Decades of Boko Haram insurgency has left Borno, one of the affected north-eastern states of Nigeria, with nutrition and health emergencies, with reported rates of severe acute malnutrition (SAM) exceeding emergency thresholds and a high burden of childhood diseases( Reference Vahyala, Minnessi and Kabiru 1 – Reference Dunn 4 ). There are a multitude of factors that led to this precarious situation, including food insecurity due to drought and armed conflicts, resulting in cessation of farming activities as farm lands were deserted by the fleeing population and breakdown of health infrastructure due to population displacement directly attributed to the insurgency( Reference Vahyala, Minnessi and Kabiru 1 , Reference Loewenberg 3 ). High mortality rates and disease outbreaks such as measles have also been documented, particularly among internally displaced persons (IDP) in Borno, while most children continue to suffer from malaria, pneumonia and diarrhoea( Reference Awosusi 5 , Reference Leidman, Tromble and Yermina 6 ). These conditions are the top causes of the under-five mortality rate, with half of the deaths having underlying malnutrition( Reference Walker, Rudan and Liu 7 , Reference Black, Morris and Bryce 8 ).
Yet the occurrence and burden of these diseases among children with SAM and their related outcomes in conflict settings in Nigeria have not been systematically and routinely reported. There is a myriad of literature showing rapid disease progression and high mortality among children, as well as adults, with malnutrition and co-morbidities( Reference Fergusson, Tomkins and Kerac 9 , Reference Ahmed, Choe and Mustad 10 ). These poor outcomes are likely to be worse in conflict settings due to precarious living conditions and limited availability of services( Reference Qirbi and Ismail 11 , Reference Polonsky, Ronsse and Ciglenecki 12 ). In protracted emergencies such as Afghanistan and Somalia, childhood wasting was a leading cause of disability-adjusted life years, whereas the crises in Yemen, Libya and Syria have all resulted in a reduction of life expectancy( Reference Mokdad, Forouzanfar and Daoud 13 ).
Addressing the co-morbidities among severely malnourished children is one of the pillars of child survival strategies and remains critical in reducing the under-five mortality rate below the emergency threshold( Reference Fergusson, Tomkins and Kerac 9 , Reference Chamla, Essajee and Young 14 , Reference Bahwere 15 ). However, in Borno State and some emergency programmes, nutrition and health services are managed separately by different agencies, leading to missed opportunities in providing comprehensive and integrated care to a sick child, who often presents with multiple conditions. The current paper presents evidence on the burden and outcomes of co-morbidities among severely malnourished children in a conflict setting with the aim of generating global discourse for joint health–nutrition programming as part of a minimum package of primary health-care services in humanitarian settings.
Materials and methods
The present study was a facility-based, retrospective review of medical records of children diagnosed with SAM and enrolled between 1 June and 30 November 2016 in the outpatient therapeutic programme (OTP) facilities supported by UNICEF in the north-eastern state of Borno, Nigeria, that has been under Boko Haram insurgency in the past decade. The eligibility criteria were restricted to all children with medical records aged 6–59 months who enrolled in OTP facilities inside and outside IDP settlements during this period. The records of these children included data from the date of admission until the date of last exit from the OTP.
In OTP, standard community management of acute malnutrition (CMAM) protocols that are based on Nigerian national guidelines and aligned with SPHERE standards were implemented. In these protocols, SAM was screened using a colour-coded measuring tape around the mid-upper arm circumference (MUAC) during the OTP admission. A child with measurement of less than 115 mm, which corresponded to red colour in the MUAC tape, with or without medical complications, was classified as SAM. Assessment to determine swelling of the feet was also required to be done and those children with swelling in both feet were classified as having oedema, a sign of protein deficiency.
Co-morbidities among children with severe acute malnutrition
In the present study, pneumonia and diarrhoea were the only co-infections analysed due to the availability and reliability of data retrieved from the medical records. In many emergencies diarrhoea and pneumonia are most common and account for the greatest number of deaths( Reference Mokdad, Forouzanfar and Daoud 13 , 16 ). Malaria, which was equally prevalent in the region( Reference Odugbemi, Ezeudu and Ekanem 17 , Reference Do, Babalola and Awantang 18 ), was not analysed due to the limited reliability of data as antimalarial drugs were also prescribed routinely for most children irrespective of the diagnosis. Similarly, a rapid diagnostic test for malaria was used inconsistently, making it difficult to analyse and interpret results.
The algorithm of integrated management of childhood illness (IMCI) was used to classify pneumonia, diarrhoea and their related complications, or disease severity such as severe pneumonia or dehydration( Reference Horwood, Vermaak and Rollins 19 ). IMCI employs a three-step process that includes: (i) assessing a child for general danger signs, common conditions, and nutrition and immunization status; (ii) classifying a child’s illnesses using a colour-coded triage system; and (iii) identifying specific treatments. Both pneumonia and diarrhoea were classified based on the IMCI booklet and using data from the medical records showing history of cough or difficult breathing and fast breathing, and passage of watery stool in a 24h period with various degrees of dehydration, respectively.
Sampling and data sources
A selection of facilities offering outpatient therapeutic treatment commenced with purposive selection of six local government areas, representing four geographical zones, based on IDP population density and malnutrition rate. This was followed by selection of two OTP facilities from each local government area. The list of IDP camps and OTP facilities was drawn from State and UNICEF health facility directories. The list of the local government areas, IDP camps and health facilities can be found in the online supplementary material, Supplemental Table 1. Lastly, all children in the selected facilities enrolled in the above-mentioned period were included in the sample.
The main data source was the national OTP card containing information on demographic profile, anthropometry, medical history, medication/treatment and follow-up. The outpatient department register was also used to complement the information collected from the OTP card in the case of data gaps. The information from these data sources was abstracted into structured questionnaires by a trained team of data collectors. All filled questionnaires were sent to Maiduguri field office where a data manager reviewed the data for consistency and quality and entered the information in the electronic database.
Outcome measures and data analysis
The primary outcome measures were ‘recovery’, ‘defaulter’ from the OTP and ‘mortality’, comparing children with SAM only and those with SAM and co-morbidity at baseline.
Standard CMAM definitions were used for ‘recovery’ as MUAC gained and maintained above 115mm in two consecutive measurements; ‘defaulter’ was defined as absent from weekly OTP visits for three consecutive times, while ‘transfer-out’ indicated those with medical complications who were transferred from OTP to another facility for further treatment, usually in a stabilization centre. In Borno, these stabilization centres were managed by other humanitarian agencies or government. Secondary outcome measures included the burden of co-morbidities among children with SAM and the uptake of measles vaccination, antibiotics for pneumonia, and diagnostic tests for malaria and HIV.
Individual demographic and biological factors that, based on the literature, influence outcomes were selected and included for analysis( Reference Lapidus, Minetti and Djibo 20 ). These include age, sex, residence (IDP camp or non-camp), referral source to OTP, oedema, weight at admission and measles vaccination status. We also determined whether OTP admission before or after the declaration of a Level 3 (L3; severe) emergency by the UN would influence the outcomes due to the likely differences in the availability of services. Length of OTP stay/enrolment was calculated by subtracting the dates of OTP admission and OTP exit. We followed CMAM and SPHERE standards to determine thresholds such that mortality and defaulter rates below 10 and 15 %, respectively, and recovery rate of 75 % or above were considered acceptable, and 56d for the average length of stay in OTP.
Kaplan–Meier survival analysis was used to estimate survival probabilities throughout the length of stay in OTP, comparing those classified as SAM and SAM with co-morbidities. Bivariate and multivariate Cox proportion hazard models established the relationship between individual factors and primary outcomes. Correlates of co-morbidities among SAM children at baseline were assessed using OR and 95 % CI, while mean differences were determined as significant using the t test with statistical significance of α<0·05. All statistical analyses were performed using the statistical software package Stata version 14.2.
Ethical considerations
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the State Health Research Committee of Borno, Nigeria. Further approval for data abstraction from OTP cards that are facility-held was obtained from the State Ministry of Health and the State Primary Health Care Development Agency. All personal identifiers were removed from the medical records.
Results
A total of 396 children with median age of 15 months (interquartile range: 10–24 months), of whom 186 (47·0 %) were under 12 months of age and 194 (48·9 %) were female, were identified and followed up from the date of admission to their last exit from OTP. Most, 348 (87·9 %), resided in IDP camps. The mean length of stay in OTP, which is the time from admission to exit from OTP, was 61 (sd 38·3)d. The co-infected SAM children had shorter mean length of stay in OTP than their counterparts (mean difference=11d; 95 % CI 2·5, 19·6d; P=0·006).
The median weight and MUAC on admission were 6·5kg (interquartile range: 5·2–8·0kg) and 111mm (interquartile range: 106–115mm), respectively. Children with SAM and co-morbidities were more likely to have lower weight (mean difference=1100g; 95 % CI 598, 1600 g; P<0·001) and smaller MUAC (mean difference= 5·2mm; 95 % CI 2·6, 7·9mm; P<0·001).
Of 388 records with data, eighteen (4·6 %) children presented with bipedal oedema at baseline. Similarly, of 109 with documented information, forty-five (41·3 %) had received measles vaccination.
Co-morbidities
Of the total, 148 (37·4 %) had at least one co-morbidity, pneumonia or diarrhoea, of which thirty-nine (26·4 %) had both. Out of sixty-seven pneumonia cases, forty-four (65·8 %) were classified as severe pneumonia using the IMCI classification. Similarly, approximately 26·3 % (104/396) of the study population was found to have dehydration. Data also showed that less than 1 % of the study population received a rapid diagnostic test for malaria, cotrimoxazole prophylaxis or an HIV test. In contrast, 90·9 % were given antibiotics (mostly amoxicillin) and 83·6 % were given antimalarial drugs.
In the multivariable regression model (Table 1), children with SAM and co-morbidities were more likely to present with oedema (OR=6·60; 95 % CI 2·29, 9·70) and to have enrolled in OTP before the L3 declaration (OR=2·78; 95 % CI 1·57, 4·92), but were less likely to reside in IDP camps (OR=0·02; 95 % CI 0·01, 0·09). However, age, sex and measles vaccination status were not statistically associated with co-morbidities.
Table 1 Demographic and biological correlates of co-morbidities among children aged 6–59 months with severe acute malnutrition (SAM) who were admitted to outpatient therapeutic programme (OTP) facilities in the conflict setting of Borno, Nigeria, June–November 2016

aOR, adjusted OR; IDP, internally displaced persons; L3, Level 3 (severe) emergency; ref., reference category.
Outcomes
The overall outcomes and differences in recovery, defaulter and mortality rates between SAM children with or without co-morbidities are presented in Table 2. Both recovery and defaulter rates did not meet CMAM and SPHERE emergency standards (57·8 and 36·1 % v. <10 and <15 %, respectively). Similarly, 1 % of the study population was ‘transferred out’ to other facilities for further treatment as per national guidelines. Only mortality was significantly higher among SAM children with co-morbidities (OR=9·7; 95 % CI 3·4, 29·6, P<0·001).
Table 2 Outcomes of the outpatient therapeutic programme (OTP), according to presence of co-morbidities, among children aged 6–59 months with severe acute malnutrition (SAM) who were admitted to OTP facilities in the conflict setting of Borno, Nigeria, June–November 2016

* Statistically significant.
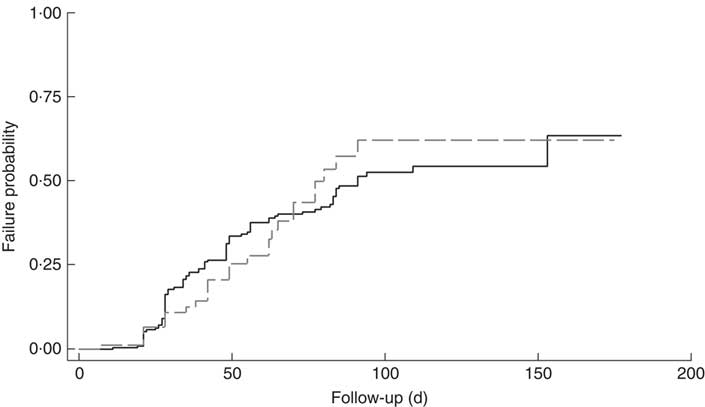
The cumulative rate of mortality during follow-up time was 9·5 (95 % CI 6·0, 15·1) per 10 000 child-days and that of defaulters was 6·7 (95 % CI 5·6, 8·0) per 1000 child-days. Following survival analysis and Cox proportional hazards modelling, children with SAM and co-morbidities were found to be less likely to survive (P<0·001), as shown in Fig. 1, and were ten times more likely to die compared with those without co-morbidities (hazard ratio (HR)= 10·2; 95 % CI 3·4, 31·0). There was no significant difference in survival probability among SAM defaulters with or without co-morbidities (HR=0·99; 95 % CI 0·65, 1·53), as shown in Fig. 2.

Fig. 1 Kaplan–Meier survival estimates for children aged 6–59 months with severe acute malnutrition (SAM) who were admitted to outpatient therapeutic programme facilities in the conflict setting of Borno, Nigeria, June–November 2016: ![]() , cases of SAM only (n 248);
, cases of SAM only (n 248); ![]() , cases of SAM with least one co-morbidity (n 148)
, cases of SAM with least one co-morbidity (n 148)
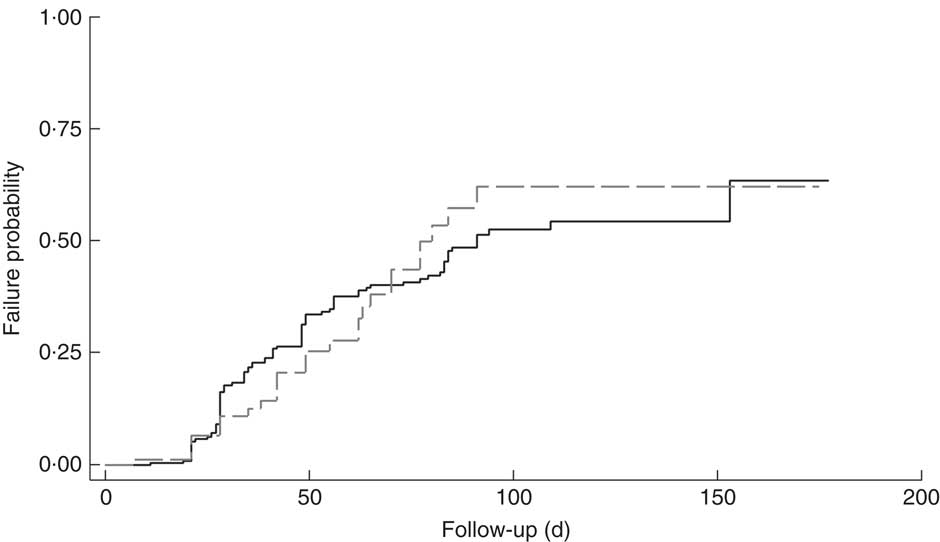
Fig. 2 Kaplan–Meier failure estimates for children aged 6–59 months with severe acute malnutrition (SAM) who were admitted to outpatient therapeutic programme facilities in the conflict setting of Borno, Nigeria, June–November 2016, and defaulted: ![]() , cases of SAM only (n 248);
, cases of SAM only (n 248); ![]() , cases of SAM with least one co-morbidity (n 148)
, cases of SAM with least one co-morbidity (n 148)
In multivariate Cox regression, those who died were likely to have a co-morbidity (adjusted HR (aHR)=4·2; 95 % CI 1·3, 13·2), oedema (aHR=7·2; 95 % CI 2·0, 25·8), dehydration (aHR=4·0; 95 % CI 1·3, 12·7) and lower weight on admission (aHR=0·6; 95 % CI 0·4, 0·9).
Discussion
The present study shows high burden of co-morbidities and associated mortality among severely malnourished children in conflict-affected northern Nigeria, conforming with the existing literature from other countries( Reference Leidman, Tromble and Yermina 6 , Reference Polonsky, Ronsse and Ciglenecki 12 , Reference Bahwere 15 , Reference Cuneo, Dansereau and Habib 21 ). There are several mechanisms and factors that increase the risk of co-morbidities such as pneumonia and diarrhoea in malnourished children, which have been published( Reference Zabihullah, Dhoubhadel and Rauf 22 – Reference Iannotti, Trehan and Clitheroe 26 ). Being underweight, which was also found in our analysis, is one of the risk factors. Other factors include deficiencies of micronutrients such as vitamin D and Zn( Reference Chowdhury, Taneja and Bhandari 27 – Reference Chowdhury, Hoque and Hossain 29 ) that are common in severely malnourished children.
The findings of the present study further suggest that children with SAM and co-morbidity were more likely to be enrolled before the L3 declaration, which was likely due to high unmet needs among the affected population, while many humanitarian actors began to develop their capacities to address the multiple health and nutrition needs during that period. After the L3 activation was done by the UN, relevant capacities across the international humanitarian system, such as improved food distribution, were scaled up to address the increasing needs caused by a multitude of factors including re-emergence of wild polio virus and widespread famine( Reference Vahyala, Minnessi and Kabiru 1 , Reference Roberts 30 ).
The burden of co-morbidity found in the present study could be higher if malaria was able to be included in the analysis, due to its high prevalence in Nigeria and other African countries( Reference Do, Babalola and Awantang 18 , Reference Rogerson, Desai and Mayor 31 – Reference Challe, Kamugisha and Mmbando 33 ). Other diseases such as tuberculosis are also common among children with severe malnutrition( Reference Chisti, Graham and Duke 34 , Reference Chisti, Ahmed and Pietroni 35 ) but there was limited documentation on the OTP cards. The limited documentation on the OTP card was linked to the absence of tuberculosis testing in the OTP; however, such testing was available in the stabilization centres for treatment of SAM children with medical complications in Borno, which were supported by other humanitarian actors. In other studies, isolates of Mycobacterium tuberculosis were identified in more than 21 % of children with SAM( Reference Chisti, Ahmed and Pietroni 35 ), underscoring the need for inclusion of tuberculosis screening among high-risk children. Similarly, the finding of suboptimal uptake of HIV testing was a missed opportunity to improve care, as malnutrition, pneumonia and diarrhoea are all common among HIV-infected children( Reference Chisti, Ahmed and Pietroni 35 – Reference Fergusson, Chinkhumba and Grijalva-Eternod 37 ). Nigeria accounts for almost 30 % of the global burden of paediatric HIV, mainly because of its low coverage of prevention of mother-to-child transmission services including in Borno State( 38 , 39 ).
Unknown HIV status among these children could partly explain low usage of cotrimoxazole, unlike other studies in Nigeria that showed high uptake of cotrimoxazole prophylaxis in antiretroviral therapy and tuberculosis programmes( Reference Ojikutu, Higgins-Biddle and Greeson 40 , Reference Chamla, Asadu and Davies 41 ). Studies have shown that malnutrition impairs child immunity( Reference Ibrahim, Zambruni and Melby 25 ) but whether this is associated with the occurrence of opportunistic infections such as atypical pneumonias requiring cotrimoxazole prophylaxis has not been well documented and is beyond the scope of the current study.
Higher mortality among co-infected children with SAM is consistent with many studies, and in countries such as Bangladesh, increased mortalities were reported among co-infected children after inpatient discharge( Reference Chisti, Graham and Duke 42 ). Our study also shows a higher average stay in OTP with those co-infected children having shorter stays, most likely due to higher mortality observed among this group. Additionally, the recovery rate was low and the defaulter rate was much higher than emergency thresholds, which is consistent with other studies in similar settings( Reference Polonsky, Ronsse and Ciglenecki 12 , Reference Cuneo, Dansereau and Habib 21 ) and is most likely due to limited follow-up mechanisms. A strong follow-up system could also have tracked the outcomes of defaulters that were not included and determined in the current study. Despite high rate of co-morbidities, only 1 % of study children were transferred out to other facilities that might likely be stabilization centres for complicated cases. Limited quality of OTP services, including limited capacity of staff, and inadequate referral or follow-up mechanisms, could partly explain why some SAM children with severe diseases were not promptly referred to stabilization centres. The limited quality of services was more likely observed before the L3 declaration when the capacity of most humanitarian agencies was still limited. This calls for further study on the quality and effectiveness of the outpatient nutrition programme in Borno that could also explain the low and high rates of recovery and defaulter, respectively.
Although the evidence of high mortality among SAM children with co-morbidity is certain, it needs to be translated into better programme designs and requires consistent implementation of current Nigerian CMAM guidelines that are linked with IMCI for case detection, treatment in OTP and prompt referral of SAM cases with any complication to the stabilization centres. This convergence of health–nutrition programming might be life-saving as co-morbidity and other factors such as dehydration have been depicted herein to be strong predictors of mortality, which is similar to the findings of other studies( Reference Lapidus, Minetti and Djibo 20 , Reference Tadesse, Worku and Berhane 43 ). The impact of IMCI in improving health outcomes has also been amply documented( Reference Horwood, Vermaak and Rollins 19 , Reference Chopra, Patel and Cloete 44 , Reference Masanja, Schellenberg and de Savigny 45 ).
The results of the present study have major programmatic implications and underscore the need for strengthening or inclusion of clinical algorithms such as IMCI during the assessment of malnutrition as part of a minimum package of services, especially in conflict settings where there is often a significant breakdown of health and nutrition infrastructure. Such programmatic shifts, however, require policy dialogue, appropriate normative tools and trained personnel for successful implementation. In Nigeria, the CMAM protocol was integrated as part of IMCI but its implementation was limited and varied across IDP camps. Strong government leadership remains critical in advancing the integrated delivery while ensuring effective coordination and alignment of a large number of humanitarian actors in north-eastern Nigeria, to ensure that standard guidelines are followed and to guarantee efficiency gains and improving child survival.
The present study findings are limited by several factors that include data gaps due to incomplete medical records and lack of randomization that could ensure wide and diverse representation of the facilities. However, efforts were made to address data gaps by using alternative data sources such as outpatient department registers. Limited diagnostic capacity and application of IMCI algorithms as part of OTP might likely have resulted in underestimation of the burden of co-morbidities. Despite these limitations, the findings of the present study are consistent with other literature of poor outcomes of children with SAM and co-morbidities.
Conclusion
In conclusion, high morbidity and mortality among malnourished children presents a major challenge in reaching the ambitious goal of ‘survive and thrive’ set forth by the global health strategy (Every Woman Every Child Every Adolescent) in complex emergencies. As the countdown to 2030 for child survival progresses, extra efforts will be needed to achieve its targets for children in humanitarian settings. The present study underscores the inextricable link between health and nutrition and calls for programmatic shifts that include OTP models which incorporate simplified clinical algorithms and diagnostic tools based on the epidemiological situation. At an upstream level, it underlines the need for a strategic discourse on the convergence between public health and nutrition that could be important in addressing the vulnerabilities and risks of co-morbidities and malnutrition.
Acknowledgements
Acknowledgements: The authors thank all staff of the OTP facilities, health teams and all members of the assessment team who collected data and assisted in data analysis in the five selected states; in particular, Ladi Ejike (state nutrition officer), Victor Adeniyi (data officer) and Dr Austin Tino (health specialist, UNICEF Borno). Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for this research. Conflict of interest: Authors declare no conflict of interest. Authorship: The protocol and design of the study including data collection tools were drafted by D.C. and reviewed by O.O., N.S. and I.M. The data collection was coordinated by O.O., I.M., S.M. and H.M. The data analysis was conceived and conducted by D.C. and reviewed by O.O., N.S. and I.M. Similarly, D.C. wrote the first draft of the paper which was reviewed by O.O., N.S., I.M., S.M. and H.M. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the State Health Research Committee of Borno, Nigeria. All personal identifiers were removed from medical records.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980018003968