suggests that a well-functioning iodized salt programme can provide adequate iodine to pregnant women, continuous monitoring of this critical target group is required and in populations where iodized salt is either not available or not adequately iodized, iodine supplementation during pregnancy should be considered. In addition, evaluation of the current recommendation to use median UIC in SAC as a proxy for the iodine status in the general population and population groups most vulnerable to iodine deficiency, such as young children and pregnant women, was recently recognized as a research priority(Reference Andersson, Benoist and Rogers61). We suggest that the current WHO/UNICEF/ICCIDD cut-off for median UIC in children indicating more-than-adequate intake may need to be reconsidered to allow the iodized salt programme to cover the increased needs of pregnant women.
Acknowledgements
Acknowledgements: The authors thank the pregnant women and their children for their participation in the study. They are thankful to the doctors, nurses and technical staff at St Martha’s Hospital for their support. The authors also acknowledge Dr Sumithra Muthayya for her contribution to the study and Ms J. Madhumitha, Ms Mary Menega, Ms Rani Usha, Ms H.P. Ramya and Ms M. Manjula for technical support. Financial support: This work was supported by the Nestlé Foundation, Lausanne, Switzerland. The Nestlé Foundation had no role in the design, analysis or writing of this article. Conflicts of interest: None. Authorship: N.J. was involved in data collection, data analysis and wrote the first draft of the manuscript; A.M.B. was involved in data analysis and writing of the manuscript; S.K.S. was involved in data collection and supervision; K.S. was involved in supervision of data collection and manuscript writing; M.B.Z. was involved in conception of the study design and manuscript writing. All authors read and approved the final manuscript. Ethics of human subject participation: The study was conducted in accordance with the guidelines laid down in the Declaration of Helsinki and all procedures involving pregnant women and their children were approved by the institutional ethical review boards at St John’s National Academy of Health Sciences and St Martha’s Hospital, Bangalore, India. The study was explained in detail to the participating women and written informed consent from each participant was obtained at recruitment.