It is well known that humans need a wide variety of foods to meet their essential nutrients’ requirements. For children, a well-balanced diet is of extreme importance because they need energy supply and nutrient-dense foods to develop physically and mentally(Reference Arimond and Ruel1). One common concern of parents is the quality of their children’s diet. In fact, many of them find it difficult to introduce healthy foods, especially fruits and vegetables, into their children’s diet during infancy(Reference Blissett, Bennett and Fogel2). One of the reasons is many children seem to dislike or refuse the food that is presented to them for the first time. The rejection of new or unfamiliar foods is termed food neophobia (FN)(Reference Birch3). It is a normal phase of the development and is not considered pathological(Reference Dovey, Staples and Gibson4). Some authors note that FN starts to show at about 18 months of age and it reaches its peak between 2 and 6 years. It then goes to decrease by late childhood and adolescence(Reference Pliner, Salvy, Shepherd and Raats5). A French study revealed that almost three-quarters of children aged between 2 and 10 years show a reluctance to try unknown foods(Reference Ayadi6). Many studies dated back FN to ancient times, when humans were exposed to large amount of toxins and the fear of trying novel foods was a way of protection against ingestion of these harmful substances(Reference Rozin, Millman and Nemeroff7).
Determinants of food neophobia
Sociodemographic characteristics
Residents of rural areas show higher levels of neophobia compared with urban residents(Reference Flight, Leppard and Cox8). Also, FN levels have been noticed to be related to the level of education of the parents. In fact, the more educated the parents, the less the child will be neophobic(Reference Meiselman, King and Gillette9). Also, studies have shown that when income increases, the levels of neophobia decrease(Reference Meiselman, King and Gillette9). It has been shown that the eating behaviours of others may influence the food choices of children(Reference Pliner and Salvy10). In other terms, neophobic behaviours can be reduced when the child observes others trying the same food.
Previous food experiences
Breastfed children have also shown more acceptance of vegetables and fruits at an older age(Reference Perrine, Galuska and Thompson11). The timing of the introduction of solid foods seems to be an important factor in determining later acceptance of fruits and vegetables. The best period to introduce solid foods for better acceptance correlates with the period recommended by the American Academy of Pediatrics (AAP) and by the WHO: between 4 and 6 months of age(Reference Longfier, Soussignan and Reissland12). The earlier a type of food is introduced (before 6 months of age), the easier it will be for the child to accept it(Reference Harris and Coulthard13).
Furthermore, many parents present with the complaint that their child is refusing to eat new food, and few are aware of the fact that the same food may need to be introduced about 10 to 15 times before the child starts accepting it(Reference De Cosmi, Scaglioni and Agostoni14).
Parental feeding styles
A parenting style is a broad term that describes the type of relationship between a child and his/her parents, the behaviours of the parents towards their children, and the emotional interaction between them. There are four types of parenting styles(Reference Shloim, Edelson and Martin15,Reference Garcia and Serra16) : (1) an authoritarian parent: a parent who is highly demanding of his/her child, with a lot of rules and little regard for the needs of his/her child; (2) an authoritative parent: a parent who is also highly demanding and sets rules but is also highly responsive to the needs of his/her child; (3) an indulgent parent: a parent who is highly responsive, less demanding, with no rules; and (4) a neglectful parent: a parent who is neither demanding nor responsive and doesn’t set rules nor listens to his/her child’s needs.
Moreover, the different feeding practices that the parents use are thought to be the manifestation of their parenting style during a specific aspect of their life: mealtimes.
Children who have parents that are highly responsive to their preferences showed greater consumption of vegetables compared with those of parents who were not(Reference Johnson17). Positive parental styles include but are not limited to the encouragement of the child while feeding, modelling of vegetable intake, well structuring the mealtime, monitoring food intake and using non-food rewards(Reference Johnson17,Reference Yuan, Rigal and Monnery-Patris18) . Authoritative feeding style is considered one of the best and is correlated with low levels of FN and a better home environment(Reference Johnson, Welk and Saint-Maurice19).
Caregivers that are uninvolved, strict or extremely permissive negatively affect a child’s food consumption and favour neophobic behaviours(Reference Johnson17,Reference Ventura and Worobey20) . Authoritarian parenting style has been linked with lower vegetable intake and more food rejection in children(Reference Rigal21,Reference Hughes, Power and Orlet Fisher22) . A common method used by parents is the rewarding system: they offer a reward that is usually a food that the child likes on the condition that the child will eat unwanted food(Reference Roberts, Marx and Musher-Eizenman23). Another common method is pressuring and forcing the child to finish his/her food before he/she can leave the table. Studies done on these practices have shown that although they might sometimes increase the consumption of the food immediately, they will end up increasing the rejection of the same food by the child in the long run(Reference Galloway, Fiorito and Francis24) .
Consequences of food neophobia
Although classified as normal, FN comes with many consequences, especially at higher levels. Research has shown that neophobic children consume a less variety of foods than non-neophobic ones(Reference Eertmans, Victoir and Vansant25). Also, FN increases the risk of chronic diseases. A study that was done on a Finish and Estonian population showed that adults who still showed traits of neophobia ate more food rich in salt and saturated fat, putting them at risk of developing CVD and type 2 diabetes(Reference Sarin, Taba and Fischer26). On another note, FN has been associated with stress and frustration during the meal as parents may begin to worry when faced with a child who refuses to eat the food presented to him/her. For this reason, mealtimes can become a cause of conflict for them(Reference Moret27).
Food neophobia v. avoidant/restrictive food intake disorder
Avoidant/restrictive food intake disorder (ARFID) is an eating disorder defined as the avoidance or restriction of foods that result in weight loss or failure to gain weight, deficiency in nutrients, dependency on supplementation and psychosocial changes. Unlike FN, ARFID tends to persist until adulthood(Reference Dovey and Reilly28). Also, FN is not characterised by weight loss or dependence on supplements. Also, there is an entity between FN and ARFID called picky-eating. A picky eater is a child who refuses to eat many familiar as well as unfamiliar foods. His/her diet is usually very selective and limited, as he/she refuses some food textures not just particular foods(Reference Dovey, Staples and Gibson4). A lot of researchers seem to believe that these three entities share a common aetiology and that a child with ARFID also manifests high levels of neophobia(Reference Dovey and Reilly28).
Measurements of FN have not been reported in Lebanon. A common scale used worldwide is the Child Food Neophobia Scale (CFNS), adapted from the Food Neophobia Scale (FNS) that was developed in 1992 by Pliner and Hobden(Reference Pliner and Hobden29). Since then, many countries have worked on validating and adapting this questionnaire to their population(Reference Laureati, Bergamaschi and Pagliarini30,Reference de Andrade Previato and Behrens31,Reference Zou, Liu and Yang32,Reference Rossbach, Foterek and Schmidt33) . Hence, it is important to validate the Arabic version of the CFNS to adapt it to our specific population. Since no studies have been done in Lebanon on FN, we aimed to validate the FNS and study the factors associated with FN in children in hopes of shedding the light on it and the ways to deal with it.
Methods
Sampling and data collection
In this cross-sectional study conducted between July and December 2019, 850 questionnaires were proportionately distributed across the Lebanese governorates. Children included were those of Lebanese nationality, those between 2 and 10 years of age, those who were born on term and those who lived with both parents. Children with documented food allergies and those who have neurodevelopmental abnormalities were not included. The total number of questionnaires collected was 850, but 194 of them were eliminated and did not make it to the data entry because of the exclusion criteria mentioned above.
Minimal sample size calculation
Using the Epi Info software (population survey), the minimal sample size needed to have enough statistical power was 544, taking a 77 % frequency of neophobia according to a previous study(Reference Moret27), a design effect of 2 and a risk of error of 5 %.
Questionnaire
The questionnaire was in Arabic. In the first part, questions concerned the sociodemographic characteristics of each individual: age, gender, BMI, area of living, number of rooms in the house and number of people living in the same house. This part also included information about the parents: their BMI, their level of education and their monthly income.
The second part of the questionnaire focused on the feeding patterns of the child since he/she was born up until this age: whether he/she was breastfed or not, whether he/she attended kindergarten or not, his/her food dislikes, the method and timing of introduction of solid foods, the place where he/she usually has his/her meals and if he/she sits with his/her family during mealtimes. Some of the questions included about food refusal were: if the child dislikes a food before tasting it, if he/she refuses a certain type of food (specified as vegetables, fruits, fish, meat, etc.), if he/she asks to try before tasting, if he/she refuses certain textures and if he/she is on any supplements. The third and final part asked questions about the feeding style adopted by the parents: whether they are controlling, too permissive, use rewards or encourage their child to eat. The questionnaire included two validated scales that served our study purpose (CFNS in the second part and Parental Feeding Style Questionnaire in the third part).
The child food neophobia scale
The FNS is a 10-item scale that determines the level of FN in adults. It was developed by Pliner and Hobden in 1992(Reference Pliner and Hobden29). In 1994, Pliner adapted this questionnaire to children and it was called the CFNS(Reference Pliner34). Parents of the children are meant to answer the questions. Each question is answered on a Likert scale: ranging from 1 (strongly agree) to 7 (strongly disagree). The minimal score is 10 and the highest is 70, and higher scores reflected higher levels of neophobia. The CFNS has often been used in the literature to measure the level of neophobia in children(Reference Rhee, Lumeng and Appugliese35) (α Cronbach = 0·739).
The parental feeding style questionnaire
Parental Feeding Style Questionnaire is a questionnaire that has a total of twenty-seven questions tackling four dimensions of the feeding styles adopted by parents: ‘instrumental feeding’, ‘control over eating’, ‘encouragement to eat’ and ‘emotional eating’. The questions regarding emotional eating were omitted in our questionnaire because these questions were not relevant to our dependent variable (FN). The Parental Feeding Style Questionnaire assesses the level of control of the parents regarding their children’s meals, the use of instrumental feeding such as offering foods as a reward and the level of encouragement that parents adopt to get their child to eat a refused food. The questions are answered on a 5-point Likert scale (ranging from never to always). Higher levels of each dimension indicate higher use of this method by parents. This specific scale for measuring parental feeding styles has been widely used in many research(Reference Jansen, Mallan and Nicholson36,Reference Özçetin, Yılmaz and Erkorkmaz37) (α Cronbach = 0·706).
Parental attitude score
Parental attitude score is a tool to evaluate the parent’s attitude towards a child’s novel food refusal. It was validated to measure different attitudes. It contains actions such as offering the same food multiple times to the child, presenting it with a food that he/she already likes, eating the food with the child at mealtimes and punishing the child when he/she refuses or showing signs of anger in front of the child. Answers were coded on a 5-point Likert scale ranging from 1 (never) to 5 (always). Higher levels mean higher use of the method by parents (α Cronbach = 0·843).
Forward and back-translation procedure
One healthcare professional translated from English to Arabic. This forward translation was then translated by a second healthcare professional back to Arabic. No major differences were found between the two English versions, with discrepancies resolved by consensus. A pilot test of the Arabic version was performed on twenty parents, before launching data collection. The pilot sample’s results were included in the final datasheet.
Statistical analyses
The statistical data analysis was conducted using the 23rd version of the SPSS software. Two different methods were used to confirm the FNS construct validity. First, factor analysis was run using the principal component analysis technique and run on sample 1 (n 328). Since the extracted factors were found not to be significantly correlated, the varimax rotation technique was used. To ensure the model’s adequacy, the Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy and Bartlett’s test of sphericity were calculated. Factors with an Eigenvalue >1 were retained. Moreover, Cronbach’s α was recorded for reliability analysis for the total scale and its subscales. Second, confirmatory factor analysis was carried out on sample 2 (n 328). To assess the structure of the instrument, the maximum likelihood method for discrepancy function was used. Several goodness-of-fit indicators were reported: relative chi-square (χ 2/df), root mean square error of approximation, goodness-of-fit index and the adjusted goodness-of-fit index. The index of goodness of fit was calculated by the value of χ 2 divided by the df (χ 2/df) (cut-off values <2–5). The root mean square error of approximation tests the fit of the model to the covariance matrix. As a guideline, values of <0·05 indicate a close fit and values below 0·11 an acceptable fit. The goodness of fit index and adjusted goodness-of-fit index are chi-square-based calculations independent of df. The recommended thresholds for acceptable values are ≥0·90(Reference Marsh, Hau and Wen38).
The sample did not have a normal distribution; non-parametric tests were used during the analysis. The Mann–Whitney test was used to compare two means, the Kruskal-Wallis test to compare three or more means, whereas the Spearman correlation test was used for the comparison of continuous variables. Finally, a stepwise linear regression was conducted taking the neophobia score as the dependent variable; independent variables were variables exhibiting a significant association with the neophobia score in the bivariate analysis. A P < 0·05 was considered significant.
Results
Sociodemographic and other characteristics
The mean age of the children was 5·34 ± 2·20 years (50·8 % females). The highest percentage of fathers (53·4 %) and mothers (52·9 %) had a university level of education. Other characteristics are summarised in Table 1. The mean neophobia score was 39·09 ± 8·32; the visual binning option in SPSS showed that 238 (36·4 %) had low neophobia (scores ≤37), whereas 219 (33·5 %) and 197 (30·1 %) had moderate (scores between 38 and 41) and severe (scores ≥42) neophobia, respectively.
Table 1 Sociodemographic and other characteristics of the participants (n 656)

Table 2 Factor analysis of the Food Neophobia Scale items
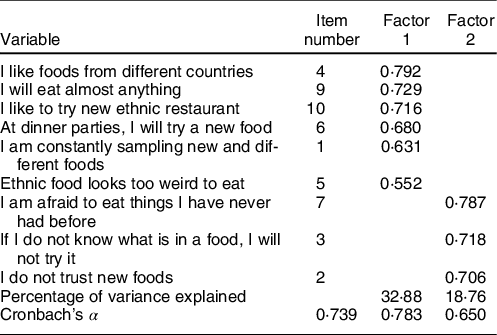
Factor 1: the absence of neophobia; Factor 2: the presence of neophobia.
Exploratory factor analysis
Sample 1 was used for the factor analysis; all items of the FNS scale were extracted except item 8 and yielded a two-factor solution with Eigenvalues > 1 (variance explained = 51·64 %; KMO = 0·746; Bartlett’s sphericity test P < 0·001; α Cronbach = 0·739) (Table 2).
Confirmatory factor analysis
A confirmatory factor analysis was run on sample 2, using the structure obtained in sample 1. The following results were obtained: the maximum likelihood chi-square = 209·95 and df = 57·21, which gave an x2/df = 3·67. For non-centrality fit indices, the Steiger-Lind root mean square error of approximation was on 0·143 (0·125, 0·162). Moreover, the Joreskog goodness-of-fit index equaled 0·876 and adjusted goodness-of-fit index equaled 0·894.
Bivariate analysis
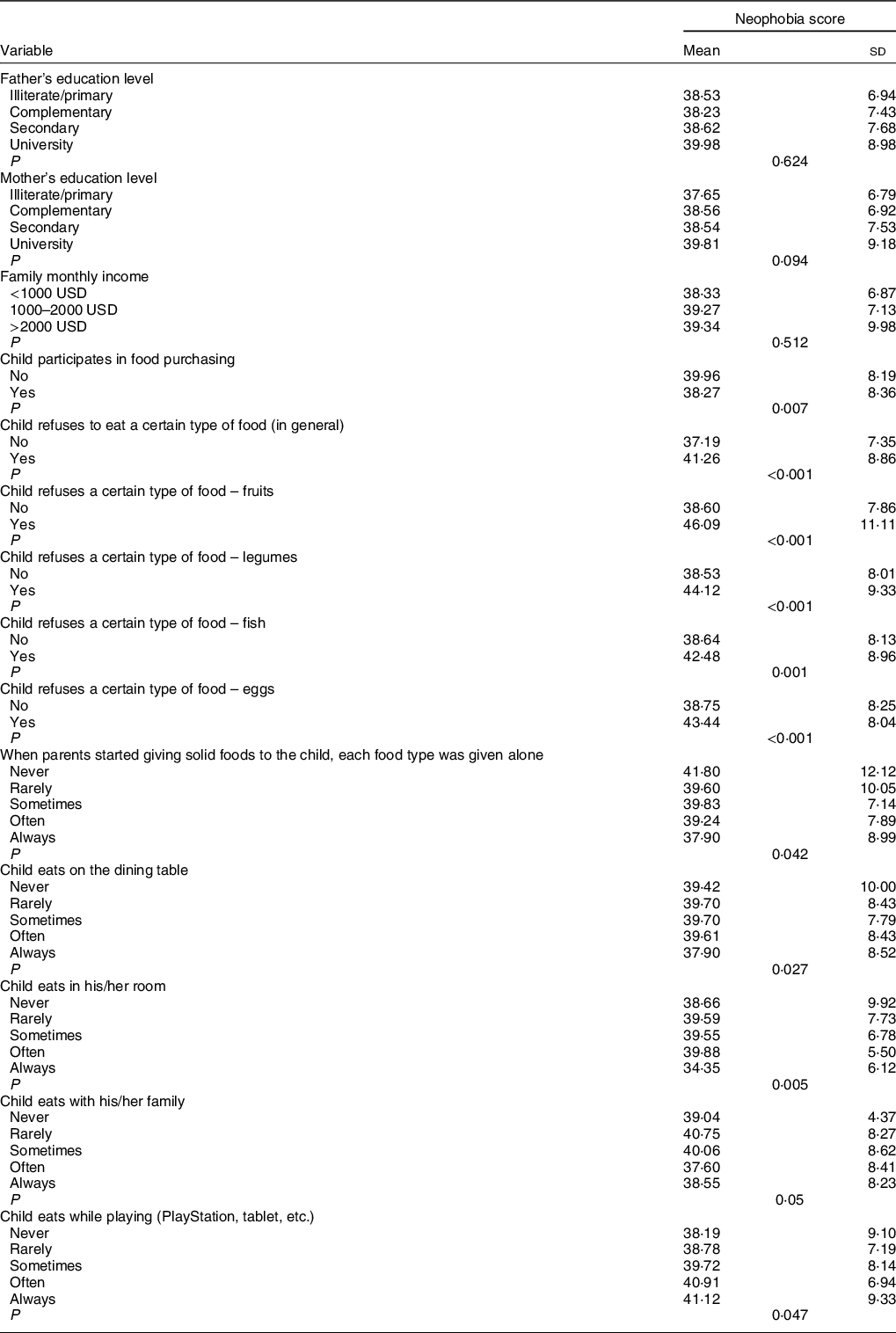
A significantly higher mean neophobia score was seen in children who do not participate in food purchasing compared with those who do (39·96 v. 38·27), in those who refuse to eat a certain type of food (in general), particularly fruits, legumes, fish and eggs. Furthermore, children whose parents have never introduced each type of solid food alone, those who eat on the dining table rarely and sometimes, those who often eat in their rooms, rarely with their families, and those who always eat while playing (PlayStation, tablet, etc.) had higher neophobia scores in children (Table 3).
Table 3 Bivariate analysis of factors associated with neophobia
Higher control scores (r = −0·117; P = 0·003), higher encouragement scores (r = −0·122; P = 0·002) and higher parents’ attitude scores (r = −0·118; P = 0·003) were significantly, but weakly, associated with lower neophobia, whereas higher instrumental feeding (r = 0·193; P < 0·001) was significantly, but weakly, associated with higher neophobia scores in children. No association was found between age and neophobia (r = −0·039; P = 0·324). All other variables did not show a significant difference with the neophobia score.
Multivariable analysis
The results of linear regression, taking the neophobia score as the dependent variable, showed that children who refuse to eat vegetables (β = 5·51), fish (β = 4·57), fruits (β = 4·75), and eggs (β = 2·99) and higher parents’ instrumental feeding scores (β = 0·3) were significantly associated with higher neophobia scores, whereas higher parents’ encouragement scores (β = −0·21) were significantly associated with lower neophobia scores in children (Table 4).
Table 4 Multivariable analysis: linear regression taking the neophobia score as the dependent variable

* Reference group.
Discussion
This study is the first of its kind in Lebanon that aims to validate the FNS and evaluate factors associated with FN. Overall, 63·6 % of the children had moderate to severe neophobia; those who refused to eat certain types of foods, specifically fruits, vegetables, fish and eggs and having parents who use rewards to get their children to eat had higher levels of FN. The encouraging attitude adopted by some parents was significantly associated with lower neophobia.
The factor structure obtained was satisfying, dividing the scale’s items into the absence and presence of neophobia, with the confirmatory analysis yielding satisfactory results as well. The Cronbach’s α value of the Arabic CFNS version was adequate (0·739), but lower than that of previous validated scale’s versions: German (0·79)(Reference Rossbach, Foterek and Schmidt33), Chinese (0·91)(Reference Zou, Liu and Yang32), Brazilian Portuguese (0·916)(Reference de Andrade Previato and Behrens31) and Italian (0·89)(Reference Laureati, Bergamaschi and Pagliarini30). Nevertheless, the Arabic version implies good reliability of the scale in the Lebanese population. Discrepancies related to Cronbach’s α values can be related to differences in the perception and patterns of neophobia among different cultures/populations(Reference Zou, Liu and Yang32). Furthermore, FN was shown to differ between urban and rural areas(Reference Flight, Leppard and Cox8), another possible explanation for this discrepancy.
Our results showed a percentage of 63·6 % of moderate to severe in our children, similar to previous studies. Some studies even noticed that neophobia is highly present until the age of 10 years, then it declines until adolescence or early adulthood(Reference Pliner, Salvy, Shepherd and Raats5). As mentioned earlier, one study showed that three-quarters of children aged between 2 and 10 years showed neophobic behaviours(Reference Ayadi6). Another study done in France revealed 77 % of neophobic children within this age range(Reference Moret27). The high prevalence of neophobia at this age could be due to the fact that children tend to show assertive behaviours and try to establish independence from their parents. Therefore, refusing certain foods is a way to affirm their authority and presence. Another reason for this higher number may be because older children are subject to peer and familial influences making them more prone to accept new foods(Reference Pliner and Salvy10). Also, they are more experienced with food and will not find a lot of novel foods to reject(Reference Dovey and Reilly28). Furthermore, as they grow older, children begin to develop an idea of the importance of eating and having a diversified diet(Reference Birch and Fisher39).
Children who had lower variety in their diets, particularly those who ate fewer fruits (β = 4·75), vegetables (β = 5·51), fish (β = 4·57) and eggs (β = 2·99), showed more neophobic behaviours than those who ate these types of food. These results support the findings of previous research(Reference Larson and Story40,Reference Ball, Timperio and Crawford41,Reference Guzek, Głąbska and Mellová42) . Many studies talked about the association between less consumption of fruits and vegetables and the presence of neophobia in children(Reference Larson and Story40,Reference Ball, Timperio and Crawford41,Reference Guzek, Głąbska and Mellová42) . When studying the diet of neophobic children, literature shed the light on the consumption of fruits and vegetables specifically. Not many talked about the attitude towards fish and eggs. These results go with the fact that children dislike bitter-tasting foods and favour sweet and energy-dense foods(Reference Cowart43). Furthermore, some children are genetically determined to dislike bitter foods, which may contribute to the presence of neophobic behaviours(Reference Mennella, Pepino and Reed44). Since FN is common, our results that associate neophobic children with lower consumption of vegetables, fruits, eggs and fish shed the light on the importance of managing neophobic behaviours to help diversify the diet of children. Furthermore, inadequate nutrition and diversification may be risk factors for the development of ARFID.
Additionally, our results showed that parents who used rewards to get their children to eat food, such as promising them a treat or something they like, had children with higher neophobia levels. This is consistent with findings from previous studies(Reference Roberts, Marx and Musher-Eizenman23,Reference Galloway, Fiorito and Francis24) done on this parental feeding style. Using rewards has been linked with the presence of neophobia(Reference Roberts, Marx and Musher-Eizenman23,Reference Galloway, Fiorito and Francis24) . This might be explained by the fact that when a certain food is promised as a reward for eating an undesired food, this may reinforce the child’s negative perception of the non-preferred food and may lead to the child not being willing to eat it in the absence of rewards in the future. Parents may think that this method will make the child consume the refused food because they only see the immediate effect. In the long run, the child will link the food he/she is forced to eat with a bad experience or a punishment, which will further enhance his dislike towards this food(Reference Lobos and Januszewicz45).
At last, the results showed a negative association between parents who encourage their children to eat the refused food and the level of neophobia; the more encouraging the parents are, the less the children have neophobic behaviours. Reaffirming previous studies, parents who encourage their children to consume or enjoy the mealtime and to try different tastes will not have to deal with neophobia as much as parents who pressure the child to eat his/her meal(Reference Johnson17,Reference Yuan, Rigal and Monnery-Patris18) . This might be because linking mealtimes to positive experiences discourage neophobia. Mealtimes will not be seen as a punishment, rather a time of bonding between the child and his/her parents. Our results highlight the importance of educating the parents on ways to handle the neophobic behaviours of their children.
Clinical implications
FN is common in children, and it concerns the majority of them aged between 2 and 10 years. It is a normal phase of the development of the child, and parents should be aware of it to deal with it the right way. Furthermore, paediatricians should advise the parents during their visits to the best ways to deal with a child who refuses to eat certain types of food. They could advise parents to encourage their children during mealtimes and avoid pressuring them or offering them rewards.
Limitations
There are some limitations to our research. Since this is a cross-sectional study, it is difficult to evaluate causal relationships. Also, our study type is subject to some biases. Recall bias could have occurred since parents may have incomplete recollections regarding their children’s past. Parents could also under- or overestimate a question leading to a non-differential information bias. Furthermore, some factors such as genetic predisposition and the personality of the child could have acted as confounders since they were not measured but were found in previous studies to affect eating behaviours. Also, the Arabic versions of the questionnaires have not yet been independently validated, but our results point to their having convergent validity. Overall, our results are compatible with the majority of the literature findings. Given the methodology used during the data collection, we believe that our results can be generalised to the whole population.
Conclusion
FN, or the avoidance of new foods, is a common period in the development of the child. Our results are consistent with those in the literature and prove that neophobia is an important issue that parents have to deal with daily. The parent must know the right methods to use to help the child get familiarised with the food. The use of encouraging words and attitudes was found to be associated with low levels of food avoidance in children. In contrast, children who were offered food as a reward to get them to eat showed more signs of avoidance. Using the results of this study in our daily lives, we can help parents worry less about this normal behaviour and offer them ways on how to handle it. Many studies should be conducted in the future to understand the level of awareness of Lebanese parents and paediatricians on FN and to see if using more encouragement and less instrumental feeding would be an effective intervention.
Acknowledgements
Acknowledgements: The authors would like to thank all parents for their help in the data collection. Financial support: This study was funded by the Lebanese University. Conflict of interest: The authors disclose no conflicts of interest. Authorship: M.C.F.K. conceived and designed the study. D.M., R.E.M. and M.A. performed the data collection and entry. S.H. involved to data interpretation and statistical analysis. R.E.M. wrote the manuscript. All authors critically revised the manuscript for intellectual content. All authors read and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Holy Spirit University ethics committee. Written informed consent was obtained from all children’s parents.
Availability of data and materials
There is no public access to all data generated or analysed during this study to preserve the privacy of the identities of the individuals. The dataset that supports the conclusions is available to the corresponding author upon request.







