Adequate nutrition during infancy and early childhood is essential to ensure growth, health and development of children to reach their full potential. Breast milk strongly contributes to good health and nutrition of infants. Apart from being an excellent nutritional source for the growing child, breast milk is associated with a reduced risk of many diseases in infants and mothers. A recent review by Duijts et al.(Reference Duijts, Ramadhani and Moll1) points out that a number of studies in industrialised countries suggest that breast-feeding protects infants against overall infections, gastrointestinal and respiratory tract infections. Moreover, Ip et al.(Reference Ip, Chung and Raman2) reported long-term benefits of breast-feeding for infants to be reduced risk of obesity and type 2 diabetes in later life, and long-term benefits for breast-feeding mothers to be reduced risk of breast and ovarian cancers.
The initiation and duration of exclusive breast-feeding and breast-feeding are influenced by a number of factors. Although the factors that influence the initiation and duration of breast-feeding in developed countries have been broadly studied(Reference Dennis3–Reference Thulier and Mercer5), previous studies in these countries have rarely examined the factors associated with exclusive breast-feeding. Breast-feeding and exclusive breast-feeding are, in general, reported to be associated with maternal age, marital status, parental educational level and smoking(Reference Lande, Andersen and Baerug6–Reference Michaelsen, Larsen and Thomsen8). In addition, breast-feeding duration is found to be associated with a wide range of other factors like social status, insufficient milk supply, parity, maternal work situation, infant health problems as well as health service-related factors(Reference Thulier and Mercer5, Reference Ludvigsson and Ludvigsson7).
Since 2001, the WHO(9) has recommended exclusive breast-feeding for the first 6 months of life. Exclusive breast-feeding is defined as feeding the infant only breast milk without any additional food or drink(10). Norwegian health authorities also recommend exclusive breast-feeding for the first 6 months of life and thereafter gradual introduction of appropriate complementary foods with continued breast-feeding(11). Norway has an extensive and positive breast-feeding tradition(Reference Liestol, Rosenberg and Walloe12) and long paid maternal leave, which support the possibility of breast-feeding during the first year of life. The parental benefit period is either 46 weeks at 100 % benefit or 56 weeks at 80 % benefit(13).
Based on the data from a large national dietary survey on infant feeding, we have explored factors associated with exclusive breast-feeding and breast-feeding during the first year of life in Norway.
Methods
Subjects and design
A nation-wide sample of about 3000 Norwegian infants was established by Statistics Norway. The sample included all infants born in Norway during a 3-week period from 17 April to 8 May in 2006. We assumed that the diet of infants born in April–May was similar to the diet of infants born at other times of the year. The mothers should be born in Norway, Sweden or Denmark. If the child was a twin or a triplet, the parents were asked only to include the oldest. The study had a longitudinal design and was carried out in October–November 2006 and April–May 2007, when the infants were 6 and 12 months of age, respectively. At 6 months, 1986 (67 %) mothers/infants participated. Those who gave a written refusal to participate at 6 months were not invited to participate at 12 months. At 12 months, 1635 (57 %) mothers/infants participated. In the present study, data from 1490 mothers/infants who participated at both 6 and 12 months of age were used for analysis, resulting in a response rate of 52 %.
The Regional Committees for Medical Research Ethics approved the study, and informed consent was obtained from the mother/parents.
The mothers received an invitation and a semi-quantitative FFQ (SFFQ) by mail about 2 weeks before the child turned 6 and 12 months of age. To obtain data on the infant’s weight and length, parents were asked to bring the questionnaire to the regular 6- and 12-month check-up at the child’s health clinic, and then return the completed questionnaire in a pre-paid envelope. At 6 months, one combined thanks/reminder letter and one reminder letter with the questionnaire enclosed were sent out. At 12 months, mothers were contacted once by telephone and received one reminder with the questionnaire enclosed. At 6 and 12 months, those who returned a completed questionnaire were entered in a lottery of ten cheques of approximately US$800 each and ten cheques of approximately US$1600 each.
The semi-quantitative FFQ
Two SFFQ were designed to describe feeding practices at 6 and 12 months of age, respectively, and also to retrospectively describe feeding practices from birth up to the given age. They were based on the SFFQ used in the first Norwegian national dietary survey among infants in 1998–1999(Reference Lande14, Reference Lande and Andersen15), and the SFFQ used among 12-month-olds had been validated(Reference Andersen, Lande and Arsky16). Parents were asked to complete the questionnaires on the day as closely to the child’s 6 months or 12 months of age as possible and to describe habitual feeding practices at the given age. Both SFFQ were tested in pilot studies and then revised.
The final SFFQ included forty-two questions at 6 months and fifty-one questions at 12 months. The questions on breast-feeding were related to whether or not the child received breast milk and to the breast milk frequency. Breast milk intake was not quantified. It was also asked when the child stopped receiving breast milk, when it started receiving infant formula/other milk and when the child was introduced to solid or semi-solid foods for the first time. The questions on complementary foods at 6 and 12 months covered approximately fifty and 160 different food items, respectively.
Both SFFQ also provided information on parental educational levels, maternal age, maternal work situation, maternal marital status, maternal smoking and number of children/parity, asthma/allergy in the family, infant gender, infant birth weight, gestational age and day care (only at 12 months).
Classification of breast-feeding
Based on the WHO definitions on breast-feeding(10), breast-fed infants were categorised into exclusively breast-fed and breast-fed. Exclusively breast-fed infants at a given age received only breast milk and had not been introduced to any additional food or drink, not even water, but could have received vitamin–mineral supplements. Breast-fed infants included all infants who received breast milk; both those exclusively breast-fed and those who had been introduced to other drinks than breast milk and/or complementary foods.
Data analysis
Multiple logistic regression analysis was applied to study exclusive breast-feeding at every month up to 5·5 months of age and breast-feeding at every month up to 12 months of age, in relation to selected parental and infant characteristics. Furthermore, factors associated with the introduction of solid foods were also studied using multiple logistic regression analysis. Multiple logistic regression analysis was applied to those who had information on all maternal/infant characteristics. Results are presented as adjusted OR with 95 % CI. Potential interaction effects were assessed. Statistical significance was tested by the likelihood ratio test. Tests for trends across categories were performed by treating the categories as continuous variables in logistic regression analysis. Maternal and paternal educational levels were coded by eight categories and combined into four categories in the logistic regression analysis: primary and secondary schools, comprehensive school, academy/college/university of ≤4 years and academy/college/university of >4 years. Maternal age, reported as a continuous variable, was categorised into three groups: ≤24, 25–34 and ≥35 years. Maternal smoking status in pregnancy was coded as yes, yes but quitted and no. Maternal smoking status when the infant was at 6 months old was coded as yes and no. Maternal marital status was coded as married, cohabitant and not married/cohabitant. Ten categories of maternal work situation before childbirth were combined into four categories: full-time, part-time, student and other (including mothers working at home/housewives, mothers on sick leave, unemployed, disabled, on rehabilitation, etc.). Ten categories of maternal work situation when the infant was 12 months were combined into four categories: full-time, part-time, maternity leave and other. Four categories of the number of children/parity were categorised into three groups: one child, two children and three or more children. Five categories of day care at 12 months by others, than parents, were categorised as no (day care by the mother and/or the father) and yes (day care by childminder, kindergarten or grandparents/other care persons). Geographic region was combined into six categories: Capital and surroundings, East, South, West, Middle and North regions. Infant birth weight, reported as a continuous variable, was categorised as >3500, 2500–3500 and <2500 g. Asthma/allergy in the family was categorised as yes (the infant’s mother, father and/or sibling(s) have or have had asthma/allergy) and no.
With regard to analysis of exclusive breast-feeding at 5·5 months of age, there was a need of collapsing categories for maternal education and maternal age to avoid small subgroups.
We used both results from the univariate analyses (with a criterion of P < 0·10) and evidence from the literature to decide which variables should be examined in the multivariate analyses. In the final models, significant variables (P < 0·05) were included. However, regardless of the statistical significance level, we decided to include maternal age and maternal education in all the final models. All P values are two-sided, and a 5 % level of significance was used. All statistical analyses were performed with the Statistical Package for Social Sciences statistical software package version 16·0 (SPSS Inc., Chicago, IL, USA).
The results of the analyses of exclusive breast-feeding at 4 and 5·5 months of age, of breast-feeding at 6 and 12 months of age and the introduction of solid foods before 4 months of age are presented. These ages were chosen to study adherence to the recommendations on infant feeding and gave a possibility of comparison with earlier national data from Norway(Reference Lande, Andersen and Baerug6).
Results
Table 1 presents selected characteristics of the infants and their parents. Of the 1490 mothers/infants who participated at both 6 and 12 months of age, 93 % of the mothers completed the questionnaires.
Table 1 Characteristics of infants and their parents (n 1490)

†Percentages for categorical variables, and means with sd for continuous variables.
‡When the infant was at the age of 6 months.
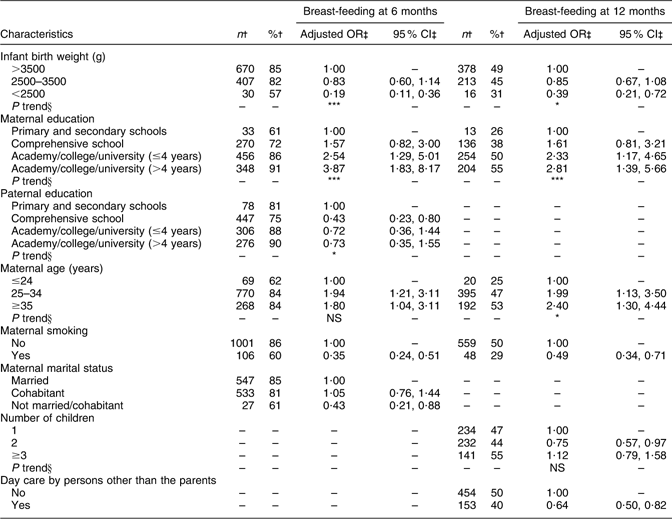
Ninety-two per cent of the infants were exclusively breast-fed at 1 week of age. The proportion of exclusively breast-fed infants was 84 % at 1 month of age, 65 % at 3 months and decreased to 48 % at 4 months of age and further down to 13 % at 5·5 months of age (Fig. 1). Only 1·5 % of the infants had never been breast-fed. The breast-feeding level slowly decreased from 96 % at 1 month of age, to 82 % at 6 months of age and to 46 % at 12 months of age (Fig. 1).
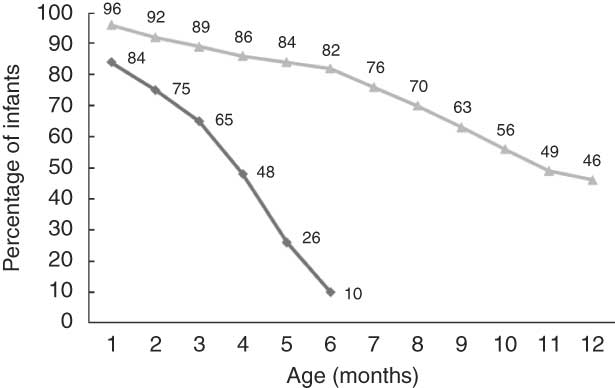
Fig. 1 Exclusive breast-feeding (![]() ) during the first 6 months of life and breast-feeding (
) during the first 6 months of life and breast-feeding (![]() ) during the first year of life (n 1490)
) during the first year of life (n 1490)
Among those who ceased breast-feeding before 6 months, the three most important reasons reported were insufficient milk (40 %), the infant did not want to have breast milk (17 %) and sucking problems (10 %). Between 6 and 12 months of age, the three most important reasons reported for breast-feeding cessation were that the infant did not want to have breast milk (37 %), insufficient milk (20 %) and no specific problems, but did not want to breast-feed any longer (10 %).
Factors associated with exclusive breast-feeding
Maternal and paternal education, number of children, geographical region and maternal smoking were significantly associated with exclusive breast-feeding at 4 months of age (Table 2). Significant positive trends were found for maternal education and number of children. Maternal age was significantly associated with exclusive breast-feeding at 5·5 months of age (Table 3) and a significant positive trend was observed. At both 4 and 5·5 months of age the odds of exclusive breast-feeding were significantly (P < 0·01) lower for smoking mothers compared with non-smoking mothers.
Table 2 Adjusted OR of exclusive breast-feeding at 4 months of age
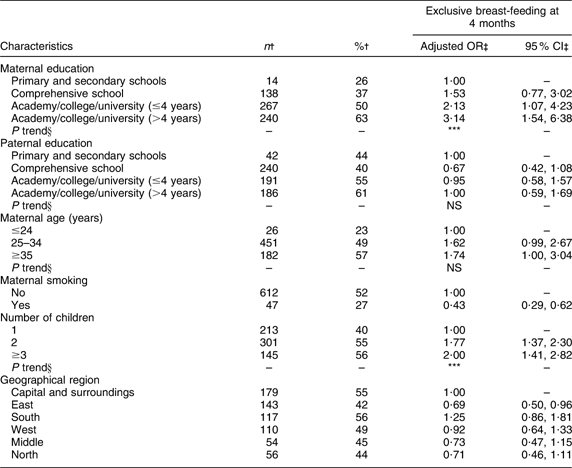
†Number and percentage of exclusive breast-fed infants within current independent variable. Total number of infants at 4 months of age (n 1343).
‡OR and 95 % CI are adjusted for all other variables in the table.
§Test for linear trend: NS; *P < 0·05, **P < 0·01, ***P < 0·001.
Table 3 Adjusted OR of exclusive breast-feeding at 5·5 months of age
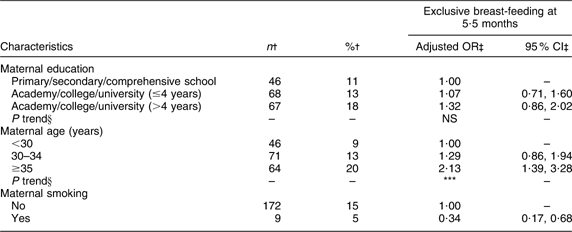
†Number and percentage of exclusive breast-fed infants within current independent variable. Total number of infants at 5·5 months of age (n 1343).
‡OR and 95 % CI are adjusted for all other variables in the table.
§Test for linear trend: NS; *P < 0·05, **P < 0·01, ***P < 0·001.
In addition, multivariate regression analyses were performed for exclusive breast-feeding at ages 1, 2, 3 and 5 months and the results are summarised in Table 4. Maternal education turned out to be the most stable variable as it was significantly associated with exclusive breast-feeding at most ages. From 3 months of age, maternal smoking was significantly associated with exclusive breast-feeding.
Table 4 Factors associated with exclusive breast-feeding during the first 6 months of lifeFootnote †

† Total number of infants (n 1343), with x indicating adjusted significant associations (P < 0·05).
Factors associated with breast-feeding
Maternal and paternal education, maternal age and marital status were significantly associated with breast-feeding at 6 months (Table 5). Breast-feeding at 12 months was significantly associated with maternal education, maternal age and number of children (Table 5). At both ages, significant negative associations with breast-feeding were observed for maternal smoking and for descending infant birth weight, and at 12 months a negative association was also observed for having day care by other than the parents.
Table 5 Adjusted OR of breast-feeding at 6 and 12 months of age
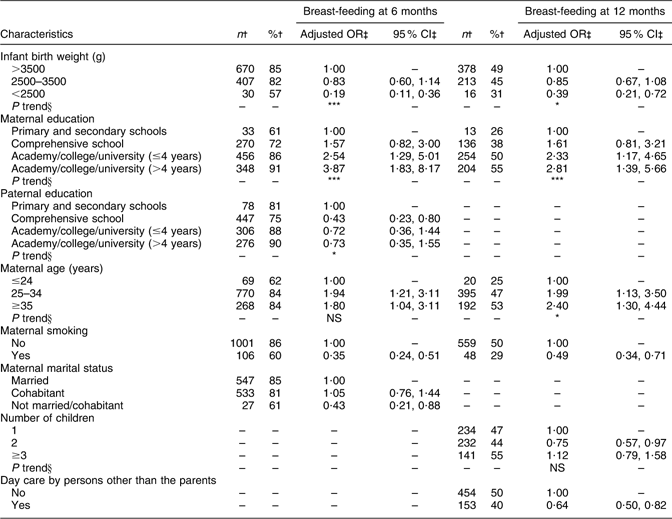
†Number and percentage of breast-fed infants within the current independent variable. Total number of infants at 6 (n 1343) and at 12 (n 1285) months of age.
‡OR and 95 % CI are adjusted for all other variables in the table, with the exception of day care by persons other than the parents at 6 months of age.
§Test for linear trend: NS; *P < 0·05, **P < 0·01, ***P < 0·001.
In addition, multivariate regression analyses were performed for breast-feeding at every month from 1 to 5 months of age and from 7 to 11 months of age and the results are summarised in Table 6. Maternal education turned out to be the most stable variable as it was significantly associated with breast-feeding at all ages. Maternal age, maternal smoking, paternal education and infant birth weight also turned out to be stable variables as they showed significant associations with breast-feeding at most ages.
Table 6 Factors associated with breast-feeding during the first year of lifeFootnote *

* Total number of infants from 1 to 6 months of age (n 1343) and from 7 to 12 months of age (n 1285), with x indicating adjusted significant associations (P < 0·05).
Factors associated with introduction of solid foods
Ten per cent of the infants were introduced to solid foods before 4 months of age (i.e. at 3·5 months of age or earlier). In this group, the most common first food was porridge made of maize/rice/millet (63 % had consumed this), while 25 % had consumed porridge made of oat/wheat/barley and 24 % had consumed fruits/berries before the age of 4 months. Only a few had been introduced to vegetables, meat or yoghurt before 4 months of age.
A significant linear trend of increasing odds of not introducing solid foods before 4 months of age was found with increasing maternal education (Table 7). Girls had significantly higher odds of not being introduced to solid foods before 4 months of age than boys, and significant associations were also found for number of children and geographical region. The odds of not introducing solid foods before 4 months of age was significantly lower (P < 0·001) for smoking mothers compared with non-smoking mothers.
Table 7 Adjusted OR of not receiving solid foods before 4 months of age
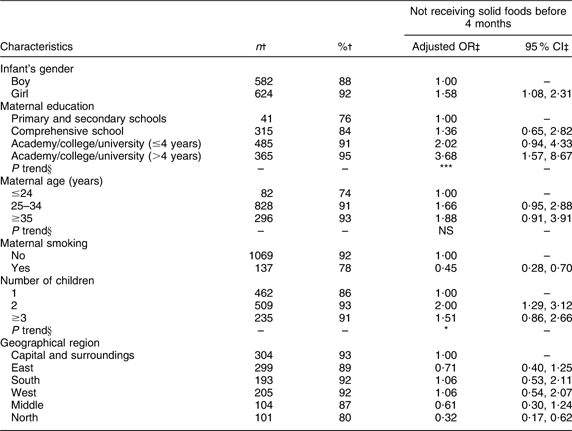
†Number and percentage of infants not receiving solid foods before 4 months of age within current independent variable. Total number of infants (n 1343).
‡OR and 95 % CI are adjusted for all other variables in the table.
§Test for linear trend: NS; *P < 0·05, **P < 0·01, ***P < 0·001.
Discussion
Based on the data from a large national infant dietary survey, factors associated with exclusive breast-feeding and breast-feeding during the first year of life among Norwegian infants were explored. We found that exclusive breast-feeding and breast-feeding in early infant age overall were high. In multivariate regression analyses, exclusive breast-feeding was significantly associated with maternal education, while breast-feeding was significantly associated with maternal education and maternal age. Both exclusive breast-feeding and breast-feeding were negatively associated with maternal smoking.
Exclusive breast-feeding and breast-feeding levels
More than 90 % of the infants in the present study were exclusively breast-fed at 1 week of age, and the level of exclusive breast-feeding was high during the first 3 months of life, but then declined to 10 % at 6 months of age. These data are consistent with earlier national data from Norway(Reference Lande14). National data from Sweden show the same level of exclusive breast-feeding at 1 week of age compared to our findings (88 %), while 15 % of the infants were exclusively breast-fed at 6 months of age(17). In the USA, national data show a low level of exclusive breast-feeding at 1 week of age (50 %), while 14 % of the infants were exclusively breast-fed at 6 months of age(18).
Only 1·5 % of the infants in our study had never been breast-fed. Breast-feeding level slowly decreased from 92 % at 2 months of age, to 82 % at 6 months and further down to 46 % at 12 months of age. A high level of breast-feeding initiation has also been reported in Sweden where 98 % of the mothers initiated breast-feeding(17). At 2 months of age, 90 % were breast-fed, while 69 % and 17 % of the infants were breast-fed at 6 and 12 months of age, respectively(17). The breast-feeding initiation reported from the USA was 74 % and at 2, 6 and 12 months of age the corresponding breast-feeding rates were 63 %, 43 % and 23 %, respectively(18).
Maternal and paternal education
A number of studies have observed that maternal education is significantly associated with exclusive breast-feeding and breast-feeding duration(Reference Scott and Binns4, Reference Thulier and Mercer5, Reference Ludvigsson and Ludvigsson7, Reference Michaelsen, Larsen and Thomsen8, Reference Susin, Giugliani and Kummer19–Reference Yngve and Sjostrom21). In our study, the odds of exclusive breast-feeding and breast-feeding increased significantly with increasing maternal educational level. We found a significant association between exclusive breast-feeding at 4 months of age and paternal education, but no significant association was found at 5·5 months of age. In the AIBS study (All Babies in Southeast Sweden), data on exclusive breast-feeding were available for more than 10 000 infants born between 1997 and 1999(Reference Ludvigsson and Ludvigsson7), and low paternal education was reported to be a risk factor for short exclusive breast-feeding (<4 months). Paternal education was found to be significantly associated with breast-feeding at every month from 2 to 9 months of age in the present study. In a study among 10 500 Californian women, Heck et al.(Reference Heck, Braveman and Cubbin22) showed positive associations between breast-feeding and paternal education, and Lanting et al.(Reference Lanting, Van Wouwe and Reijneveld20) reported that women in The Netherlands who initiated breast-feeding were more likely to have a higher-educated partner.
Maternal age
Maternal age is a powerful variable that has been associated with exclusive breast-feeding and breast-feeding initiation and duration(Reference Dennis3–Reference Thulier and Mercer5, Reference Ludvigsson and Ludvigsson7). In contrast to what others have reported(Reference Lande, Andersen and Baerug6, Reference Ludvigsson and Ludvigsson7), we did not find a significant association between exclusive breast-feeding and increasing maternal age at 4 months of age. However, at 5·5 months of age a significant association was observed. In the present study, multivariate analyses of breast-feeding during the first year of life showed that maternal age turned out to be a stable variable as it was significantly associated with breast-feeding at every month from 3 to 12 months of age.
Maternal smoking
In Sweden, Ludvigsson and Ludvigsson(Reference Ludvigsson and Ludvigsson7) reported smoking to be associated with increased risk of short exclusive breast-feeding (<4 months). In the previous national dietary survey among infants in Norway, Lande et al.(Reference Lande, Andersen and Baerug6) reported significantly lower odds of exclusive breast-feeding at 4 months of age for mothers who smoked compared with non-smoking mothers (adjusted OR = 0·40; 95 % CI 0·32, 0·50). This is similar to the results we observed for maternal smoking with regard to exclusive breast-feeding at 4 and 5·5 months of age.
In the present study, maternal smoking was an important adverse factor on the duration of breast-feeding. The odds of breast-feeding at both 6 and 12 months of age were lower for mothers who smoked compared with non-smoking mothers. For breast-feeding duration and maternal smoking, a consistent negative association has been reported in the literature(Reference Dennis3–Reference Thulier and Mercer5, Reference Horta, Kramer and Platt23).
In our analyses, maternal smoking turned out to be a stable variable as it was negatively associated with exclusive breast-feeding at every month from 3 to 5·5 months of age and with breast-feeding from 2 to 12 months of age.
Marital status
We found a significant association between marital status and breast-feeding at 6 months of age, but no such association was observed for breast-feeding at 12 months of age or for exclusive breast-feeding at 4 or 5·5 months of age. Some studies have shown that breast-feeding occurs more frequently among married women and that married women breast-feed for longer periods of time(Reference Thulier and Mercer5), while others have not found this association(Reference Scott and Binns4).
Birth weight
We found no significant association between infant birth weight and exclusive breast-feeding; however, the odds of breast-feeding decreased significantly with decreasing birth weight at both 6 and 12 months of age. The same was observed by Lande et al.(Reference Lande, Andersen and Baerug6).
Parity
In the literature, the association between parity and breast-feeding is inconsistent(Reference Scott and Binns4, Reference Thulier and Mercer5). In our study, the odds of exclusive breast-feeding at 4 months of age increased with increasing number of children. We did not observe a consistent pattern between exclusive breast-feeding or breast-feeding and parity.
Day care
Due to the Norwegian parental leave system, the majority of Norwegian infants are in parental care at the first year of life. At the age of 12 months, 70 % of the infants in our study were in parental care, while 30 % were taken care of by childminders, kindergartens, grandparents or other care persons. For breast-feeding at 12 months of age, we observed a significant negative association with non-parental day care and observed that infants who had non-parental care had lower odds of being breast-fed at this age. A recent study from the USA(Reference Kim and Peterson24), using a national representative sample of 8150 infants, reported that infants who attended day care before 3 months of age were less likely to ever have been breast-fed than those in parental care. In another national sample of 2500 mothers in the USA, Hendricks et al.(Reference Hendricks, Briefel and Novak25) observed that being in day care was associated with decreased duration of breast-feeding at ages 6 and 12 months.
Introduction of solid foods
Norwegian health authorities recommend that solid foods are introduced at 6 months of age, or at the earliest at 4 months of age(11). In the present study, 10 % had been introduced to solid foods before 4 months of age. National data from the USA(Reference Briefel, Reidy and Karwe26) show that about 29 % of all infants were introduced to solid foods before 4 months of age, while earlier national data from Norway(Reference Lande, Andersen and Baerug6) observed that 21 % of the infants received solid foods before the age of 4 months; the corresponding percentages reported from Sweden and Switzerland are 4 % and 5 %, respectively(Reference Brekke, Ludvigsson and van27, Reference Dratva, Merten and Ackermann-Liebrich28).
Consistent with earlier findings from Norway, we found that infants with mothers who were less educated were more likely to have been introduced to solid foods before 4 months of age and that the introduction of solid foods differed significantly among geographical regions(Reference Lande, Andersen and Baerug6). In the univariate analysis the odds of timely introduction of solid foods increased with increasing maternal age, but in contrast to earlier findings(Reference Lande, Andersen and Baerug6, Reference Hendricks, Briefel and Novak25, Reference Erkkola, Pigg and Virta-Autio29), this association was not significant in the multivariate analysis. Moreover, a significant association between the introduction of solid foods and parity was observed in the present study. Compared to associations reported in the literature, some have found an association(Reference Lande, Andersen and Baerug6, Reference Tatone-Tokuda, Dubois and Girard30), while others have not(Reference Erkkola, Pigg and Virta-Autio29, Reference Scott, Binns and Graham31). In our study, boys were more likely to be introduced to solid foods before 4 months of age than girls. Lande et al.(Reference Lande, Andersen and Baerug6) and Erkkola et al.(Reference Erkkola, Pigg and Virta-Autio29) reported similar results, while Scott et al. did not find such association(Reference Scott, Binns and Graham31). We found that mothers who smoked were more likely than non-smoking mothers to introduce solid foods early, and this is consistent with what others have reported(Reference Lande, Andersen and Baerug6, Reference Dratva, Merten and Ackermann-Liebrich28, Reference Scott, Binns and Graham31).
In the present study, we used national representative data from 1490 mothers/infants who participated in a prospective cohort study in Norway. The response rate was 52 %. When comparing the responders with available data on all Norwegian births in 2006, there were no indications of differences regarding an infant’s gender, gestational age, number of children, geographical region or maternal marital status. Small differences, which were considered unimportant, were seen for maternal age and infant birth weight, e.g. the average maternal age in our cohort was 1 year higher than the average age of all mothers giving birth that year. The only information we had on the non-responders was geographical region, and this did not differ from the responders.
Data on exclusive breast-feeding and breast-feeding practices were collected retrospectively, but within a maximum of 6 months after cessation. Li et al.(Reference Li, Scanlon and Serdula32) concluded in their review that mothers seems to provide accurate estimates of initiation and duration of any breast-feeding, especially when the duration is recalled over a period of 3 years or less. The validity and reliability of maternal recall for the age at introduction of foods and fluids other than breast milk seems to be less satisfactory(Reference Li, Scanlon and Serdula32). We used several questions to assess exclusive breast-feeding, breast-feeding and introduction of solid foods, but we cannot fully exclude recall bias.
For exclusive breast-feeding at 4 months of age, we observed a tendency towards stronger effects of maternal age on the number of children in the lowest age groups compared with those in the highest age groups. For breast-feeding at 6 months of age we observed a tendency towards stronger effects of maternal age on infant birth weight among mothers in the age group 25–34 years compared with the two other age groups. For breast-feeding at 6 months of age we also observed a tendency towards stronger effects of maternal smoking among mothers in the lowest educational groups compared with the higher educational groups (data not shown). These interactions need to be investigated further.
Conclusion
Even though Norway has an extensive and positive breast-feeding tradition and a maternal leave system which support the possibility to breast-feed, factors like maternal education, maternal age and maternal smoking are strongly associated with duration of exclusive breast-feeding and breast-feeding. Research to better understand the reasons for inequalities in breast-feeding is needed to facilitate the development of more effective breast-feeding promotion strategies. This, again, may improve compliance with recommendations and reduce inequalities in infant feeding practices.
Acknowledgements
The present study was generously supported by EXTRA funds from the Norwegian Foundation for Health and Rehabilitation. The authors declare no conflict of interest. A.L.K. carried out the data analyses and wrote the manuscript; B.L., N.C.Ø. and L.F.A. assisted and provided advice during all the stages of the work. All authors contributed in the discussion and interpretation of the results, and in the drafting and editing of the manuscript. The authors are grateful to all children and their parents who participated in the national dietary survey ‘Spedkost 2006–2007’. They thank all health professionals who assisted the parents in getting data on the infants’ weight and height, and also thank Tron Anders Moger who provided advice for the statistical analyses.