Serum alanine aminotransferase (ALT) is a common and inexpensive laboratory assay for detecting liver diseases such as non-alcoholic fatty liver disease (NAFLD)( Reference Welsh, Karpen and Vos 1 ). Serum ALT elevation is also regarded as a hepatic manifestation of the metabolic syndrome (MetS)( Reference Park, Kim and Park 2 – Reference Liu, Que and Ning 6 ). Recently, a meta-analysis on the impact of serum ALT activity on MetS incidence in thirty-nine studies in adults showed a greater pooled relative risk of incident MetS for those with the highest v. the lowest ALT levels( Reference Liu, Que and Ning 6 ). Mechanistic links between serum ALT elevation and risks of MetS are still not fully understood. ALT is a cytosolic enzyme and is predominantly present in the liver. Elevated serum ALT levels (>40 U/l) typically reflect hepatocellular injury( Reference Adams, Angulo and Lindor 7 ). Serum ALT levels are positively correlated with BMI( Reference Welsh, Karpen and Vos 1 ) and visceral fat accumulation( Reference Song, Yun and Park 8 , Reference Ayonrinde, Olynyk and Beilin 9 ). When examining relationships between serum ALT activity and individual components of MetS in children, several authors reported a strong correlation between serum ALT activity and blood lipid profiles (e.g. TAG, LDL cholesterol and HDL cholesterol)( Reference Di Bonito, Sanguigno and Di Fraia 4 , Reference Park, Han and Choi 5 ). The predicting effects of serum ALT levels on insulin resistance seem to be stronger in adults( Reference Kunutsor, Apekey and Walley 10 , Reference Wang, Chang and Yao 11 ) than in children( Reference Di Bonito, Sanguigno and Di Fraia 4 , Reference Park, Han and Choi 5 ).
Dysmetabolic Fe overload syndrome, which is characterized by mild to moderate Fe overload, was first described by Deugnier et al. in 1992( Reference Deugnier, Loreal and Turlin 12 ). Dysmetabolic Fe overload syndrome is frequently associated with obesity and alterations of glucose and lipid metabolism( Reference Riva, Trombini and Mariani 13 ). It was estimated that dysmetabolic Fe overload syndrome is present in about a third of patients with both NAFLD and MetS( Reference Dongiovanni, Fracanzani and Fargion 14 ). Excessive body Fe can cause damage to the liver. For example, high Hb levels are positively correlated with serum ALT concentrations( Reference Bai, Owaga and Cheng 15 ) and predict the development of NAFLD( Reference Yu, Xu and Xu 16 ). The consensus view is that an obesity-related inflammatory environment( Reference Chang, Li and Lu 17 ) may alter Fe metabolism and interfere with cardiometabolic functions( Reference Yilmaz, Senates and Ayyildiz 18 ). Analysis of hepatic genes involved in the metabolism of fatty acids and Fe in NAFLD showed that the genes involved in fatty acid metabolism were attenuated as NAFLD progressed, whereas Fe-related metabolism increased( Reference Mitsuyoshi, Yasui and Harano 19 ). Fe-induced oxidative stress promotes hepatic injury, leading to the release of the intracellular ALT enzyme into the circulating blood.
Fe is an essential element for erythropoiesis and Hb synthesis. High Hb levels affect the blood’s viscosity, which may limit blood flow and oxygen delivery to tissues. Disorders of blood viscosity are a risk factor for cardiovascular dysfunction( Reference Metivier, Marchais and Guerin 20 ). We hypothesized that high Hb levels may interfere with cardiometabolic functions and unresolved cardiometabolic stress may lead to hepatic injury. The aim of the present study was to investigate the interactive relationships between Hb levels and cardiometabolic abnormalities in relation to risks of serum ALT elevation in a general adolescent population as part of the Nutrition and Health Survey in Taiwan (NAHSIT 2010–2011, Adolescents).
Experimental methods
Study design
The Fourth National Nutrition and Health Survey in Taiwan (NAHSIT 2010–2011) was funded by the Food and Drug Administration, Ministry of Health and Welfare in Taiwan to provide continued assessment of the health and nutrition status of the people of Taiwan. The nationwide survey was conducted using a multistage, stratified, clustered sampling technique which included a wide range of age groups across all of Taiwan( Reference Pan, Lee and Chuang 21 ). The present study analysed data on adolescents aged 13–18 years (NAHSIT 2010–2011, Adolescents). Informed parental written consent was obtained prior to enrolment into the study. The study was approved by the Research Ethics Committee of Taipei Medical University (201210005) and Academia Sinica (EC100031).
Sample inclusion and exclusion
Information on self-reported family health histories and lifestyle factors were obtained using a standardized questionnaire. Exclusion criteria were as follows: (i) individuals with missing data for clinical biochemistry (n 1236); (ii) a self-reported health history of diabetes (n 3), thyroid disease (n 3), hepatitis (n 2), nephritis (n 3), urinary tract infection (n 34) or arthritis (n 8); and (iii) >2 drinks of alcohol/d (n 1). As such, 1941 adolescent participants (963 males and 978 females) were entered for analysis.
Data collection and laboratory measurements
Three anthropometric measures were collected in the present study. Waist circumference measurements were taken at the midpoint between the lower edge of the ribcage and the top of the iliac crest. Height and weight of participants were measured simultaneously using Detecto scales (Detecto Scales, Brooklyn, NY, USA). Two blood pressure measurements were taken 30 s apart with the arm at the level of the heart. A third measurement was taken if the second measurement differed substantially from the first (e.g. >10 mmHg). The two closest blood pressure values were averaged to obtain the mean blood pressure. Biochemical data were obtained from 8-h fasting blood samples. Heparinized whole-blood samples were collected for on-site measurement of Hb. Peripheral venous blood samples were collected in tubes containing EDTA and centrifuged at 4°C, and serum was stored at −80°C until analysis. Biochemistry analyses included total cholesterol, LDL cholesterol, HDL cholesterol, TAG, fasting blood glucose, uric acid, C-reactive protein, creatine, ALT, aspartate aminotransferase and amylase.
Definitions of metabolic factors in the study population
The BMI was calculated as weight/height2 (kg/m2). Age- and sex-specific cut-off points for BMI were used to define overweight and obesity in adolescents according to guidelines of the Department of Health, Taiwan( Reference Tan, Ma and Wai 22 ). Hypertension was diagnosed as blood pressure values exceeding the 90th percentile for age and sex. Diabetes was defined as fasting serum glucose≥110 mg/dl. Dyslipidaemia was classified as adolescents with the presence of any blood lipid values exceeding the 90th percentile or below the 10th percentile: (i) TAG≥112 mg/dl; (ii) total cholesterol≥197 mg/dl; (iii) LDL cholesterol≥121·2 mg/dl; and (iv) HDL cholesterol<40 mg/dl. ALT tertile (T) cut-off points for boys were 11 and 16 U/l, and for girls were 9 and 12 U/l. Hb quartiles (Q) for boys were 14·1, 14·7 and 15·4 g/dl, and for girls were 12·4, 13·1 and 13·6 g/dl.
Statistical analyses
Statistical analyses were performed using the statistical software package SAS version 9·22. Categorical data are presented as numbers and percentages, and were assessed with a χ 2 test. Continuous data are presented as means and standard deviations, and were assessed with a two-sample t test. Differences between two independent samples were analysed by the Wilcoxon rank-sum test for non-parametric data. Logistic regression models were used to estimate the odds ratio of the dependent variable (serum ALT) and independent variables (age, sex, BMI, C-reactive protein, amylase, uric acid, creatine, Hb, diabetes, hypertension and dyslipidaemia) and the 95 % confidence interval. In Table 2, the OR was expressed as ALT T3 v. ALT T1 (reference) after adjusting for ALT T2 v. T1. To further characterize the interactive relationships between Hb and dyslipidaemia in relation to the risk of ALT elevation, a binary logistic model was employed (Table 3). P<0·05 was considered statistically significant.
Results
Baseline characteristics
In total, 1941 adolescents (963 boys and 978 girls) participated in the present study. The mean age of study participants was 15·2 (sd 1·9) years (boys, 15·3 (sd 0·1) years; girls, 15·2 (sd 0·1) years). Their mean BMI was 21·4 (sd 4·2) kg/m2 (boys, 21·9 (sd 0·2) kg/m2; girls, 21·0 (sd 0·1) kg/m2; P<0·001). Mean ALT was 14·8 (sd 13·3) U/l (boys, 17·7 (sd 16·3) U/l; girls, 12·1 (sd 8·7) U/l; P<0·001). Mean Hb was 13·8 (sd 1·5) g/dl (boys, 14·7 (sd 1·0) g/dl; girls, 12·9 (sd 1·3) g/dl; P<0·001). The prevalence of diabetes, hypertension and dyslipidaemia were 11·4 % (boys, 16·0 %; girls, 9·4 %; P<0·001), 16·7 % (boys, 24·2 %; girls, 9·4 %; P<0·001) and 19·1 % (boys, 18·0 %; girls, 20·2 %), respectively. Table 1 shows characteristics of the study population in relation to hepatic injury (defined as serum ALT>40 U/l). Fifty-four of 963 boys (5·6 %) and twelve of 978 girls (1·2 %) had elevated ALT levels. Adolescents with hepatic injury were heavier and had higher levels of AST, systolic blood pressure, Hb, total cholesterol, TAG, LDL cholesterol, uric acid, creatine and C-reactive protein, and lower HDL cholesterol levels (all P<0·05; Table 1).
Table 1 Baseline characteristics of the study population in relation to hepatic injury: healthy adolescents aged 13–18 years (n 1941), Fourth National Nutrition and Health Survey in Taiwan (NAHSIT 2010–2011, Adolescents)
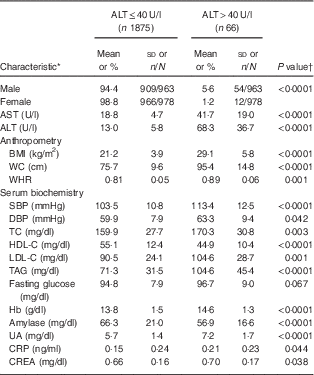
AST, aspartate aminotransferase; ALT, alanine aminotransferase; WC, waist circumference; WHR, waist-to-hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; UA, uric acid; CRP, C-reactive protein; CREA, creatine.
* Categorical data (sex) are presented as percentage and number of observations/number of participants; continuous data are presented as mean and standard deviation.
† Differences between groups were analysed by a two-sample t test.
Associations between serum biomarkers and serum alanine aminotransferase concentrations
We next investigated the associations between serum biomarkers and risk of ALT elevation in relation to sex. After adjusting for age, factors such as BMI, C-reactive protein, uric acid, Hb, hypertension and dyslipidaemia were significantly associated with serum ALT levels (model A; Table 2). After adjusting for covariates including the T2 v. T1 effect, the OR were markedly higher for dyslipidaemia (OR=1·85; 95 % CI 1·16, 2·93), BMI (OR=1·34; 95 % CI 1·27, 1·41) and Hb (OR=1·21; 95 % CI 1·02, 1·42) for boys with the highest ALT (T3) compared with the lowest (T1; multivariate model: boys; Table 2). The predictive effects of dyslipidaemia (OR=1·52; 95 % CI 1·16, 16·16), Hb (OR=1·30; 95 % CI 1·47, 2·13) and BMI (OR=1·20; 95 % CI 1·01, 1·35) for raised ALT levels in girls were similar to those of boys (multivariate model: girls; Table 2).
Table 2 Comparisons of odds ratios and 95 % confidence intervals for risk factors associated with serum alanine aminotransferase elevation among healthy adolescents aged 13–18 years (n 1941), Fourth National Nutrition and Health Survey in Taiwan (NAHSIT 2010–2011, Adolescents)
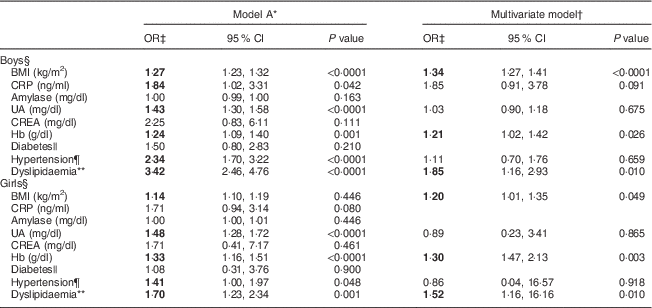
CRP, C-reactive protein; UA, uric acid; CREA, creatine; ALT, alanine aminotransferase; T, tertile.
Significant OR are indicated in bold font.
* Model A is adjusted for age.
† Multivariate model is adjusted for age, BMI, inflammatory markers (CRP, amylase, UA and CREA), Hb, diabetes, hypertension and dyslipidaemia.
‡ The OR was expressed as ALT T3 v. ALT T1 (reference) after adjusting for ALT T2 v. T1.
§ Serum ALT T by sex: males, 11 and 16 U/l; females, 9 and 12 U/l.
|| Diabetes: fasting serum glucose≥110 mg/dl.
¶ Hypertension: values exceeding the 90th percentile for age and sex.
** Dyslipidaemia defined as presence of any of the following criteria: (i) TAG≥112 mg/dl; (ii) total cholesterol≥197 mg/dl; (iii) LDL cholesterol≥121·2 mg/dl; or (iv) HDL cholesterol<40 mg/dl.
Interactive relationships of Hb and dyslipidaemia with the risk of serum alanine aminotransferase elevation
To further classify the interactive relationships of Hb and dyslipidaemia with the risk of ALT elevation, a categorical logistic model was employed. Adjusted OR for the risk of serum ALT elevation in relation to the Hb level and dyslipidaemia are shown in Table 3. Girls without dyslipidaemia and presenting in the highest quartile (Q4) of Hb (>13·6 g/dl) were 1·89 and 3·76 times more likely to have raised serum ALT (9 and >12 U/l, respectively) than the reference (lowest quartile (Q1) of Hb, <12·4 g/dl). Moreover, for those girls with dyslipidaemia, serum ALT seemed to increase with an increase in Hb levels. Specifically, girls with dyslipidaemia and Hb levels of 12·4, 13·1 and 13·6 g/dl were, respectively, 2·86, 3·53 and 5·64 times more likely to have elevated serum ALT levels (>12 U/l) than the reference (Q1 of Hb, <12·4 g/dl). The only effect found in boys was for those who had dyslipidaemia and presenting in Q4 of Hb (>15·4 g/dl), who were 7·40 times more likely to have elevated serum ALT of >16 U/l than the reference (Q1 of Hb, <14·1 g/dl).
Table 3 Adjusted odds ratios and 95 % confidence intervals for risk of serum alanine aminotransferase elevation in relation to dyslipidaemia and Hb levels among healthy adolescents aged 13–18 years (n 1941), Fourth National Nutrition and Health Survey in Taiwan (NAHSIT 2010–2011, Adolescents)
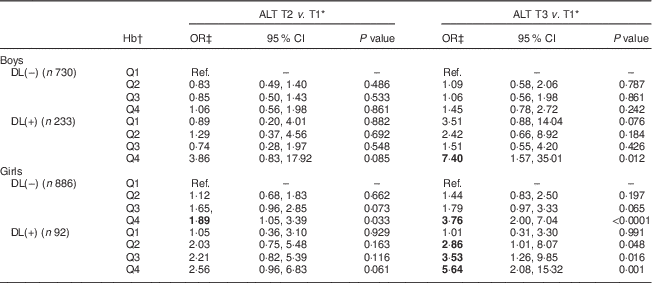
ALT, alanine aminotransferase; T, tertile; DL(–), without dyslipidaemia; DL(+), with dyslipidaemia; Q, quartile; Ref., reference category.
Significant OR are indicated in bold font.
* Serum ALT T by sex: males, 11 and 16 U/l; females, 9 and 12 U/l.
† Hb quartile by sex: males, 14·1, 14·7 and 15·4 g/dl; females, 12·4, 13·1 and 13·6 g/dl.
‡ OR adjusted for age and BMI.
Discussion
Our study found that dyslipidaemia and high Hb levels were associated with an increased risk of high ALT levels in ethnic Chinese (i.e. Taiwanese) adolescents. The hypothesized mechanisms by which Hb–lipid interactions may be associated with abnormal liver function include advanced glycation end products (AGE) and their receptors (RAGE)( Reference Kimura, Hyogo and Yamagishi 23 , Reference Moy, Jiao and Freedman 24 ); and hepcidin levels( Reference Detivaud, Nemeth and Boudjema 25 ). Obese individuals tend to replace carbohydrates with animal protein and fat as sources of energy( Reference Chang, Chen and Owaga 26 ). Meat, particularly dry-heated red meat, is a rich source of haem Fe, fat and AGE( Reference Estevez 27 ). Fu and co-workers showed that N ε-(carboxy-methyl)lysine is formed during Cu-catalysed oxidation of PUFA in the presence of protein( Reference Fu, Requena and Jenkins 28 ). Estevez et al. reported that myoglobin is a good predictive marker for protein carbonyl formation( Reference Fu, Requena and Jenkins 27 ). The interaction of AGE and RAGE plays a key role in the development and progression of NAFLD( Reference Basta, Navarra and De Simone 29 , Reference Basta, Navarra and De Simone 30 ). Lastly, the hepcidin level, a key regulator of Fe metabolism, may play an important role in Hb levels and hepatic function. By investigating thirty-six human liver specimens derived from liver carcinoma or transplantation for cirrhosis, Detivaud et al. found a positive relationship between liver hepcidin mRNA and Hb levels and a negative relationship between hepatic fibrosis status and hepcidin mRNA levels( Reference Detivaud, Nemeth and Boudjema 25 ).
Girls appear to be more sensitive to Hb–lipid interactions than boys with regard to serum ALT elevation. The reason for this sex difference is unclear. However, it is interesting to speculate that this sex difference may be due to the interactive relationship between oestrogen and erythrocytes. Reproductive-aged women have lower mean Hb levels (12 %) than men. A recent study by Hou et al. showed that oestrogen regulates Fe homeostasis via down-regulating hepatic hepcidin expression via an oestrogen response element( Reference Hou, Zhang and Wang 31 ). When hepcidin concentrations are low, Fe enters the blood plasma at a higher rate resulting in high body Fe levels. Although oestrogen seems to have beneficial effects on Fe levels, it was also associated with deleterious changes in membrane lipids of erythrocytes. For example, a study by Cho and co-workers demonstrated that oestrogen-induced dyslipidaemia resulted in changes in the fatty acid composition of membrane lipids of erythrocytes, which in turn increased osmotic fragility( Reference Cho, Smith and Park 32 ). Le Petit-Thevenin et al. showed that oestrogen increased erythrocyte susceptibility to peroxidation generated by incubation with hydrogen peroxide( Reference Le Petit-Thevenin, Lerique and Nobili 33 ). Decreased erythrocyte membrane fluidity and altered lipid compositions were associated with human liver diseases( Reference Owen, Bruckdorfer and Day 34 ).
Our study suggests that Hb testing in adolescents with dyslipidaemia may identify those with serum ALT elevation. Jiang and colleagues also proposed that Fe biomarkers (Hb and ferritin) combined with TAG could serve as an indicator for a diagnosis of NAFLD in Chinese adults( Reference Jiang, Zeng and Chen 35 ). Although a liver biopsy remains the gold standard for diagnosing NAFLD, a histological diagnosis is often not possible in community-based studies. In Taiwan, a diagnosis of NAFLD is largely based on ultrasonography and serum ALT due to the asymptomatic nature of the disease and invasiveness and discomfort associated with a liver biopsy. However, the use of ultrasonography is limited to diagnosing advanced or fully developed NAFLD. Furthermore, ultrasonography cannot identify the presence of non-alcoholic steatohepatitis or reliably distinguish changes in fibrosis from non-alcoholic steatohepatitis. Our study suggests that Hb can serve as an additional biomarker for detecting liver disease. However, there is no consensus on the ‘normal reference range’ for Hb levels in adults and adolescents. The absence of relevant cut-off points for Hb limits its clinical utility.
Several limitations of the present study should be taken into account when interpreting the results. First, the number of adolescents with abnormal ALT (>40 U/l) levels was relatively small. Second, the liver function diagnosis was based on the serum ALT threshold and not on ultrasonography due to budget constraints. Third, exclusion of hepatitis viral infections was based on a self-reported health history and not on hepatitis surface antigens. Previously, the hepatitis B virus was endemic in Taiwan, but the infection rate decreased in adolescents and young adults after the launch of the world’s first universal vaccination programme in 1984, which involved vaccinating newborns of infectious mothers and was later expanded to all newborns in 1986. A recent report investigated 8733 senior-high-school students and reported overall hepatitis B surface antigen (HBsAg) and anti-HBs-positive rates of 1·9 % and 48·3 %, respectively( Reference Wu, Lin and Wang 36 ). Lastly, our study did not measure the pubertal stage, and therefore we cannot discriminate the potential influence of sexual hormones on Hb concentrations.
Conclusions
Our findings suggest that an increased Hb level is a predictor of elevated serum ALT in adolescent girls with dyslipidaemia. Our study also highlights the importance of new studies aiming to establish cut-off points for Hb levels and its utility in diagnosing and preventing the onset of cardiometabolic abnormalities particularly in female adolescents.
Acknowledgements
Acknowledgements: The authors express their sincere appreciation to the study participants. They also wish to thank staff from the Research Center for Humanities and Social Sciences, Center for Survey Research, Academia Sinica and the directors, Dr Wen-Han Pan and Dr Su-Hao Tu, for their assistance. Financial support: Data analysed in this paper were collected in the research project ‘The Fourth Nutrition and Health Survey in Taiwan (NAHSIT 2010–2011, Adolescents)’, sponsored by the Food and Drug Administration, Ministry of Health and Welfare in Taiwan (grant numbers 99TFDA-FS-408 and 100TFDA-FS-406). J.-S.C. was supported by the Taipei Medical University Hospital (grant numbers 102TMU-TMUH-16 and 103TMU-TMUH-11) and the Ministry of Science and Technology, Taiwan (grant numbers NSC102-2320-B038-013 and MOST103-2320-B-038-015). The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: K.-C.C. participated in blood biochemistry analysis and drafted the initial manuscript. C.-C.C. participated in the supervision of the project. T.-C.H. carried out the initial data analyses. E.O. critically reviewed the manuscript. C.-H.B. participated in the supervision of the project and critically reviewed the manuscript. W.-H.P. conceptualized the National Nutrition and Health Survey. J.-S.C. conceptualized the project, drafted the manuscript and approved the final manuscript as submitted. Ethics of human subject participation: This study was approved by the Research Ethics Committee of Taipei Medical University (201210005) and Academia Sinica (EC100031).






