The Australian Dietary Guidelines recommend exclusive breast-feeding of infants up to at least 6 months, with further continued breast-feeding up to 12 months and beyond( 1 ). These recommendations are based on the numerous positive and protective short-/long-term effects of breast-feeding for both the infant and mother( 2 – Reference Kramer and Kakuma 7 ) and are in accordance with the WHO recommendation of exclusive breast-feeding up to 6 months, followed by an introduction of complementary foods and continued breast-feeding thereafter( 2 ).
In 2010, it was estimated that breast-feeding was initiated with 96 % of Australian infants; however, only 15 % of infants were exclusively breast-fed up to the recommended 6 months (21 % predominantly breast-fed)( 6 ). With 60 % of infants breast-fed at all (be it exclusive, full/predominant or complementary) at 6 months, information about breast-feeding duration based on birth order is scarce.
Maternal attributes positively associated with breast-feeding initiation and duration include: higher maternal age( 6 , Reference Callen and Pinelli 8 – Reference Heck, Braveman and Cubbin 11 ); higher maternal education( 6 , Reference Callen and Pinelli 8 – Reference Howel and Ball 13 ), although not in all studies( Reference Scott, Binns and Oddy 10 ); higher family income( Reference Callen and Pinelli 8 ); being married( Reference Thulier and Mercer 9 , Reference Heck, Braveman and Cubbin 11 ), although not in all studies( Reference Scott, Binns and Oddy 10 ); living with a partner( Reference Howel and Ball 13 ); history of prior breast-feeding( Reference Howel and Ball 13 – Reference Taylor, Geller and Risica 15 ); and having a healthy pre-pregnancy BMI( Reference Baker, Michaelsen and Sorensen 16 – Reference Amir and Donath 18 ). Higher parity has also been positively associated with breast-feeding initiation( Reference Heck, Braveman and Cubbin 11 ) and more frequently with breast-feeding duration( Reference Scott, Binns and Oddy 10 , Reference Simard, O’Brien and Beaudoin 19 , Reference Dennis 20 ); while difficulties with infant feeding in the first month postpartum are negatively associated with breast-feeding duration( Reference Scott, Binns and Oddy 10 ). Further social and demographic characteristics positively associated with breast-feeding, such as father’s preference for breast-feeding, can be found summarised in the 2013 Australian Dietary Guidelines (Table 4·2)( 21 ).
While lower education has been associated with reduced odds of breast-feeding in Australia( 6 , Reference Callen and Pinelli 8 , Reference Scott, Binns and Oddy 10 ) and other high-income countries( Reference Callen and Pinelli 8 , Reference Thulier and Mercer 9 , Reference Howel and Ball 13 , Reference Amir and Donath 18 , Reference Amir and Donath 22 ), one study found negligible social differences in ceasing exclusive breast-feeding at 3 months( Reference Yang, Platt and Dahhou 23 ). In 2004–05, an Australian study found a 26 % increase in odds of breast-feeding at 6 months in neighbourhoods of increasing socio-economic position (SEP) advantage, as measured by quintiles of SEIFA (Socio-Economic Indexes for Area; a measure of the distribution of socio-economic conditions based on neighbourhood( 24 )); these social inequalities have increased since 1995 and are similar when investigating odds of breast-feeding at 3 and 12 months( Reference Amir and Donath 25 ).
Given the association of breast-feeding with positive maternal and infant health and development outcomes, it is important to understand how SEP and parity relate to breast-feeding initiation and duration in Australia today. Such information will assist in identifying groups of women less likely to meet the guidelines and thus encourage consideration of strategies to overcome SEP-specific barriers to breast-feeding. The present study uses childhood and adulthood measures of SEP to identify whether initiation of breast-feeding and breast-feeding for at least 6 months (i) varies by parity and (ii) is socially patterned.
Methods
Study design and participants
We used data from the Australian Longitudinal Study on Women’s Health (ALSWH), a prospective cohort study comprising Australian citizens and permanent residents randomly selected from the national health and insurance database (Medicare), with an intentional oversampling of women in rural/remote areas. Women completed a self-reported questionnaire in 1996 (baseline) and at approximate 3–4 yearly intervals thereafter. The ALSWH has obtained informed consent from all study participants and is approved by the Human Research Ethics Committees of the Universities of Newcastle and Queensland. Further details about ALSWH recruitment and study design can be found elsewhere( Reference Brown, Bryson and Byles 26 , Reference Lee, Dobson and Brown 27 ).
Our sample is drawn from the ALSWH cohort born in 1973–78 (aged 18–23 years at baseline, n 14 247). Analysis of the relatively high attrition between baseline and Survey Two (n 9688; conducted in 2000; women aged 22–27 years) has concluded that possible biases due to loss to follow-up do not limit significant longitudinal analysis of these data( Reference Powers and Loxton 28 ). Since Survey Two, attrition has remained fairly stable: at Survey Three, 64 % (n 9081; 2003; aged 25–30 years); Survey Four, 64 % (n 9145; 2006; aged 28–33 years); at Survey Five, 58 % (n 8200; 2009; aged 31–36 years); and at Survey Six, 56 % (n 8010; 2012; aged 34–39 years).
In order to use the most recent child birth information, we restricted the sample to parous women who answered Surveys One and Six (n 5917). Our final sample included complete cases for all exposures (n 4777).
Measurements
Exposure: indicators of socio-economic position
Parental education (highest of mother’s or father’s), a marker of early-life SEP, was collected at Survey Two and categorised as: very low (≤10 years); low (≤12 years/equivalent); intermediate (trade/certificate/diploma); high (degree/higher); or did not know.
Own highest achieved education was collected at age 34–39 years and categorised as: low (≤12 years); intermediate (trade/apprentice/certificate/diploma); or high (degree/higher).
Area of residence at baseline was categorised as: urban (major cities); rural (inner regional); or remote (outer regional/remote). Distribution of socio-economic conditions based on neighbourhood (SEIFA score for education and occupation) was collected at baseline and divided into quintiles, with lower scores indicating greater disadvantage( 24 ), and was included as a continuous variable in the models. Financial stress was measured by asking women about their ability to manage on their income. This was collected at age 34–39 years and categorised as: always difficult (impossible/always); sometimes difficult; or easily managed (not too bad/easy).
Outcome measures
Duration of breast-feeding was taken from Survey Six (if missing, then Survey Five) from the question ‘How many complete months have you breast-fed each of your children?’ Given that 92·4 % of women had three or fewer children, we analysed breast-feeding with the first, second or third child only, categorised as: not initiated; <6 months; or ≥6 months. For each child, two dichotomous outcomes were: initiation of breast-feeding; and, among those who had initiated breast-feeding, whether they were breast-fed (at all) for at least 6 months( 1 ).
Additional covariates
Parity (total number of children) was categorised as: one; two; or three or more. Age at birth of the first child was calculated by subtracting the woman’s date of birth from that of her first child. This score was categorised as: <20·0 years; 20·0–24·99 years; 25·0–29·99 years; 30·0–34·99 years; or ≥35·0 years. Fertility issues, measured at Survey Six, were dichotomised based on the question ‘Have you and your partner (current or previous) ever had problems with infertility (that is, tried unsuccessfully to get pregnant for 12 months or more)?’
BMI at age 34–39 years was based on self-reported weight and height (kg/m2). Using the WHO’s categories, weight status was defined as: underweight (<18·50 kg/m2); healthy weight (18·50–24·99 kg/m2); overweight (25·00–29·99 kg/m2); or obese (≥30·00 kg/m2)( 29 ). Country of birth was categorised as ‘Australia’ or ‘other’, since few women were born outside Australia.
Statistical analysis
Descriptive analyses, including t tests and Pearson’s χ 2 tests, were used to describe the sample and explore the associations between maternal and own SEP characteristics with the breast-feeding patterns, with results considered statistically significant at P<0·05. ‘Lasagne plots’( Reference Jones, Hockey and Mishra 30 ) were created to show breast-feeding patterns stratified by parity, and proportions were plotted to document breast-feeding patterns stratified by highest education and total parity.
Logistic regression was used to: (i) describe the patterns of breast-feeding by parity; and investigate the association between the SEP measures and (ii) initiating breast-feeding with each child and (iii) breast-feeding each child for at least 6 months (among women who had initiated breast-feeding with that child). All models were adjusted for age at baseline (centred at the cohort mean) and the child’s year of birth (OR1). OR2 further adjusted for parental education.
We ran sensitivity analyses: (i) investigating the association between SEP and odds of breast-feeding each child for at least 6 months among all women with an index child, and not only those who had initiated breast-feeding; and (ii) for both outcomes using data imputed for all parous women (n 5917). We ran PROC MI, with twenty imputations using fully conditional specification, to impute all outcomes, exposures and covariates used in the multinomial logistic models. We also included auxiliary variables associated with missingness in the imputation model( Reference Spratt, Carpenter and Sterne 31 ).
All analyses were completed using the SAS statistical software package version 9·4.
Results
At Survey Six and a mean age of 36·8 years (median 36·9 years, interquartile range 35·6–38·1 years), approximately 79 % of the sample was multiparous, with almost half of women having two children. Almost three-quarters of the women were aged between 25 and 35 years at first birth (median 29·3 years), and half of the sample had a high education and lived in an urban area (Table 1). Approximately 60 % of women had breast-fed the first, second and third child for at least 6 months (Table 1).
Table 1 Reproductive and demographic characteristics among parous women (born 1973–78) from the Australian Longitudinal Study on Women’s Health (n 4777)
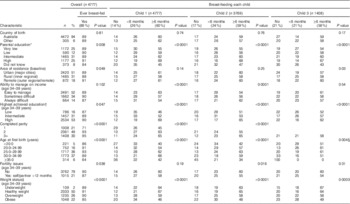
* Parental education (highest of mother’s or father’s) categorised as very low (≤10 years), low (≤12 years/equivalent), intermediate (trade/certificate/diploma), high (degree/higher) and did not know (could not recall).
† Own highest education categorised as low (≤12 years), intermediate (trade/apprentice/certificate/diploma) and high (degree/higher).
‡ Weight status defined using the WHO categories( 29 ).
§ Mantel–Haenszel χ 2.
Patterns of breast-feeding by parity
Overall, 89 % of the ALSWH population had ever breast-fed (Table 1) and 83 % of the children included in the analyses were breast-fed; while 59 % of infants were breast-fed for at least 6 months and 68 % of women had breast-fed at least one child for at least 6 months. Breast-feeding of firstborn children was more common among women who continued to deliver more children; ~71 % of primiparas initiated breast-feeding with their first child (44·9 % breast-fed for at least 6 months, 26·5 % breast-fed for less than 6 months; results not shown) compared with 90·5 % of women with two children and 88·7 % of women with three or more children (Fig. 1).

Fig. 1 Percentage of multiparous women (born 1973–78) breast-feeding each index child for at least 6 months (![]() , 6 months or longer;
, 6 months or longer; ![]() , less than 6 months;
, less than 6 months; ![]() , did not initiate), stratified by parity: (a) parity=2; (b) parity=3+, among women from the Australian Longitudinal Study on Women’s Health (n 4777). Overall, women who breast-fed their first child for at least 6 months were most likely to also do so with their second child, unless it was their youngest child; multiparous women tended to be less likely to initiate breast-feeding with their youngest child. (Note: among women with one child, 28·6 % did not initiate breast-feeding, while 44·9 % breast-fed for at least 6 months and 26·5 % breast-fed for less than 6 months; data not shown)
, did not initiate), stratified by parity: (a) parity=2; (b) parity=3+, among women from the Australian Longitudinal Study on Women’s Health (n 4777). Overall, women who breast-fed their first child for at least 6 months were most likely to also do so with their second child, unless it was their youngest child; multiparous women tended to be less likely to initiate breast-feeding with their youngest child. (Note: among women with one child, 28·6 % did not initiate breast-feeding, while 44·9 % breast-fed for at least 6 months and 26·5 % breast-fed for less than 6 months; data not shown)
With the first child, a lower percentage of primiparous women breast-fed for at least 6 months (45 %), compared with almost two-thirds of multiparous women (Fig. 1). Among women with three or more children, breast-feeding initiation and duration tended to be similar with the first and second child (Fig. 1). However, 20 % of multiparous women did not initiate breast-feeding with their youngest child (Fig. 1).
Socio-economic position and breast-feeding initiation and duration
A slightly higher percentage of high-educated women initiated breast-feeding with the first child compared with lower educated women (Fig. 2). Further analysis stratified by total parity showed this was apparent only among multiparous women (see online supplementary material, Supplemental Fig. 1). With regard to the second and third child, the percentage of women initiating breast-feeding was highest among women with an intermediate education (Fig. 2 and Supplemental Table 1). Overall, women were less likely to initiate breast-feeding with their youngest child (Fig. 2). Stratification by total parity showed that the largest absolute decrease in the percentage of women initiating breast-feeding with their youngest, compared with oldest child, was among high-educated women (Supplemental Fig. 1).
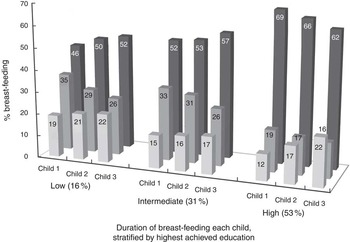
Fig. 2 Percentage of women (born 1973–78) initiating breast-feeding and breast-feeding for at least 6 months (![]() , 6 months or longer;
, 6 months or longer; ![]() , less than 6 months;
, less than 6 months; ![]() , did not initiate), stratified by highest achieved education, among women from the Australian Longitudinal Study on Women’s Health (n 4777)
, did not initiate), stratified by highest achieved education, among women from the Australian Longitudinal Study on Women’s Health (n 4777)
Stronger social patterning was found in breast-feeding for at least 6 months, where higher educated women were more likely to do so with each child (Fig. 2). However, while the percentage of low- and intermediate-educated women breast-feeding for at least 6 months increased slightly from the first to the third child, this was not the case with high-educated women (Fig. 2). These women were less likely to breast-feed their youngest child for at least 6 months.
Results from logistic regression models adjusted for woman’s age and birth year of the index child (OR1), and further adjusted for parental education (OR2), confirmed that compared with high-educated women, low-educated women had 1·5–2 times increased odds of not having initiated breast-feeding with any of their children (Table 2). As well as intermediate-educated women, low-educated women also had increased odds of not breast-feeding their first, second and third child for at least 6 months (Table 3).
Table 2 Socio-economic position and odds of not initiating breast-feeding with the first, second and third child among women (born 1973–78), among women from the Australian Longitudinal Study on Women’s Health (n 4777)

OR1, minimally adjusted for baseline age and birth year of the index child; OR2, minimally adjusted + parent’s education; SEIFA, Socio-Economic Indexes for Areas (education and occupation); Ref., reference category.
Significant results are indicated in bold font.
When we further adjusted for the most recent birth interval, estimates were very similar (fractionally lower).
* Parental education (highest of mother’s or father’s) categorised as very low (≤10 years), low (≤12 years/equivalent), intermediate (trade/certificate/diploma), high (degree/higher) and did not know (could not recall).
† Own education categorised as low (≤12 years), intermediate (trade/apprentice/certificate/diploma) and high (degree/higher).
Table 3 Socio-economic position and odds of not breast-feeding for at least 6 months among women (born 1973–78) who had initiated breast-feeding with each index child, among women from the Australian Longitudinal Study on Women’s Health (n 4777)

OR1, minimally adjusted for baseline age and birth year of the index child; OR2, minimally adjusted + parent’s education; SEIFA, Socio-Economic Indexes for Areas (education and occupation); Ref., reference category.
Significant results are indicated in bold font.
When we further adjusted for the most recent birth interval, estimates were very similar (fractionally lower).
* Parental education (highest of mother’s or father’s) categorised as very low (≤10 years), low (≤12 years/equivalent), intermediate (trade/certificate/diploma), high (degree/higher) and did not know (could not recall).
† Own education categorised as low (≤12 years), intermediate (trade/apprentice/certificate/diploma) and high (degree/higher).
Additionally, women with a very low-educated parent had increased odds of not initiating breast-feeding with their first and second child (Table 2) and also of not breast-feeding their first, second or third child for at least 6 months (Table 3). Women who did not know their parent’s education had approximately twice the odds of not initiating breast-feeding with their first and second child, and ~3·7 times increased odds with the third child (Table 2). These women also had at least 2·5 times increased odds of not breast-feeding for at least 6 months (Table 3). Women who found it difficult to manage on their income had 1·3–1·4 times increased odds of not breast-feeding for at least 6 months with their first and second child (Table 3). Area of residence and SEIFA score of SEP were not significantly associated with breast-feeding initiation or duration.
Sensitivity analyses showed marginally stronger associations between SEP and breast-feeding for at least 6 months when analysed among all parous women (see online supplementary material, Supplemental Table 1) and not just among those who had initiated breast-feeding (Table 3). Additionally, analyses using multiply imputed data for all exposures and outcomes showed comparable estimates for the association between SEP and breast-feeding initiation (Supplemental Table 2) and breast-feeding for at least 6 months (Supplemental Table 3).
Discussion
The present study investigated the association between SEP and parity on breast-feeding initiation and duration among a cohort of Australian women. While 60 % of women breast-fed their first, second and third child for at least 6 months, we found differences based on completed parity, where multiparous women were more likely to have met this target. While a higher percentage of high-educated women breast-fed each child for at least 6 months, women were less likely to initiate breast-feeding with their youngest child; a difference which was greatest among higher educated women. Overall, women with a lower education were less likely to initiate breast-feeding or to breast-feed for at least 6 months.
Among our sample, 89 % of women had initiated breast-feeding. While a previous Australian study suggested there was an increase in the rate of breast-feeding initiation of infants (to 96 % in 2010)( 6 ), our finding of 83 % of infants being breast-fed is in accordance with a 2001 estimate( Reference Donath and Amir 32 ). Despite this result, we found an increase in the percentage of infants who were breast-fed for at least 6 months; 59 % in our study compared with the 2001 Australian estimate of 50 % of infants being breast-fed at 6 months (be it exclusive, full/predominant or complementary)( Reference Donath and Amir 32 ). Our results indicate that greater support is required in the early phase to assist women in successfully initiating breast-feeding, but more importantly to overcome difficulties in sustaining breast-feeding, be they medical or otherwise.
Consistent with previous Australian findings( 6 ), we found that low-educated women were less likely to initiate breast-feeding or to breast-feed for at least 6 months. This may be a result of higher educated women being more receptive to advised health behaviours, or conversely to reduced family support and assistance for breast-feeding among the disadvantaged. While adequate milk supply and no feeding difficulties in the first month postpartum( Reference Scott, Binns and Oddy 10 ) are positively associated with breast-feeding duration, other factors positively associated with breast-feeding initiation and duration, which may also be socially patterned, include: maternal positive attitude towards breast-feeding( Reference Scott, Binns and Oddy 10 ); not smoking while breast-feeding( Reference Thulier and Mercer 9 , Reference Scott, Binns and Oddy 10 , Reference Baker, Michaelsen and Sorensen 16 ); and gestational weight gain within the Institute of Medicine’s guidelines( Reference Hilson, Rasmussen and Kjolhede 17 ). Higher rates of breast-feeding have been found in Europe and Australia, compared with the USA and Canada( Reference Callen and Pinelli 8 ), which encourages us to consider the importance of social/political contexts in shaping features of the home, work and community environments that may support breast-feeding. This includes not returning to employment early( Reference Scott, Binns and Oddy 10 , Reference Bai, Fong and Tarrant 14 ), parental leave policies and flexible working conditions( Reference Bai, Fong and Tarrant 12 ); as well as social support, positive cultural norms surrounding breast-feeding and the visibility of breast-feeding in public( 1 ). Our finding that all women, particularly those with a high education, were less likely to breast-feed their youngest child may be due to women returning to work soon after having reached their desired number of children, in order to limit their absence from the workforce.
The timing and type of breast-feeding intervention can also influence effectiveness, with a combination of antenatal and postnatal interventions, as well as involving the partner/significant caregiver, being important( 1 ). However, since population-wide interventions can potentially increase social inequalities through greater uptake and improvements among advantaged individuals( Reference Macintyre, Chalmers and Horton 33 ), it is important to identify specific barriers to breast-feeding among the most disadvantaged. Evidence suggests that peer support programmes in combination with professional support are effective in increasing breast-feeding rates( Reference Kaunonen, Hannula and Tarkka 34 ). With high maternal BMI being associated with socio-economic disadvantage( Reference Baker, Michaelsen and Sorensen 16 – Reference Amir and Donath 18 ) as well as difficulties in breast-feeding( Reference Skouteris, Nagle and Fowler 35 ), we should not discount this as another key area in reducing inequalities in breast-feeding.
We also found that women with a very low-educated parent, as well as those who did not know their parent’s education, were more likely to not initiate breast-feeding. These women, and additionally those with an intermediate- or low-educated parent, were also more likely to not breast-feed for at least 6 months. This is a concern which highlights possible intergenerational chains of risk, with previous studies showing that women who were themselves breast-fed as an infant were more likely to intend to, initiate and persevere with breast-feeding( Reference Di Manno, Macdonald and Knight 36 ). Not knowing their parent’s education level may reflect a poor relationship with their parent or increased family dysfunction, or possibly a low level of education which they do not want to disclose.
Despite potential bias in using income measures as a proxy for SEP among women of reproductive age, it is important to estimate the extent to which material circumstances make it easier or more difficult for women to breast-feed. While we acknowledge that our measurement of financial stress is not ideal (due to the uniform timing of data collection, which does not take account of the individual reproductive histories), our results indicate that material circumstances are important for breast-feeding duration. Women who found it difficult to manage on their income were more likely to not breast-feed their first or second child for at least 6 months. We speculate this may be due to single mothers and those on a low income being forced back into the labour market earlier than women with the resources to remain at home. Sensitivity analyses using this same measure of financial stress at earlier time points found that, if anything, the significant estimates reported possibly underestimate the association.
Having a child may introduce financial strain on the family, through additional costs of care and preclusion from the labour market. As such the legislative and regulatory environment, as well as social context, is important for providing parents with the support required to make positive choices for their offspring. This includes adequate employment leave and entitlements, marketing restrictions for infant formula, reducing discrimination towards those who breast-feed, and creating breast-feeding friendly workplaces and communities( 1 ).
With a higher percentage of multiparous women having initiated breast-feeding and breast-fed their first child for at least 6 months, we further speculate that women with hormonal imbalances may find it more difficult to breast-feed and may also be less fertile.
Strengths and limitations
Despite a higher representation of high-educated women, the ALSWH 1973–78 cohort is a nationally representative sample providing longitudinal data over 16 years for women of reproductive age. Very few studies and registers provide information about breast-feeding patterns and behaviours, and while we lack information for some WHO infant feeding categories (exclusive, predominant and complementary), our analyses still provide valuable information on a national level regarding breast-feeding initiation and duration. From an Australian perspective this is particularly important, since there is a lack of national monitoring of breast-feeding which would be particularly useful for priority groups (i.e. young mothers, low SEP, indigenous Australians)( 1 ). Additionally, despite rates of breast-feeding initiation and duration being available in most Australian states/territories, consistency in the measurement and collection of these data varies( 1 ). We acknowledge the potential for recall bias in using breast-feeding duration recorded when women were in their thirties, particularly for women who may have had their child many years earlier.
Conclusion
While overall rates of infant breast-feeding initiation have not increased substantially in Australia since 2001, a greater percentage of infants were breast-fed for at least 6 months. Despite this, high-educated multiparous women were less likely to breast-feed their youngest child and disadvantaged women (with a lower education or a low-educated parent) were less likely to initiate breast-feeding or to breast-feed for at least 6 months. These groups may need greater attention from health-care professionals in the antenatal and postnatal periods, in order to gain a greater understanding of and overcome SEP-specific barriers to breast-feeding initiation and sustained duration, which may assist in reducing existing inequalities in infant breast-feeding.
Acknowledgements
Acknowledgements: The authors would like to thank all ALSWH participants. Financial support: This work was supported by the Australian Commonwealth Department of Health. The ALSWH was conceived and developed by researchers at the University of Newcastle and the University of Queensland, Australia. N.H. is supported by an Australian Postgraduate Award Scholarship. G.M. is supported by an Australian Research Council Future Fellowship (FT 120100812). I.K. is supported by the Swedish Research Council for Health, Working Life and Welfare (project 2006–1518). The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: All authors conceived and designed the study. Data were analysed by N.H., M.J. and G.M., while all authors were involved in interpreting the results. N.H. drafted the article. All authors critically revised the manuscript for important intellectual content and have read and approved the final manuscript. Ethics of human subject participation: The ALSWH was approved by the Human Research Ethics Committees of the Universities of Newcastle and Queensland, and all study participants provided informed consent.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1368980016000367








