The recent Public Health Nutrition paper by Nikooyeh and colleagues(
Reference Nikooyeh, Abdollahi and Hajifaraji
1
) reports on seasonal latitudinal variations in serum 25-hydroxyvitamin D (25(OH)D) concentration, serum lipid status, height-for-age Z-score and BMI for 530 apparently healthy children aged 5–18 years in six regions of Iran. Serum 25(OH)D concentrations increased from 31 (sd 16) to 50 (sd 30) nmol/l for boys and from 27 (sd 18) to 43 (sd 29) nmol/l for girls. The primary reason for this difference was that boys spent more time in the sun and were less likely to use sunscreen than girls. More importantly, HDL-cholesterol (HDL-C) concentration, a frequently used biomarker of cardiovascular health, increased significantly in summer compared with winter for both boys and girls in Iran. In addition, there were inverse correlations between seasonal changes in serum 25(OH)D concentration and changes in BMI, TAG and total cholesterol, and a direct correlation with height-for-age Z-score; all healthy cardiometabolic risk factor changes.
The changes in weight and height with season may reflect increased exercise and growth rates in summer compared with winter; however, other observed healthy risk factor changes are likely due to the measured increase in serum 25(OH)D concentration. In this commentary, we describe how the known functions of vitamin D impact these changes in cardiovascular risk markers and this builds confidence in the prospect that the beneficial observed risk factor changes are driven by increases in vitamin D status.
The reduction in TAG in summer may reflect the changes in weight, but increases in vitamin D status could contribute directly to this effect since calcitriol, the active hormonal metabolite of vitamin D, reduces NEFA synthesis(
Reference Ji, Gupta and Feldman
2
) and can inhibit hepatic TAG synthesis(
Reference Cheng, So and Zhang
3
). One randomized controlled trial has shown that vitamin D enhanced the beneficial effects of weight loss on serum TAG(
Reference Zittermann, Frisch and Berthold
4
).
In an earlier cross-sectional Norwegian study involving 17 083 individuals older than 40 years, HDL-C concentration was also significantly correlated with 25(OH)D concentration(
Reference Jorde and Grimnes
5
), where mean concentration ranged from ~18 to 110 nmol/l. Graphical comparison of these studies shows that the Norwegian 25(OH)D concentration–HDL-C concentration relationship matches the Iranian relationship reasonably well, although the HDL-C concentration for Iranian boys in summer(
Reference Nikooyeh, Abdollahi and Hajifaraji
1
) was below the linear fit to Jorde and Grimnes’ data(
Reference Jorde and Grimnes
5
) for low fish oil intake by ~2 mg/dl (Fig. 1). This finding may be due to a non-linear relationship between UVB exposure and HDL-C concentration as reported in two studies in Pune, India (18·5°N), since low UVB exposure increased HDL-C concentration but high UVB exposure reduced HDL-C concentration(
Reference Patwardhan, Khadilkar and Chiplonkar
6
,
Reference Patwardhan, Mughal and Padidela
7
).
The implications of these findings warrant consideration. First, is HDL-C an important risk factor for CHD? A recent review found that HDL-C concentration >51 mg/dl was not a significant risk-reduction factor after adjustment for other recognized risk factors(
Reference Gaksch, Jorde and Grimnes
8
), although HDL-C concentration <51 mg/dl was a significant risk factor, after adjustment for other recognized risk factors.
Vitamin D deficiency has been shown to be linked to the metabolic syndrome(
Reference Boucher
9
,
Reference Boucher
10
) or syndrome ‘X’(
Reference Reaven
11
,
Reference Reaven
12
). The hallmarks of the metabolic syndrome include three or more of large waist size, raised TAG, low HDL-C concentration, hypertension, hyperglycaemia, increased insulin resistance and non-alcoholic fatty liver disease(
Reference Alberti, Eckel and Grundy
13
). Raising serum 25(OH)D concentration can raise HDL-C concentration, reduce the risk of hypertension(
Reference Forman, Curhan and Taylor
14
,
Reference Mirhosseini, Vatanparast and Kimball
15
) and reduce fasting glucose(
Reference Al-Khalidi, Kimball and Rotondi
16
). Serum 25(OH)D concentration has been found to be inversely correlated with CVD in a meta-analysis of observational studies, finding a pooled risk ratio of 0·90 (95 % CI 0·86, 0·94) for each 25 nmol/l increment in 25(OH)D concentration(
Reference Zhang, Li and Gao
17
).
The first mechanism to consider in explaining the effects of increased vitamin D status on HDL-C concentration is vitamin D’s role in changing gene expression, which can be induced by higher serum 25(OH)D concentrations. It is well established that virtually all biological activities of the active metabolite of vitamin D are mediated by the vitamin D receptor, a nuclear receptor protein that functions to control expression of genes and gene networks in specific cell types(
Reference Pike and Christakos
18
). However, other effects considered to be non-genomic induction are driven by the active form of vitamin D through an increased intracellular Ca2+ concentration(
Reference Trochoutsou, Kloukina and Samitas
19
).
A second genetic explanation stems from vitamin D supplementation studies that have shown up- and down-regulation of many genes. In one study, three individuals were given 10 µg (400 IU) of cholecalciferol (vitamin D3) daily and five were given 50 µg (2000 IU) of cholecalciferol daily for two months, and white blood cells were sampled to monitor changes. The cholecalciferol supplementation that improved serum 25(OH)D concentrations was associated with at least a 1·5-fold alteration in the expression of 291 genes in these white blood cells. There also was a significant difference in the expression of sixty-six genes at baseline between individuals with vitamin D deficiency (serum 25(OH)D<50 nmol/l) and those without (serum 25(OH)D>50 nmol/l). After cholecalciferol supplementation, gene expression of these sixty-six genes was similar for both groups(
Reference Hossein-nezhad, Spira and Holick
20
). In another study, twenty-five adults were randomized to receive placebo or a single oral dose of cholecalciferol of 1250 µg (50 000 IU), 250 µg (100 000 IU) or 5000 µg (200 000 IU)(
Reference Scott, Das and Ahsanuddin
21
). Adults receiving 5000 µg were much more likely to have expression of many genes flipped from up- to down- or from down- to up-regulated. The results of gene expression flipping were grouped into two clusters for skin barrier repair genes. Those with increased expression of the skin barrier repair genes had a mean 25(OH)D concentration of ~106 nmol/l, while those with no increase in skin barrier repair gene expression had a mean 25(OH)D concentration of ~75 nmol/l.
A third possible mechanism is described in two papers that identified 25(OH)D-dependent effects of APOA5 polymorphisms on circulating HDL-C(
Reference Shirts, Howard and Hasstedt
22
,
Reference Vimaleswaran, Cavadino and Hypponen
23
). Shirts et al.(
Reference Shirts, Howard and Hasstedt
22
) described a 25(OH)D receptor binding site-modifying APOA5-promoter polymorphism associated with lower HDL-C in 25(OH)D-deficient individuals(
Reference Shirts, Howard and Hasstedt
22
). The other identified a borderline interaction between the SNP rs12272004 (near the APOA5) and serum 25(OH)D on HDL-C (P for interaction=0·05)(
Reference Vimaleswaran, Cavadino and Hypponen
23
).
A fourth line of evidence supporting vitamin D’s role in the observed changes in cardiometabolic risk factors concerns the widespread seasonal variations in gene expression that have been shown, including genes recognized as biomarkers of increased risks of CVD(
Reference Dopico, Evangelou and Ferreira
24
). While Dopico et al.’s study(
Reference Dopico, Evangelou and Ferreira
24
) did not identify changes in 25(OH)D concentration as the driver, based on the preceding discussion, it seems likely that it is a contributor.
Studies of the seasonality of disease and mortality rates find that rates are often higher in winter than in summer. For example, death rates in the USA are 25 % higher in winter than in summer, with CVD being a major contributor to that seasonal variation(
Reference Grant, Bhattoa and Boucher
25
), suggesting that keeping serum 25(OH)D concentration >90 nmol/l year-round may reduce death rates significantly. This estimate was supported by calculations based on increasing 25(OH)D concentration from 54 to 110 nmol/l globally(
Reference Grant
26
), as well as a meta-analysis of mortality rates found in relationship to serum 25(OH)D concentrations in thirty-two independent studies(
Reference Garland, Kim and Mohr
27
). Thus, the study by Nikooyeh et al.(
Reference Nikooyeh, Abdollahi and Hajifaraji
1
) provides further support for beneficial effects of seasonal changes in available UVB dose, and in 25(OH)D concentration, that may have a role in reducing mortality rates.
Clinical trials conducted by 2017 had not shown that vitamin D supplementation reduces the risks of CVD(
Reference Veloudi, Jones and Sharman
28
) but this finding fails to question the appropriateness of the design of the clinical trials. Most vitamin D clinical trials have been based on the assumptions implicit in trials of pharmaceutical drugs, namely that the only source of the agent is that provided in the trial and that there is a linear dose–response relationship; however, neither assumption is satisfied for vitamin D. The primary determinant of health outcomes in clinical trials with high doses of vitamin D is the achieved 25(OH)D concentration, not the vitamin D dose used. A recent clinical trial supplementing with high-dose cholecalciferol supports this concept that clinical trial efficacy should not be based on the success of the dose used to alter disease biomarkers, but on the improved change in 25(OH)D concentration associated with improved disease risk(
Reference Lappe, Watson and Travers-Gustafson
29
,
Reference Grant and Boucher
30
). A recent paper outlined how to base clinical trials of vitamin D on measurements of 25(OH)D concentrations(
Reference Grant, Boucher and Bhattoa
31
). However, results of two clinical trials reported after those papers had been published have shown reductions in biological risk factors for CVD. In one, hypertensive patients who increased cholecalciferol doses from ~50 to ~150 µg/d (~2000 to ~6000 IU/d), with increases in their 25(OH)D concentrations from ~82 to 110 nmol/l, did show reductions in systolic blood pressure by ~16 mmHg and in diastolic blood pressure by ~12 mmHg(
Reference Mirhosseini, Vatanparast and Kimball
15
), while blood pressure changes in normotensive subjects were not significant. In the second trial, seventy overweight African Americans (aged 13–45 years) with serum 25(OH)D concentrations <50 nmol/l were given monthly cholecalciferol doses equivalent to 15, 50 or 100 µg/d (600, 2000 or 4000 IU/d) v. placebo(
Reference Raed, Bhagatwala and Zhu
32
). The beneficial change in carotid–femoral pulse wave velocity was significantly greater for 100 µg/d (−10·4 %; 25(OH)D=33 nmol/l at baseline, 89 nmol/l at 8 weeks, 87 nmol/l at 16 weeks) than for 50 µg/d (−2·0 %; 25(OH)D=40 nmol/l at baseline, 76 nmol/l at 8 weeks, 90 nmol/l at 16 weeks); as was the change in carotid–radial pulse wave velocity at −8·0 % after 100 µg/d v. +1·2 % after 15 µg/d (25(OH)D=35 nmol/l at baseline, 53 nmol/l at 8 weeks, 57 nmol/l at 16 weeks). While these two clinical trials did not assess HDL-C concentrations, they did show that vitamin D supplementation at high enough doses can reduce biological risk markers for CVD in those at increased risk; furthermore, these two trials also demonstrate that it is not always necessary to enrol people with deficiency in order to be able to find significant beneficial effects of cholecalciferol supplementation if the cholecalciferol dose is high enough. In the vitamin D3 plus calcium trial for cancer, mean serum 25(OH)D concentration increased from 83 to 109 nmol/l in the treatment arm but decreased from 82 to 79 nmol/l in the control arm(
Reference Lappe, Watson and Travers-Gustafson
29
).
We are currently in the ‘golden age’ of vitamin D research with many exciting findings being reported each year on the non-skeletal effects of vitamin D. Given the difficulty of conducting vitamin D clinical trials, observational studies such as the one reported by Nikooyeh et al.(
Reference Nikooyeh, Abdollahi and Hajifaraji
1
) play very important roles in identifying and quantifying effects and paving the way for additional studies, as well as serving to raise awareness of the importance of sun exposure and cholecalciferol for human health.
The recent Public Health Nutrition paper by Nikooyeh and colleagues( Reference Nikooyeh, Abdollahi and Hajifaraji 1 ) reports on seasonal latitudinal variations in serum 25-hydroxyvitamin D (25(OH)D) concentration, serum lipid status, height-for-age Z-score and BMI for 530 apparently healthy children aged 5–18 years in six regions of Iran. Serum 25(OH)D concentrations increased from 31 (sd 16) to 50 (sd 30) nmol/l for boys and from 27 (sd 18) to 43 (sd 29) nmol/l for girls. The primary reason for this difference was that boys spent more time in the sun and were less likely to use sunscreen than girls. More importantly, HDL-cholesterol (HDL-C) concentration, a frequently used biomarker of cardiovascular health, increased significantly in summer compared with winter for both boys and girls in Iran. In addition, there were inverse correlations between seasonal changes in serum 25(OH)D concentration and changes in BMI, TAG and total cholesterol, and a direct correlation with height-for-age Z-score; all healthy cardiometabolic risk factor changes.
The changes in weight and height with season may reflect increased exercise and growth rates in summer compared with winter; however, other observed healthy risk factor changes are likely due to the measured increase in serum 25(OH)D concentration. In this commentary, we describe how the known functions of vitamin D impact these changes in cardiovascular risk markers and this builds confidence in the prospect that the beneficial observed risk factor changes are driven by increases in vitamin D status.
The reduction in TAG in summer may reflect the changes in weight, but increases in vitamin D status could contribute directly to this effect since calcitriol, the active hormonal metabolite of vitamin D, reduces NEFA synthesis( Reference Ji, Gupta and Feldman 2 ) and can inhibit hepatic TAG synthesis( Reference Cheng, So and Zhang 3 ). One randomized controlled trial has shown that vitamin D enhanced the beneficial effects of weight loss on serum TAG( Reference Zittermann, Frisch and Berthold 4 ).
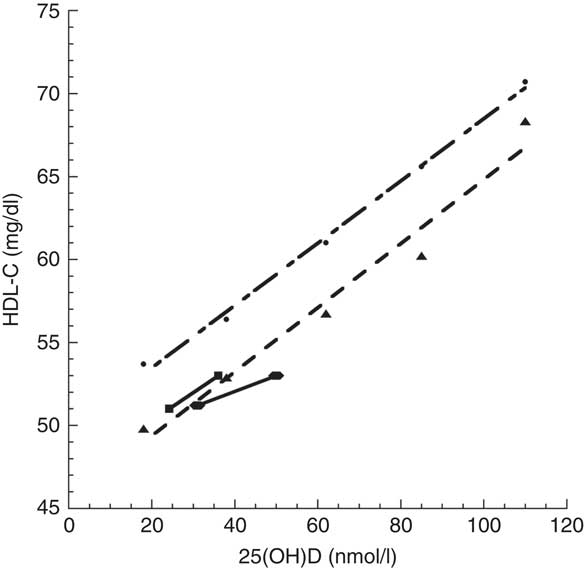
In an earlier cross-sectional Norwegian study involving 17 083 individuals older than 40 years, HDL-C concentration was also significantly correlated with 25(OH)D concentration( Reference Jorde and Grimnes 5 ), where mean concentration ranged from ~18 to 110 nmol/l. Graphical comparison of these studies shows that the Norwegian 25(OH)D concentration–HDL-C concentration relationship matches the Iranian relationship reasonably well, although the HDL-C concentration for Iranian boys in summer( Reference Nikooyeh, Abdollahi and Hajifaraji 1 ) was below the linear fit to Jorde and Grimnes’ data( Reference Jorde and Grimnes 5 ) for low fish oil intake by ~2 mg/dl (Fig. 1). This finding may be due to a non-linear relationship between UVB exposure and HDL-C concentration as reported in two studies in Pune, India (18·5°N), since low UVB exposure increased HDL-C concentration but high UVB exposure reduced HDL-C concentration( Reference Patwardhan, Khadilkar and Chiplonkar 6 , Reference Patwardhan, Mughal and Padidela 7 ).
Fig. 1 Relationship of HDL-cholesterol (HDL-C) concentration with 25-hydroxyvitamin D (25(OH)D) concentration: comparison between Jorde and Grimnes’ study( Reference Jorde and Grimnes 5 ) ( , Norway, standard diet;
, Norway, standard diet;  , Norway, low fish oils) and Nikooyeh et al.’s study(
Reference Nikooyeh, Abdollahi and Hajifaraji
1
) (
, Norway, low fish oils) and Nikooyeh et al.’s study(
Reference Nikooyeh, Abdollahi and Hajifaraji
1
) ( , Iran, boys;
, Iran, boys;  , Iran, girls)
, Iran, girls)
The implications of these findings warrant consideration. First, is HDL-C an important risk factor for CHD? A recent review found that HDL-C concentration >51 mg/dl was not a significant risk-reduction factor after adjustment for other recognized risk factors( Reference Gaksch, Jorde and Grimnes 8 ), although HDL-C concentration <51 mg/dl was a significant risk factor, after adjustment for other recognized risk factors.
Vitamin D deficiency has been shown to be linked to the metabolic syndrome( Reference Boucher 9 , Reference Boucher 10 ) or syndrome ‘X’( Reference Reaven 11 , Reference Reaven 12 ). The hallmarks of the metabolic syndrome include three or more of large waist size, raised TAG, low HDL-C concentration, hypertension, hyperglycaemia, increased insulin resistance and non-alcoholic fatty liver disease( Reference Alberti, Eckel and Grundy 13 ). Raising serum 25(OH)D concentration can raise HDL-C concentration, reduce the risk of hypertension( Reference Forman, Curhan and Taylor 14 , Reference Mirhosseini, Vatanparast and Kimball 15 ) and reduce fasting glucose( Reference Al-Khalidi, Kimball and Rotondi 16 ). Serum 25(OH)D concentration has been found to be inversely correlated with CVD in a meta-analysis of observational studies, finding a pooled risk ratio of 0·90 (95 % CI 0·86, 0·94) for each 25 nmol/l increment in 25(OH)D concentration( Reference Zhang, Li and Gao 17 ).
The first mechanism to consider in explaining the effects of increased vitamin D status on HDL-C concentration is vitamin D’s role in changing gene expression, which can be induced by higher serum 25(OH)D concentrations. It is well established that virtually all biological activities of the active metabolite of vitamin D are mediated by the vitamin D receptor, a nuclear receptor protein that functions to control expression of genes and gene networks in specific cell types( Reference Pike and Christakos 18 ). However, other effects considered to be non-genomic induction are driven by the active form of vitamin D through an increased intracellular Ca2+ concentration( Reference Trochoutsou, Kloukina and Samitas 19 ).
A second genetic explanation stems from vitamin D supplementation studies that have shown up- and down-regulation of many genes. In one study, three individuals were given 10 µg (400 IU) of cholecalciferol (vitamin D3) daily and five were given 50 µg (2000 IU) of cholecalciferol daily for two months, and white blood cells were sampled to monitor changes. The cholecalciferol supplementation that improved serum 25(OH)D concentrations was associated with at least a 1·5-fold alteration in the expression of 291 genes in these white blood cells. There also was a significant difference in the expression of sixty-six genes at baseline between individuals with vitamin D deficiency (serum 25(OH)D<50 nmol/l) and those without (serum 25(OH)D>50 nmol/l). After cholecalciferol supplementation, gene expression of these sixty-six genes was similar for both groups( Reference Hossein-nezhad, Spira and Holick 20 ). In another study, twenty-five adults were randomized to receive placebo or a single oral dose of cholecalciferol of 1250 µg (50 000 IU), 250 µg (100 000 IU) or 5000 µg (200 000 IU)( Reference Scott, Das and Ahsanuddin 21 ). Adults receiving 5000 µg were much more likely to have expression of many genes flipped from up- to down- or from down- to up-regulated. The results of gene expression flipping were grouped into two clusters for skin barrier repair genes. Those with increased expression of the skin barrier repair genes had a mean 25(OH)D concentration of ~106 nmol/l, while those with no increase in skin barrier repair gene expression had a mean 25(OH)D concentration of ~75 nmol/l.
A third possible mechanism is described in two papers that identified 25(OH)D-dependent effects of APOA5 polymorphisms on circulating HDL-C( Reference Shirts, Howard and Hasstedt 22 , Reference Vimaleswaran, Cavadino and Hypponen 23 ). Shirts et al.( Reference Shirts, Howard and Hasstedt 22 ) described a 25(OH)D receptor binding site-modifying APOA5-promoter polymorphism associated with lower HDL-C in 25(OH)D-deficient individuals( Reference Shirts, Howard and Hasstedt 22 ). The other identified a borderline interaction between the SNP rs12272004 (near the APOA5) and serum 25(OH)D on HDL-C (P for interaction=0·05)( Reference Vimaleswaran, Cavadino and Hypponen 23 ).
A fourth line of evidence supporting vitamin D’s role in the observed changes in cardiometabolic risk factors concerns the widespread seasonal variations in gene expression that have been shown, including genes recognized as biomarkers of increased risks of CVD( Reference Dopico, Evangelou and Ferreira 24 ). While Dopico et al.’s study( Reference Dopico, Evangelou and Ferreira 24 ) did not identify changes in 25(OH)D concentration as the driver, based on the preceding discussion, it seems likely that it is a contributor.
Studies of the seasonality of disease and mortality rates find that rates are often higher in winter than in summer. For example, death rates in the USA are 25 % higher in winter than in summer, with CVD being a major contributor to that seasonal variation( Reference Grant, Bhattoa and Boucher 25 ), suggesting that keeping serum 25(OH)D concentration >90 nmol/l year-round may reduce death rates significantly. This estimate was supported by calculations based on increasing 25(OH)D concentration from 54 to 110 nmol/l globally( Reference Grant 26 ), as well as a meta-analysis of mortality rates found in relationship to serum 25(OH)D concentrations in thirty-two independent studies( Reference Garland, Kim and Mohr 27 ). Thus, the study by Nikooyeh et al.( Reference Nikooyeh, Abdollahi and Hajifaraji 1 ) provides further support for beneficial effects of seasonal changes in available UVB dose, and in 25(OH)D concentration, that may have a role in reducing mortality rates.
Clinical trials conducted by 2017 had not shown that vitamin D supplementation reduces the risks of CVD( Reference Veloudi, Jones and Sharman 28 ) but this finding fails to question the appropriateness of the design of the clinical trials. Most vitamin D clinical trials have been based on the assumptions implicit in trials of pharmaceutical drugs, namely that the only source of the agent is that provided in the trial and that there is a linear dose–response relationship; however, neither assumption is satisfied for vitamin D. The primary determinant of health outcomes in clinical trials with high doses of vitamin D is the achieved 25(OH)D concentration, not the vitamin D dose used. A recent clinical trial supplementing with high-dose cholecalciferol supports this concept that clinical trial efficacy should not be based on the success of the dose used to alter disease biomarkers, but on the improved change in 25(OH)D concentration associated with improved disease risk( Reference Lappe, Watson and Travers-Gustafson 29 , Reference Grant and Boucher 30 ). A recent paper outlined how to base clinical trials of vitamin D on measurements of 25(OH)D concentrations( Reference Grant, Boucher and Bhattoa 31 ). However, results of two clinical trials reported after those papers had been published have shown reductions in biological risk factors for CVD. In one, hypertensive patients who increased cholecalciferol doses from ~50 to ~150 µg/d (~2000 to ~6000 IU/d), with increases in their 25(OH)D concentrations from ~82 to 110 nmol/l, did show reductions in systolic blood pressure by ~16 mmHg and in diastolic blood pressure by ~12 mmHg( Reference Mirhosseini, Vatanparast and Kimball 15 ), while blood pressure changes in normotensive subjects were not significant. In the second trial, seventy overweight African Americans (aged 13–45 years) with serum 25(OH)D concentrations <50 nmol/l were given monthly cholecalciferol doses equivalent to 15, 50 or 100 µg/d (600, 2000 or 4000 IU/d) v. placebo( Reference Raed, Bhagatwala and Zhu 32 ). The beneficial change in carotid–femoral pulse wave velocity was significantly greater for 100 µg/d (−10·4 %; 25(OH)D=33 nmol/l at baseline, 89 nmol/l at 8 weeks, 87 nmol/l at 16 weeks) than for 50 µg/d (−2·0 %; 25(OH)D=40 nmol/l at baseline, 76 nmol/l at 8 weeks, 90 nmol/l at 16 weeks); as was the change in carotid–radial pulse wave velocity at −8·0 % after 100 µg/d v. +1·2 % after 15 µg/d (25(OH)D=35 nmol/l at baseline, 53 nmol/l at 8 weeks, 57 nmol/l at 16 weeks). While these two clinical trials did not assess HDL-C concentrations, they did show that vitamin D supplementation at high enough doses can reduce biological risk markers for CVD in those at increased risk; furthermore, these two trials also demonstrate that it is not always necessary to enrol people with deficiency in order to be able to find significant beneficial effects of cholecalciferol supplementation if the cholecalciferol dose is high enough. In the vitamin D3 plus calcium trial for cancer, mean serum 25(OH)D concentration increased from 83 to 109 nmol/l in the treatment arm but decreased from 82 to 79 nmol/l in the control arm( Reference Lappe, Watson and Travers-Gustafson 29 ).
We are currently in the ‘golden age’ of vitamin D research with many exciting findings being reported each year on the non-skeletal effects of vitamin D. Given the difficulty of conducting vitamin D clinical trials, observational studies such as the one reported by Nikooyeh et al.( Reference Nikooyeh, Abdollahi and Hajifaraji 1 ) play very important roles in identifying and quantifying effects and paving the way for additional studies, as well as serving to raise awareness of the importance of sun exposure and cholecalciferol for human health.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: W.B.G. receives general research funding from Bio-Tech Pharmacal, Inc. (Fayetteville, AR, USA). Authorship: W.B.G. wrote the first draft of this commentary, which was revised by W.B.G. and B.J.B. Ethics of human subject participation: Not applicable.