Older adults are the fastest-growing age group, and the number of adults aged 65 years and older worldwide is expected to more than double from 727 million in 2020 to over 1·5 billion in 2050(1). As the population ages, the prevalence of chronic diseases, multimorbidity and frailty will also increase(1–Reference Raina, Gilsing and Mayhew3). Several modifiable risk factors are associated with an increased risk of disability and disease with aging, one of which is poor diet quality(Reference Bassim, Mayhew and Ma4,Reference Raina, Ali and Joshi5) . Unfortunately, many older adults do not meet current age-specific nutrition guidelines(6,7) concerning both diet quality and quantity(Reference Leslie and Hankey8,Reference Choi, Crimmins and Kim9) . As individuals age, many decrease their total food intake(Reference Wakimoto and Block10), in part due to reduced appetite, sensory impairment, hormonal imbalance and changes in the gastrointestinal tract and dentition(Reference Kaur, Rasane and Singh11). Age-related changes in living situations, retirement, social isolation and loss of relationships can also negatively impact food intake and diet quality(Reference Conklin, Forouhi and Surtees12,Reference Vesnaver and Keller13) . The intersection of financial, psychosocial, environmental, physical, cognitive, gender and cultural factors are known to influence eating behaviour(Reference Vesnaver and Keller13), food access(Reference Keller, Dwyer and Senson14) and mobility(Reference Webber, Porter and Menec15) among older adults.
The relationship between mobility (the ability to move oneself within the immediate environment and broader community(Reference Webber, Porter and Menec15)) and nutrition has been shown to be bidirectional in older adults. One’s mobility can impact food access (e.g. ability to transport oneself to locations with high-quality food sources)(Reference Schwartz, Buliung and Wilson16) and may also be influenced by dietary quality. Reduced intake of both micronutrients and macronutrients may lead to sarcopaenia(Reference Morley, Abbatecola and Argiles17–Reference Cruz-Jentoft, Kiesswetter and Drey19), and the loss of muscle mass in aging may result in mobility limitations and impaired quality of life(Reference Milaneschi, Tanaka and Ferrucci20). Proper nutrition also plays an important role in maintaining skeletal strength and preventing falls and chronic diseases among older adults(Reference Milaneschi, Tanaka and Ferrucci20–Reference Webb22). Given this, promoting healthier eating and reducing nutrition risk is necessary to maintain and improve health and mobility among community-dwelling older adults. However, many older adults perceive functional decline as an inevitable part of ageing and may experience difficulties accessing available programmes and services(Reference Srivarathan, Jensen and Kristiansen23).
Group-based nutrition interventions, including education, interactive discussion and hands-on activities, have demonstrated benefits in supporting older adults to learn from each other’s knowledge and experiences, overcome psychosocial and environmental barriers to healthy eating, enhance motivation and promote dietary behavioural change(Reference Higgins and Clarke Barkley24–Reference Keller, Hedley and Hadley26). Group-based interventions among older adults also foster a sense of group cohesion(Reference Agronin27), allowing individuals to feel acknowledged and form bonds with others who understand their experiences firsthand. Although many group-based nutrition interventions exist, some of which have been formally evaluated for effectiveness(Reference Keller, Gibbs and Wong28,Reference Keller, Hedley and Wong29) , these interventions vary widely and optimal design features remain unclear.
In a previous umbrella review of systematic reviews to identify existing synthesised evidence regarding group-based physical activity and/or nutrition interventions for community-dwelling older adults, only nine reviews evaluated interventions with a nutrition component (namely protein supplementation combined with physical activity)(Reference Neil-Sztramko, Teggart and Moore30). No systematic reviews of group-based nutrition interventions alone were identified, and there was no benefit observed for the addition of protein supplementation with physical activity in this population. Further, none of the nutrition interventions evaluated at the review level extended beyond supplementation, highlighting a lack of synthesised evidence to identify the effectiveness of group-based interventions targeting healthy eating. This understanding is key to informing the development and implementation of evidence-informed, group-based community programmes to promote healthy eating and mobility among older adults.
To address this gap, our team initiated a systematic review of single studies focussed on group-based nutrition interventions targeting healthy eating in community-dwelling older adults. We specifically aimed to understand whether group-based interventions targeting healthy eating in community-dwelling older adults (≥ 55 years) improved access to nutrition, affected nutritional intake or changed markers of physical mobility.
Methods
This systematic review was registered with PROSPERO (CRD42020205045). The reporting of this review is based on PRISMA guidelines(Reference Page, McKenzie and Bossuyt31).
Search strategy
The electronic databases MEDLINE, CINAHL, EMBASE, PsycINFO and Sociological Abstracts were searched on July 15, 2020, by a research librarian trained in building search strategies for systematic reviews (see online supplementary material, Supplemental Table 1–5). To focus on interventions germane to the current context and nutrition guidelines, database searches were limited to studies published from January 2010. Only English language studies were eligible due to the research team’s capacity. Reference lists of all identified systematic reviews were screened for potentially relevant and eligible studies; experts in the field were contacted to locate any additional studies not identified in our search.
Study selection
Citations were uploaded into Covidence (Veritas Health Innovation Ltd., Melbourne, Australia), and duplicates were removed. Following a pilot test, titles and abstracts were screened in duplicate by two independent reviewers against predetermined eligibility criteria. Full texts of potentially relevant studies were retrieved and screened for eligibility in duplicate by two independent reviewers. Disagreements were resolved through discussion or with the input of a third reviewer.
Eligibility criteria
Types of studies
This review included experimental and quasi-experimental study designs, including randomised controlled trials (RCT), non-RCT, before and after studies and interrupted time-series studies. Mixed methods studies with quantitative designs cited above were also included, although only quantitative data were extracted and analysed. Theses and dissertations were eligible; publication status was not a criteria for inclusion. Conference abstracts, reviews, observational designs and qualitative studies were excluded.
Participants
Eligible studies must have included community-dwelling older adults ≥ 55 years old or reported a mean age of participants as ≥ 55 years. Studies focussed on disease-specific populations were excluded, although included participants could report risk factors for or the presence of chronic diseases.
Interventions
Studies that evaluated group-based interventions targeting healthy eating were eligible. Examples of modes of delivery included interventions based on nutrition, education, gardening and congregate dining. If studies reported on interventions with multiple delivery modes, only group-based interventions were extracted and analysed. Programmes focussed on weight management or weight loss were excluded. Interventions delivered in any community-based setting were eligible, including seniors’ and community centres. Studies that took place in acute or long-term care settings were excluded.
Comparators
Studies that compared an intervention to any comparison group (including single group pre-test/post-test) were eligible. Examples of comparator groups included pre-intervention, other intervention or non-exposed control groups.
Outcomes
Studies that reported on a change in nutrition outcomes from pre- to post-intervention were eligible for inclusion. Nutrition outcomes were grouped retrospectively into three categories: (1) food and fluid intake (e.g. vegetables and fruit, whole grain foods and protein), (2) nutrition risk, defined as factors that impact food intake(Reference Keller, Goy and Kane32) (e.g. dietary habits, food access) and (3) healthy eating knowledge (e.g. nutrient functions, recommended servings). Physical mobility outcomes were considered secondary outcomes and were retrospectively grouped into two categories: (1) physical activity and (2) functional outcomes (e.g. Timed Up and Go test, gait speed).
Assessment of methodological quality
Two independent reviewers critically appraised all eligible studies for methodological quality using the Joanna Briggs Institute critical appraisal instruments for experimental or quasi-experimental studies(33). Overall scores for each study were calculated by responses to the questions. Any disagreements between reviewers were resolved through discussion or input from a third reviewer.
Data extraction
Two independent reviewers performed data extraction in duplicate using a pre-developed and tested data extraction form. This form included general study information (i.e. study aim, design, country, start/end dates), population (i.e. age, sex, number of participants, ethnicity, socioeconomic status), intervention details (including duration, frequency, who delivered, how it was delivered, where it was delivered and theoretical framework, with questions framed according to the Template for Intervention Description and Replication (TIDieR) checklist and guide(Reference Hoffmann, Glasziou and Boutron34)), comparison groups, limitations and conclusions reported by study authors. Relevant nutrition and mobility outcomes were also extracted for all time points reported in the individual studies. When measures of overall food and fluid intake were reported (e.g. Food Frequency Score, Dietary Variety Score), these were extracted over specific food group intake results. Any disagreements between reviewers were resolved through discussion or by a third reviewer. Data collection forms and extracted data used for analyses are available upon request.
Data synthesis
A meta-analysis was not possible given the variation in intervention types and outcomes across included studies. A narrative approach was used to synthesise included studies(Reference Tufanaru, Munn and Aromataris35), with data summarised and presented in supporting tables. Results tables with effect size measures, including mean differences, odds ratio, effect sizes and proportional changes, were structured by intervention category and outcome measures to explore variation and possible sources of heterogeneity. When only pre-test/post-test means or percentages were reported, mean or percent differences between groups were calculated. When missing, mean differences, confidence intervals and/or standard deviations of the changes were calculated using accepted equations(Reference Higgins, Li and Deeks36) and RevMan software(37). A correlation coefficient of 0·5 was estimated for both food and fluid intake outcomes(38–Reference Cade, Thompson and Burley42) and physical activity outcomes(Reference Cleland, Ferguson and Ellis43–Reference Topolski, LoGerfo and Patrick45), based on available literature. Reporting bias was not explored as most studies did not cite a protocol or trial registration. Sensitivity analyses were not performed. A comprehensive approach to assess the overall certainty of the evidence for each outcome was not used due to high heterogeneity across interventions and outcomes.
Results
Description of included studies
The search resulted in 4482 unique records, of which 309 were identified as potentially relevant and underwent full-text review (Fig. 1). A total of thirty-one studies met all eligibility criteria and were included in the analysis (Table 1), including eleven single group, pre-test/post-test studies(Reference Abusabha, Namjoshi and Klein46–Reference Wunderlich, Bai and Piemonte56), ten RCT(Reference Francis, MacNab and Shelley57–Reference Uemura, Yamada and Okamoto66) and ten non-randomised, two group study designs(Reference Brewer, Dickens and Humphrey67–Reference Smith, Lee and Towne76). A list of excluded studies with reasons for exclusion is provided in Supplemental Appendix 1. Studies were most often conducted in North America (n 20, 65 %), with the remainder in Asia (n 7, 23 %), Europe (n 3, 9 %) and Australia (n 1, 3 %). The total number of participants enrolled was 6723 (Range: 10–761), with high loss to follow-up noted (Range: 0–65 % where reported; 48 % (n 15) reported > 20 % attrition). Mean age ranged from 64 to 82 years (range 50–98 years when mean age was not reported). Most participants were female, with 74 % (n 23) of studies reporting > 70 % female participants. Nine studies (29 %) explicitly targeted low-income or economically disadvantaged populations(Reference Abusabha, Namjoshi and Klein46,Reference Schwingel, Galvez and Linares51,Reference Strout, Jemison and O’Brien53–Reference Turk, Elci and Resick55,Reference Meethien, Pothiban and Ostwald62,Reference Salehi, Mohammad and Montazeri64,Reference Chung and Chung68,Reference Hersey, Cates and Blitstein70) .

Fig. 1 PRISMA flow diagram
Table 1 Characteristics of included studies (n 31)

NR, not reported; PA, physical activity; FV, fruits and vegetables; RCT, randomised controlled trial; SES, socio-economic status; CCAA, Canadian Center for Activity and Aging, NIA, National Institute of Aging.
Four main intervention categories were identified: (1) nutrition education with behaviour change techniques (BCT) (n 21, 68 %)(Reference Manafo, Jose and Silverberg48,Reference Pogge and Eddings50–Reference Smith, Ory and Jiang52,Reference Wunderlich, Bai and Piemonte56–Reference Kimura, Moriyasu and Kumagai60,Reference Meethien, Pothiban and Ostwald62–Reference Uemura, Yamada and Okamoto66,Reference Gallois, Buck and Dreas69–Reference Lillehoj, Yap and Montgomery72,Reference MacNab, Davis and Francis74–Reference Smith, Lee and Towne76) , (2) didactic nutrition education (e.g. lectures, handouts) (n 4, 13 %)(Reference Thomas, Almanza and Ghiselli54,Reference Turk, Elci and Resick55,Reference Brewer, Dickens and Humphrey67,Reference Luten, Reijneveld and Dijkstra73) , (3) interactive nutrition education (e.g. workshops, discussion) (n 2, 6 %)(Reference Beasley, Kirshner and Wylie-Rosett47,Reference Lara, Turbett and McKevic61) and (4) food access (e.g. mobile markets, gardening, food samples) (n 2, 6 %)(Reference Abusabha, Namjoshi and Klein46,Reference Strout, Jemison and O’Brien53) . Two studies (6 %) combined nutrition education with BCT and food access(Reference Moreau, Plourde and Hendrickson-Nelson49,Reference Chung and Chung68) . The BCT Taxonomy(Reference Michie, Richardson and Johnston77) was used to identify interventions that incorporated BCT when components such as goal setting, action planning, feedback and monitoring, social support (e.g. motivational interviewing), shaping knowledge through instruction on how to perform a behaviour (e.g. cooking demonstrations) and behavioural practice/rehearsal (e.g. healthy food selection or recognition activities) were explicitly described. Physical activity education was reported as a co-intervention in 9 (29 %) studies(Reference Beasley, Kirshner and Wylie-Rosett47,Reference Pogge and Eddings50,Reference Thomas, Almanza and Ghiselli54,Reference Francis, MacNab and Shelley57–Reference Jancey, Holt and Lee59,Reference Gallois, Buck and Dreas69,Reference Hersey, Cates and Blitstein70,Reference Luten, Reijneveld and Dijkstra73) , and physical activity participation within sessions (e.g. strengthening, walking, step tracking) was reported in 10 (32 %) studies(Reference Schwingel, Galvez and Linares51,Reference Smith, Ory and Jiang52,Reference Turk, Elci and Resick55,Reference Kimura, Moriyasu and Kumagai60,Reference Mendoza-Ruvalcaba and Arias-Merino63,Reference Silva-Smith, Fleury and Belyea65,Reference Uemura, Yamada and Okamoto66,Reference Hsu, Wang and Chen71,Reference Lillehoj, Yap and Montgomery72,Reference Smith, Lee and Towne76) .
Median intervention duration was 12 weeks (range 1 day to 2 years). Session frequency was variable, with weekly delivery most common (n 13, 42 %)(Reference Abusabha, Namjoshi and Klein46–Reference Moreau, Plourde and Hendrickson-Nelson49,Reference Strout, Jemison and O’Brien53,Reference Turk, Elci and Resick55,Reference Meethien, Pothiban and Ostwald62,Reference Salehi, Mohammad and Montazeri64–Reference Uemura, Yamada and Okamoto66,Reference Chung and Chung68,Reference Hersey, Cates and Blitstein70,Reference MacNab, Davis and Francis74) . Interventionists included trained facilitators (n 6, 19 %)(Reference Beasley, Kirshner and Wylie-Rosett47,Reference Schwingel, Galvez and Linares51,Reference Silva-Smith, Fleury and Belyea65,Reference Gallois, Buck and Dreas69,Reference Lillehoj, Yap and Montgomery72,Reference MacNab, Davis and Francis74) , research personnel (n 4, 13 %)(Reference Thomas, Almanza and Ghiselli54,Reference Turk, Elci and Resick55,Reference Kimura, Moriyasu and Kumagai60,Reference Brewer, Dickens and Humphrey67) , educators (n 3, 10 %)(Reference Strout, Jemison and O’Brien53,Reference Francis, MacNab and Shelley57,Reference Hersey, Cates and Blitstein70) , nutritionists (n 3, 10 %)(Reference Wunderlich, Bai and Piemonte56,Reference Lara, Turbett and McKevic61,Reference Chung and Chung68) , physiotherapists and/or trainers (n 3, 10 %)(Reference Mendoza-Ruvalcaba and Arias-Merino63,Reference Uemura, Yamada and Okamoto66,Reference Hsu, Wang and Chen71) , registered dietitians (n 2, 6 %)(Reference Moreau, Plourde and Hendrickson-Nelson49,Reference Pogge and Eddings50) , healthcare providers (n 2, 6 %)(Reference Meethien, Pothiban and Ostwald62,Reference Murayama, Taguchi and Spencer75) , lay leaders (n 2, 6 %)(Reference Smith, Ory and Jiang52,Reference Smith, Lee and Towne76) and peer leaders (n 2, 6 %)(Reference Jancey, Holt and Lee59,Reference Luten, Reijneveld and Dijkstra73) . Four studies (13 %) did not report interventionist details. Programmes were delivered within congregate meal sites (n 5, 16 %)(Reference Thomas, Almanza and Ghiselli54,Reference Wunderlich, Bai and Piemonte56,Reference Francis, MacNab and Shelley57,Reference Brewer, Dickens and Humphrey67,Reference Lillehoj, Yap and Montgomery72) , seniors’ housing sites (n 5, 16 %)(Reference Abusabha, Namjoshi and Klein46,Reference Pogge and Eddings50,Reference Strout, Jemison and O’Brien53,Reference Geller, Mendoza and Timbobolan58,Reference Jancey, Holt and Lee59) , seniors’ centers (n 4, 13 %)(Reference Beasley, Kirshner and Wylie-Rosett47,Reference Mendoza-Ruvalcaba and Arias-Merino63,Reference Salehi, Mohammad and Montazeri64,Reference Hersey, Cates and Blitstein70) , community health centers (n 3, 10 %)(Reference Silva-Smith, Fleury and Belyea65,Reference Chung and Chung68,Reference Hsu, Wang and Chen71) , community centers/kitchen (n 3, 10 %)(Reference Moreau, Plourde and Hendrickson-Nelson49,Reference Kimura, Moriyasu and Kumagai60,Reference Murayama, Taguchi and Spencer75) , a church facility (n 1, 3 %)(Reference Schwingel, Galvez and Linares51) and a university (n 1, 3 %)(Reference Lara, Turbett and McKevic61), with 7 (23 %)(Reference Smith, Ory and Jiang52,Reference Turk, Elci and Resick55,Reference Meethien, Pothiban and Ostwald62,Reference Gallois, Buck and Dreas69,Reference Luten, Reijneveld and Dijkstra73,Reference MacNab, Davis and Francis74,Reference Smith, Lee and Towne76) delivered across multiple community settings and 2 (6 %) not reporting setting. Theoretical models were applied in 45 % of studies (n 14); the most common were Social Cognitive Theory (n 4, 13 %)(Reference Schwingel, Galvez and Linares51,Reference Smith, Ory and Jiang52,Reference Jancey, Holt and Lee59,Reference Smith, Lee and Towne76) , Social Marketing Theory (n 2, 6 %)(Reference Francis, MacNab and Shelley57,Reference MacNab, Davis and Francis74) , Health Belief Model (n 2, 6 %)(Reference Francis, MacNab and Shelley57,Reference Lillehoj, Yap and Montgomery72) and the Transtheoretical Model (n 2, 6 %)(Reference Schwingel, Galvez and Linares51,Reference Salehi, Mohammad and Montazeri64) . Multiple theories were often combined within studies, although none applied them in the same manner.
Methodological quality
The ten RCT had a generally unclear or high risk of bias (Fig. 2). Only one study reported blinding of participants and delivery personnel(Reference Lara, Turbett and McKevic61). There was unclear or no blinding of outcome assessors in 70 % of RCT (n 7)(Reference Francis, MacNab and Shelley57,Reference Geller, Mendoza and Timbobolan58,Reference Kimura, Moriyasu and Kumagai60–Reference Salehi, Mohammad and Montazeri64) , and 70 % (n 7) did not adequately describe or analyse differences between groups when incomplete follow-up was reported(Reference Francis, MacNab and Shelley57–Reference Kimura, Moriyasu and Kumagai60,Reference Meethien, Pothiban and Ostwald62–Reference Salehi, Mohammad and Montazeri64) . Selection bias was a concern, given that 60 % of the RCT (n 6) did not adequately report procedures for randomisation(Reference Francis, MacNab and Shelley57,Reference Geller, Mendoza and Timbobolan58,Reference Kimura, Moriyasu and Kumagai60,Reference Meethien, Pothiban and Ostwald62–Reference Salehi, Mohammad and Montazeri64) and allocation concealment(Reference Francis, MacNab and Shelley57,Reference Geller, Mendoza and Timbobolan58,Reference Meethien, Pothiban and Ostwald62–Reference Salehi, Mohammad and Montazeri64,Reference Uemura, Yamada and Okamoto66) . Similarly, the twenty-one quasi-experimental studies had an unclear or high risk of bias (Fig. 3) due to lack of a comparator group (n 13, 62 %)(Reference Abusabha, Namjoshi and Klein46–Reference Wunderlich, Bai and Piemonte56,Reference Chung and Chung68,Reference MacNab, Davis and Francis74) , inadequate description and analysis of groups when incomplete follow-up was reported (n 14, 67 %)(Reference Abusabha, Namjoshi and Klein46,Reference Manafo, Jose and Silverberg48–Reference Smith, Ory and Jiang52,Reference Thomas, Almanza and Ghiselli54,Reference Wunderlich, Bai and Piemonte56,Reference Brewer, Dickens and Humphrey67,Reference Gallois, Buck and Dreas69,Reference Hsu, Wang and Chen71,Reference Lillehoj, Yap and Montgomery72,Reference MacNab, Davis and Francis74,Reference Smith, Lee and Towne76) and unreliable outcome measurements (n 14, 67 %)(Reference Abusabha, Namjoshi and Klein46,Reference Manafo, Jose and Silverberg48–Reference Pogge and Eddings50,Reference Smith, Ory and Jiang52–Reference Wunderlich, Bai and Piemonte56,Reference Hsu, Wang and Chen71,Reference Luten, Reijneveld and Dijkstra73–Reference Smith, Lee and Towne76) . Full critical appraisal findings for each study are available in Supplemental Tables 6–7.

Fig. 2 Summary of risk of bias in randomised controlled trials (n 10). Assessed using JBI Critical Appraisal Checklist for Randomised Controlled Trials

Fig. 3 Summary of risk of bias in quasi-experimental studies (n 21). Assessed using JBI Critical Appraisal Checklist for Quasi-Experimental Studies (includes single-group, pre-test/post-test and two-group, non-randomised study designs)
Nutrition outcomes
Food and fluid intake
The twenty-two interventions assessing food and fluid intake included nutrition education with BCT (n 14, 64 %)(Reference Schwingel, Galvez and Linares51,Reference Smith, Ory and Jiang52,Reference Wunderlich, Bai and Piemonte56,Reference Geller, Mendoza and Timbobolan58–Reference Kimura, Moriyasu and Kumagai60,Reference Salehi, Mohammad and Montazeri64–Reference Uemura, Yamada and Okamoto66,Reference Gallois, Buck and Dreas69,Reference Hersey, Cates and Blitstein70,Reference MacNab, Davis and Francis74–Reference Smith, Lee and Towne76) , didactic nutrition education (n 3, 14 %)(Reference Turk, Elci and Resick55,Reference Brewer, Dickens and Humphrey67,Reference Luten, Reijneveld and Dijkstra73) , interactive nutrition education (n 2, 9 %)(Reference Beasley, Kirshner and Wylie-Rosett47,Reference Lara, Turbett and McKevic61) , food access (n 2, 9 %)(Reference Abusabha, Namjoshi and Klein46,Reference Strout, Jemison and O’Brien53) and nutrition education with BCT and food access (n 1, 4 %)(Reference Moreau, Plourde and Hendrickson-Nelson49). Food and fluid intake (e.g. vegetables and fruit, water and whole grains) were captured using a variety of tools, such as FFQ (n 10, 45 %)(Reference Moreau, Plourde and Hendrickson-Nelson49,Reference Schwingel, Galvez and Linares51,Reference Smith, Ory and Jiang52,Reference Turk, Elci and Resick55,Reference Kimura, Moriyasu and Kumagai60,Reference Salehi, Mohammad and Montazeri64,Reference Uemura, Yamada and Okamoto66,Reference Brewer, Dickens and Humphrey67,Reference Gallois, Buck and Dreas69,Reference Murayama, Taguchi and Spencer75) , 24-hour diet recalls (n 3, 14 %)(Reference Schwingel, Galvez and Linares51,Reference Silva-Smith, Fleury and Belyea65,Reference Gallois, Buck and Dreas69) and food records (n 2, 9 %)(Reference Beasley, Kirshner and Wylie-Rosett47,Reference Lara, Turbett and McKevic61) (Table 2).
Table 2 Food and fluid intake (n 22)

I, intervention group; C, comparator group; FV, fruits and vegetables; PA, physical activity; MD, mean difference; NR, not reported.
* Asterisks indicate interventions that also included a physical activity component. Bold text indicates statistical significance.
Between and within intervention categories, inconsistent findings were reported. Although the greatest number of studies utilised nutrition education with BCT interventions, findings were mixed. Five interventions found consistently positive changes in food and/or fluid intake(Reference Kimura, Moriyasu and Kumagai60,Reference Salehi, Mohammad and Montazeri64,Reference Uemura, Yamada and Okamoto66,Reference Hersey, Cates and Blitstein70,Reference MacNab, Davis and Francis74) . The Sumida TAKE10 programme (3 months of bi-weekly lectures, take-home activities, monitoring and feedback) (moderate risk of bias)(Reference Kimura, Moriyasu and Kumagai60), and a 24-week intervention incorporating nutrition education, skill-building activities and planning/implementing behavioural change (low risk of bias)(Reference Uemura, Yamada and Okamoto66) improved both food intake frequency and dietary variety compared with a cross-over control and no-intervention comparator group, respectively. The ‘Eat Smart, Live Strong’ intervention (four weekly interactive nutrition education sessions with goal setting) improved vegetable and fruit intake when compared with a waitlist control (low risk of bias)(Reference Hersey, Cates and Blitstein70). Tailored nutrition education based on the stages of change with goal setting, action planning, and reinforcement resulted in increased vegetable and fruit consumption after four weekly sessions compared with general health education (high risk of bias)(Reference Salehi, Mohammad and Montazeri64). Two modes of delivery of a whole grain education programme (both including skill-building activities and taste testing) increased total and whole-grain intake frequency after three weekly sessions compared with baseline (moderate risk of bias)(Reference MacNab, Davis and Francis74).
Five studies showed improvements in some but not all aspects of food and fluid intake following nutrition education with BCT, as findings were inconsistent across outcomes(Reference Schwingel, Galvez and Linares51,Reference Smith, Ory and Jiang52,Reference Jancey, Holt and Lee59,Reference Murayama, Taguchi and Spencer75,Reference Smith, Lee and Towne76) . Physical activity and nutrition education with goal setting and skill-building components increased the percentage of participants meeting recommended fruit intake, but not other food groups and macronutrients, as compared to no intervention (low risk of bias)(Reference Jancey, Holt and Lee59). Nutrition education and culturally tailored lifestyle programme incorporating goal setting, action planning and hands-on activities increased the number of participants consuming ≥ 3 meals/d and decreased fried food consumption, but also decreased vegetable intake and found no change in fruit intake as compared to baseline (low risk of bias)(Reference Schwingel, Galvez and Linares51). Eight weeks of bi-weekly drama-style lectures, food tasting and group discussion improved dietary variety compared to control, but inconsistent findings were noted for macronutrient consumption (low risk of bias)(Reference Murayama, Taguchi and Spencer75). Two studies evaluated the effects of the Texercise Select intervention (10 weeks of twice-weekly education, physical activity, goal setting and action planning). In the first study, Texercise Select increased the likelihood of vegetable and fruit consumption and decreased the likelihood of fast-food intake but did not change soda or water consumption compared with a non-randomised waitlist control (moderate risk of bias)(Reference Smith, Lee and Towne76); improvements were not sustained at 6-month follow-up. Texercise Select improved vegetable, fruit and water consumption but not soda and fast food consumption compared to baseline in the second study (moderate risk of bias)(Reference Smith, Ory and Jiang52).
Although heterogeneity across interventions was evident, similar nutrition education with BCT interventions was used in four studies (low to high risk of bias) that found no significant changes in food and fluid intake(Reference Wunderlich, Bai and Piemonte56,Reference Geller, Mendoza and Timbobolan58,Reference Silva-Smith, Fleury and Belyea65,Reference Gallois, Buck and Dreas69) . Didactic nutrition education(Reference Turk, Elci and Resick55,Reference Brewer, Dickens and Humphrey67,Reference Luten, Reijneveld and Dijkstra73) , interactive nutrition education(Reference Beasley, Kirshner and Wylie-Rosett47,Reference Lara, Turbett and McKevic61) and food access(Reference Abusabha, Namjoshi and Klein46,Reference Strout, Jemison and O’Brien53) interventions alone did not appear to change food and fluid intake for the better. Only one study evaluated a nutrition education with BCT and food access (take-home meal portions) intervention and found improved consumption of recommended portions of all food groups (moderate risk of bias)(Reference Moreau, Plourde and Hendrickson-Nelson49).
Nutrition risk
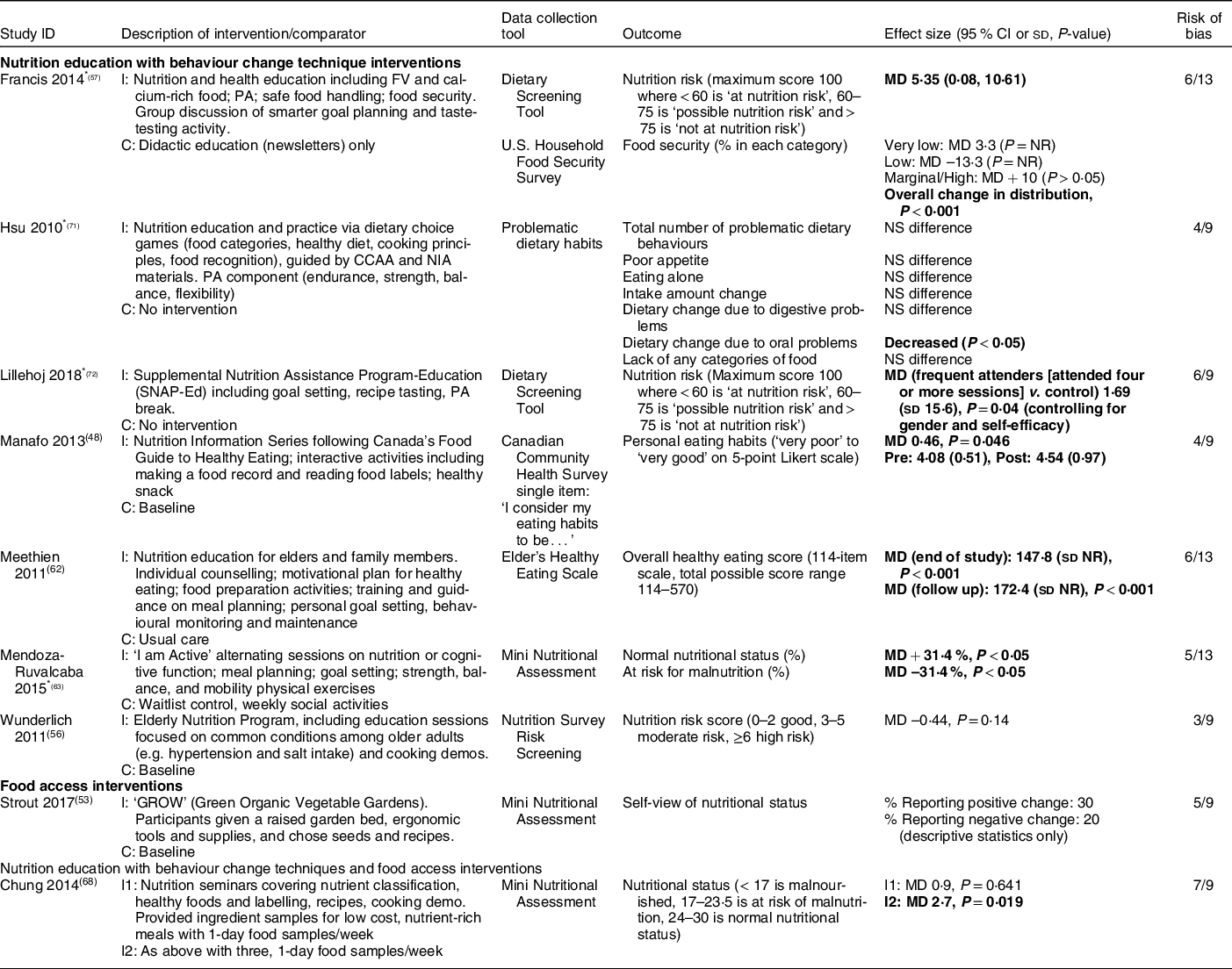
Nine studies evaluated the effectiveness of nutrition education with BCT (n 7, 78 %)(Reference Manafo, Jose and Silverberg48,Reference Wunderlich, Bai and Piemonte56,Reference Francis, MacNab and Shelley57,Reference Meethien, Pothiban and Ostwald62,Reference Mendoza-Ruvalcaba and Arias-Merino63,Reference Hsu, Wang and Chen71,Reference Lillehoj, Yap and Montgomery72) , food access (n 1, 11 %)(Reference Strout, Jemison and O’Brien53) and nutrition education with BCT and food access (n 1, 11 %)(Reference Chung and Chung68) for decreasing nutrition risk. Measures such as the Mini Nutritional Assessment (n 3, 33 %)(Reference Strout, Jemison and O’Brien53,Reference Mendoza-Ruvalcaba and Arias-Merino63,Reference Chung and Chung68) , Dietary Screening Tool (n 2, 22 %)(Reference Francis, MacNab and Shelley57,Reference Lillehoj, Yap and Montgomery72) , problematic dietary habits (n 2, 22 %)(Reference Manafo, Jose and Silverberg48,Reference Hsu, Wang and Chen71) and food security (n 1, 11 %)(Reference Francis, MacNab and Shelley57) were used (Table 3). Heterogeneous interventions and outcomes and inconsistent results were found.
Table 3 Nutrition risk (factors impacting food intake) (n 9)
I, intervention group; C, comparator group; FV, fruits and vegetables; PA, physical activity; MD, mean difference; NR, not reported; CCAA, Canadian Center for Activity and Aging; NIA, National Institute of Aging.
* Asterisks indicate interventions that also included a physical activity component.
Bold text indicates statistical significance.
Among seven studies that combined nutrition education with BCT, five demonstrated consistently positive effects(Reference Manafo, Jose and Silverberg48,Reference Francis, MacNab and Shelley57,Reference Meethien, Pothiban and Ostwald62,Reference Mendoza-Ruvalcaba and Arias-Merino63,Reference Lillehoj, Yap and Montgomery72) . The ‘I am Active’ intervention (twice weekly nutrition sessions including meal planning and goal setting for two months) increased the percentage of participants with ‘normal’ nutritional status (as defined by the Mini Nutritional Assessment) and decreased the number at risk for malnutrition compared to waitlist control (moderate risk of bias)(Reference Mendoza-Ruvalcaba and Arias-Merino63). Compared to didactic education alone, 6-monthly nutrition and health education sessions incorporating goal setting and taste testing decreased nutrition risk (moderate risk of bias)(Reference Francis, MacNab and Shelley57). People who frequently attended Supplemental Nutrition Assistant Program-Education (SNAP-Ed) nutrition education sessions with goal setting and recipe tasting decreased their nutrition risk status as compared to control (moderate risk of bias)(Reference Lillehoj, Yap and Montgomery72); however, it is important to note that only those attending four or more sessions were included in the analysis. Compared to usual care, weekly nutrition education that incorporated counselling, food preparation, goal setting and behavioural monitoring improved overall healthy eating scores after 3 months (moderate risk of bias)(Reference Meethien, Pothiban and Ostwald62). Finally, interactive nutrition education and skill-building activities also improved personal eating habits as compared to baseline (moderate risk of bias)(Reference Manafo, Jose and Silverberg48).
Conversely, two additional studies that combined nutrition education with BCT did not improve nutritional status (moderate to high risk of bias)(Reference Wunderlich, Bai and Piemonte56,Reference Hsu, Wang and Chen71) . Two studies (low to moderate risk of bias) evaluated food access or nutrition education with BCT and food access(Reference Strout, Jemison and O’Brien53,Reference Chung and Chung68) ; these did not consistently reduce nutrition risk.
Healthy eating knowledge
Five studies reported changes in healthy eating knowledge, generally using study-specific single-item questions (e.g. roles of nutrients, recommended servings) following nutrition education with BCT (n 3, 60 %)(Reference Pogge and Eddings50,Reference MacNab, Davis and Francis74,Reference Murayama, Taguchi and Spencer75) , nutrition education with BCT and food access (n 1, 20 %)(Reference Moreau, Plourde and Hendrickson-Nelson49), and didactic nutrition education (n 1, 20 %)(Reference Thomas, Almanza and Ghiselli54). Nutrition education with BCT may improve healthy eating knowledge, as found in four studies (low to moderate risk of bias) that incorporated skill-building activities into nutrition education interventions(Reference Moreau, Plourde and Hendrickson-Nelson49,Reference Pogge and Eddings50,Reference MacNab, Davis and Francis74,Reference Murayama, Taguchi and Spencer75) (Table 4).
Table 4 Healthy eating knowledge (n 5)

I, intervention group; C, comparator group; MD, mean difference; NR, not reported; PA, physical activity.
* Asterisks indicate interventions that also included a physical activity component.
Bold text indicates statistical significance.
Physical mobility outcomes
Physical activity
Physical activity outcomes were assessed in thirteen studies consisting of nutrition education with BCT (n 10, 77 %)(Reference Schwingel, Galvez and Linares51,Reference Smith, Ory and Jiang52,Reference Geller, Mendoza and Timbobolan58–Reference Kimura, Moriyasu and Kumagai60,Reference Silva-Smith, Fleury and Belyea65,Reference Uemura, Yamada and Okamoto66,Reference Gallois, Buck and Dreas69,Reference Hsu, Wang and Chen71,Reference Smith, Lee and Towne76) , didactic nutrition education (n 2, 15 %)(Reference Turk, Elci and Resick55,Reference Luten, Reijneveld and Dijkstra73) and interactive nutrition education (n 1, 8 %)(Reference Beasley, Kirshner and Wylie-Rosett47) interventions (Table 5). These were captured through both self-reported (e.g. International Physical Activity Questionnaire, 24-hour/7-day recall) and objective measurements (e.g. pedometers, accelerometers). All interventions included a physical activity component either through education or participation during the group-based sessions.
Table 5 Physical activity (n 13)

I, intervention group; FV, fruits and vegetable; PA, physical activity;. C, comparator group; IPAQ, International Physical Activity Questionnaire short form; MD, mean difference; CCAA, Canadian Centre for Activity and Aging; NIA, National Institute of Aging; MVPA, moderate to vigorous intensity physical activity; NR, not reported; NS, not significant; RAPA, Rapid Assessment of Physical Activity; CHAMP, Cardiovascular Healthy Activities Model Program for Seniors. Bold text indicates statistical significance.
Across ten studies evaluating nutrition education with BCT, findings were mixed. Four studies found a consistent increase, including participation in regular exercise(Reference Hsu, Wang and Chen71) aerobic/strength training(Reference Smith, Ory and Jiang52), steps per day(Reference Uemura, Yamada and Okamoto66) and time spent in light, moderate or vigorous physical activity(Reference Smith, Lee and Towne76) (low to moderate risk of bias); each of these included physical activity participation within group-based sessions. Six other studies (low to moderate risk of bias) did not report consistent improvements, with three studies including physical activity education only(Reference Geller, Mendoza and Timbobolan58,Reference Jancey, Holt and Lee59,Reference Gallois, Buck and Dreas69) and three(Reference Schwingel, Galvez and Linares51,Reference Kimura, Moriyasu and Kumagai60,Reference Silva-Smith, Fleury and Belyea65) including physical activity participation. Didactic nutrition education(Reference Turk, Elci and Resick55,Reference Luten, Reijneveld and Dijkstra73) and interactive nutrition education(Reference Beasley, Kirshner and Wylie-Rosett47) interventions alone did not appear to increase physical activity.
Functional outcomes
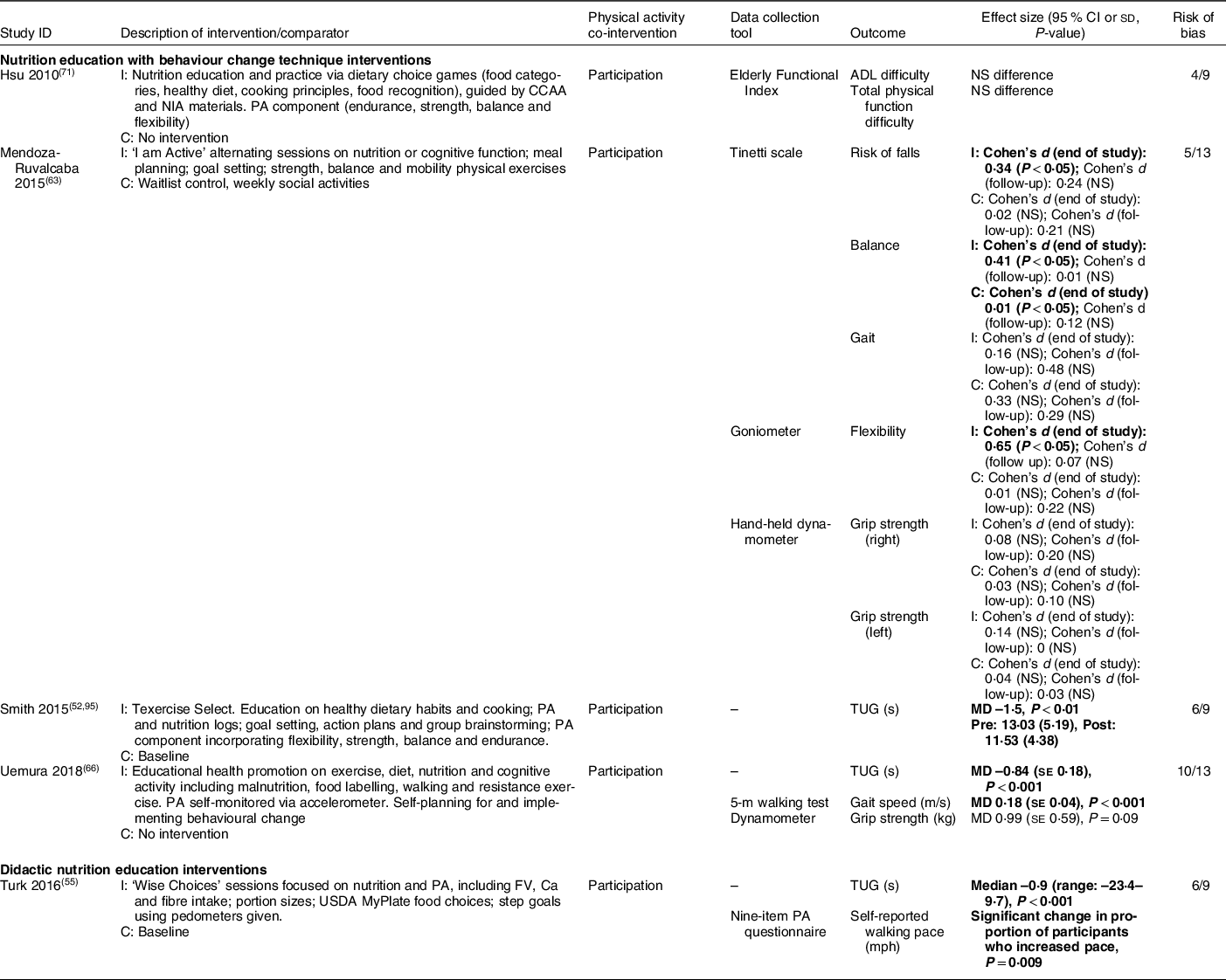
Five studies reported the impact of nutrition education with BCT (n 4, 80 %)(Reference Smith, Ory and Jiang52,Reference Mendoza-Ruvalcaba and Arias-Merino63,Reference Uemura, Yamada and Okamoto66,Reference Hsu, Wang and Chen71) and didactic nutrition education (n 1, 20 %)(Reference Turk, Elci and Resick55) on functional mobility (Table 6). Although heterogeneous intervention and outcome types were again noted, both nutrition education with BCT and didactic nutrition education generally improved functional outcomes (e.g. Timed Up and Go, gait speed), as noted in four studies (low to moderate risk of bias)(Reference Smith, Ory and Jiang52,Reference Turk, Elci and Resick55,Reference Mendoza-Ruvalcaba and Arias-Merino63,Reference Uemura, Yamada and Okamoto66) . Each of these also included participation in physical activity as a co-intervention.
Table 6 Functional outcomes (n 5)
I, intervention group; ADL, activities of daily living; C, comparator group; CCAA, Canadian Centre for Activity and Aging; NIA, National Institute of Aging; PA, physical activity; TUG, Timed Up and Go test; MD, mean difference; NS, not significant; FV, fruits and vegetable. Bold text indicates statistical significance.
Discussion
Given the wide heterogeneity and inconsistent findings across this body of literature, our certainty in the effectiveness of group-based community nutrition interventions to improve food and fluid intake, nutritional status, healthy eating knowledge and measures of physical activity or physical function in older adults is low. The available evidence suggests that nutrition education with BCT may be the most promising approach to improving food and fluid intake, nutritional status and healthy eating knowledge. Given the variation across interventions and outcomes, it is unclear which intervention is optimal for implementation in community-based settings. Both intervention duration and frequency varied widely across studies, with no discernable patterns to suggest a minimally or optimally effective intervention ‘dose’. While one would suspect that longer programmes or more frequent sessions would have a greater impact, this did not appear to be the case in the studies included in this review. Overall, these conclusions should be interpreted with caution related to high variability among intervention components and outcome measurements, in addition to unclear to high risk of bias within the studies themselves.
Most of the interventions combined nutrition education with BCT. Although we broadly grouped interventions as either including BCT or not, we did not explicitly code these based on the BCT Taxonomy(Reference Michie, Richardson and Johnston77) to identify the discrete strategies used. The effectiveness of nutrition education with BCT, particularly concerning food and fluid intake and nutrition risk, remains unclear; there is a lack of evidence on which specific BCT are required to elicit significant change. Given wide heterogeneity across intervention components, duration, frequency, interventionists, locations and theoretical frameworks used, we could not distinguish any noticeable patterns among nutrition education with BCT interventions that were consistently effective v. those that were not. Interventions that described nutrition education with BCT appeared to be more intensive than interventions that focussed on didactic or interactive nutrition education alone. However, it is conceivable that individuals who consent to participate in a more intensive programme could perhaps be more committed to overall behavioural change. Appropriately selecting and evaluating the effectiveness of BCT remains an emerging area of inquiry(Reference Michie, West and Sheals78); thus, understanding the most relevant and effective BCT to improve nutrition and mobility outcomes among community-dwelling older adults is an important next step. More fulsome reporting of intervention components following definitions from the BCT Taxonomy(Reference Michie, Richardson and Johnston77) or using a recognised framework such as the TIDIeR checklist(Reference Hoffmann, Glasziou and Boutron34) would allow future exploration of key intervention components.
We explored physical activity and functional outcomes gave the established link between adequate nutritional intake and mobility in older adults; however, all studies that explored mobility outcomes also included a physical activity co-intervention. The existence of a co-intervention made it difficult to determine which component(s) of these multifaceted interventions were driving change when observed. Although we hypothesised that comprehensive healthy lifestyle programmes might have a greater impact on behavioural change overall, we did not observe any clear trends to indicate whether the interventions that included both nutrition and physical activity components were more effective for either nutrition or mobility-related outcomes than those focussed on nutrition alone (Table 2–6). There is limited available evidence regarding the effectiveness of single v. multiple health behaviour change interventions in older adults(Reference Nigg and Long79), highlighting a potential area for further investigation(Reference Geller, Lippke and Nigg80).
Given the complex factors (e.g. financial, environmental, cultural) known to impact older adults’ ability to maintain a healthy diet(Reference Keller, Dwyer and Senson14), it is important to recognise that while nutrition education and skill building may be effective at increasing healthy eating knowledge and intentions, they may be insufficient to change outcomes such as food and fluid intake or nutrition risk. Using an equity lens, we assessed the nine studies included in this review that explicitly targeted populations with low socio-economic status (e.g. recruitment from low-income housing). Overall, findings were inconsistent, with improvements following education with BCT noted in some but not others. This may not be surprising if the primary barriers to quality food intake (e.g. vegetable and fruit consumption) are cost or ease of access(Reference Choi, Crimmins and Kim9). Environmental support and policy-level public health interventions are likely needed to ensure equitable access to healthy food before nutrition education and skill building can be expected to make a meaningful difference(Reference Menezes, Diez Roux and Costa81–Reference Blumenthal, Hoffnagle and Leung83).
To our knowledge, this review is the first to systematically identify, appraise and synthesise evidence regarding the effectiveness of nutrition-focused group-based interventions targeting food and fluid intake, nutrition risk and mobility outcomes in community-dwelling older adults. However, our results are consistent with recommendations from a pair of evidence syntheses and an expert commentary published in 2003 that concluded nutrition education alone was insufficient to improve nutritional status among older adults(Reference Higgins and Clarke Barkley24,Reference Higgins and Barkley84,Reference Higgins and Barkley85) . In line with our findings, the authors recommended that education be paired with behaviour change strategies and community participation to enhance programme effectiveness. Similarly, a 2007 review of Canadian research highlighted successful components of community nutrition programmes for older adults, including cooking classes, recipe exchanges, counselling, social support and engagement, motivation and interactivity(Reference Keller86). Consistent with our findings, these strategies would also be considered techniques to support behaviour change.
Several important considerations should be made while interpreting the findings from this review. Although our search strategy was comprehensive, it was restricted to studies published in English since 2010, which may be a limitation. However, our results are consistent with findings from older, related reviews described above that considered single studies dating back to 1993(Reference Higgins and Clarke Barkley24,Reference Higgins and Barkley84–Reference Keller86) . Further, despite the updated Consolidated Standards of Reporting Trials (CONSORT) 2010 guidelines(Reference Schulz, Altman and Moher87), methodological and reporting challenges contributed to the unclear to high risk of bias in the studies included in this review. Therefore, it is unlikely that studies published before 2010 would be of higher methodological quality or change our overall conclusions. Given that the aim of this review was to explore the effectiveness of group-based interventions, it was appropriate to focus on intervention studies only. Qualitative data may highlight important insights into reasons for variable intervention effectiveness (e.g. implementation insights). While we did include two mixed-methods studies, only quantitative data were extracted. Further, although we did endeavour to integrate considerations about study quality, consistency and directness throughout the wide variability in outcomes across included studies limited us from applying a formal approach, such as GRADE(Reference Schünemann, Brożek and Guyatt88) to assess certainty in this body of evidence.
Our conclusions are also limited by the nature of the primarily quasi-experimental single studies with incomplete follow-up included within the review. We did not observe any differences in the types of interventions or findings among the studies that reported > 20 % attrition. The large dropout rate observed might be attributed to the population; researchers often face difficulties recruiting and retaining older adults in research due to health and mobility challenges among this population(Reference Cherubini and Gasperini89). When considering intervention context, it is also possible that participation may have been fluid because of the nature of delivery in settings such as congregate meal sites and seniors’ centres that may operate on a drop-in basis. Lack of reliable outcome measurement tools may explain some of the inconsistency across studies. Challenges associated with measuring the impact of community nutrition programmes have previously been documented(Reference Higgins and Barkley90); given the nature of self-reported data, outcomes such as food intake, dietary behaviour and knowledge are notoriously complex constructs to measure accurately. Despite previous calls for community nutrition interventions for older adults based on behaviour change theories(Reference Higgins and Barkley85), less than half of the studies in this review used a theoretical framework to inform intervention delivery; this might further explain some of the variability noted in our results. We also observed variability in the content of the nutrition education provided across interventions. It is unclear if recommendations were consistently based on current, evidence-based healthy eating guidelines for older adults, further explaining the inconsistent effectiveness observed.
Implications for research
More studies using RCT designs are needed to increase confidence in the impact of group-based community nutrition interventions. Although blinding of participants and interventionists is nearly impossible given the nature of the interventions, future studies should strive to blind outcome assessors and data analysts to enhance internal validity. Authors using quasi-experimental approaches should include control groups to facilitate stronger comparisons. In an attempt to overcome potential attrition bias due to incomplete follow up with older adult participants in community settings, future studies may consider strategies such as providing transportation and involving older adults/community providers during intervention planning to ensure issues that may lead to decreased retention are considered and addressed(Reference Cherubini and Gasperini89). Given that community-based nutrition programming tends to be delivered via public health initiatives and not always through funded programmes of research, challenges noted with intervention design, outcome assessment, study quality and inappropriate statistical analyses might be attributed to the probable lack of resources available to support community programme development and evaluation. Prioritising research funding to support the development and evaluation of community-based nutrition programmes for older adults is necessary to improve the quality of the evidence base.
Implications for practice
For organisations looking to design and implement community-based nutrition programming for older adults, nutrition education with embedded BCT (e.g. goal setting, hands-on skill-building activities, taste testing) demonstrated the most promise to improve healthy eating outcomes. However, there is wide heterogeneity in the available evidence, including programme length and session frequency. The discrete techniques and intervention components that might be most important to include have yet to be determined. These will likely need to be tailored based on the needs and preferences of the community and local context. Future programme design should be based on recognised theories of behaviour change. There is a potential to draw upon significant recent advancements in behaviour change theory(Reference Michie, van Stralen and West91,Reference French, Green and O’Connor92) , which have been applied in developing complex interventions for healthy eating(Reference Moore, Rivas and Stanton-Fay93,Reference Atkins and Michie94) .
Conclusion
Group-based nutrition education with BCT demonstrated the most promise in improving food and fluid intake, nutritional status and healthy eating knowledge among community-dwelling older adults. The impact of these programmes on mobility outcomes is less clear. These findings should be interpreted with caution, given the generally unclear to high risk of bias and low quality, heterogeneous evidence base. We have highlighted several key takeaways regarding how the quality of this body of literature could be improved. Future group and community-based programmes should use recognised behavioural change theories to develop and implement evidence-based nutrition education with skill-building activities to improve healthy eating among older adults.
Acknowledgements
Acknowledgements: We gratefully acknowledge Mainka Tandon and Allison Branston for their contributions to study selection, as well as the larger EMBOLDEN principal and co-investigator team. Financial support: This work is supported by funding received from the Labarge Centre for Mobility in Aging within the McMaster Institute for Research on Aging at McMaster University, Canada Research Chairs Program, the Canadian Institutes of Health Research (Grant number 169 395) and in-kind support from the Aging, Community and Health Research Unit at McMaster University. The funders had no role in the design, analysis or writing of this article. Authorship: R.G and S.E.N-S. conceptualised the study; R.G., D.S., C.M. and S.E.N-S. designed the study and carried out the search and study selection. K.T., D.S. and C.M. completed data collection and critical appraisal. K.T. led data analysis and writing of the manuscript with substantial contributions from R.G., D.S., C.M., H.K., C.S., S.M.P. and S.E.N-S. All authors were involved in critically revising the manuscript and have approved the final version. Ethics of human subject participation: Not applicable.
Conflict of interest:
S.M.P. declares that he is a named inventor on a patent held by Exerkine, but receives no fees/payment, and is an unpaid member of the Scientific Advisory Board for Enhanced Recovery. The other authors have no conflicts of interest to disclose.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S136898002200115X












