Hypertension is the most common medical problem in pregnancy, occurring in up to 15 % of all pregnancies( Reference James and Nelson-Piercy 1 ). Pre-eclampsia is a severe pregnancy-related hypertensive syndrome characterized by hypertension and proteinuria( 2 ). Pre-eclampsia develops from mid-pregnancy and affects 3–5 % of all pregnancies. The incidence rate is 1·5- to 2·0-fold higher among first-time pregnancies compared with subsequent pregnancies( Reference Mol, Roberts and Thangaratinam 3 – Reference Skjaerven, Wilcox and Lie 5 ). On rare occasions pre-eclampsia can develop into eclampsia or haemolysis, elevated liver enzymes, low platelets (HELLP) syndrome, and other severe complications( 2 , Reference Tranquilli, Dekker and Magee 6 ). Pre-eclampsia is associated with increased morbidity and mortality in both the pregnant women and the offspring, with long-term negative health consequences such as CVD and diabetes( Reference Mol, Roberts and Thangaratinam 3 , Reference Chen, Jaffe and Karumanchi 7 – Reference Alsnes, Janszky and Forman 11 ).
The aetiology and pathophysiology of pre-eclampsia have not been fully elucidated. Abnormal placentation, oxidative stress and thrombosis are all thought to contribute to the development of pre-eclampsia( Reference Williams and Broughton Pipkin 12 ). Young and old age, high BMI, multiple pregnancies, autoimmune and infectious diseases, family history of pre-eclampsia and male offspring, as well as low maternal socio-economic status( Reference Autier, Boniol and Pizot 13 ), are described risk factors associated with pre-eclampsia( Reference Mol, Roberts and Thangaratinam 3 , Reference Roberts, Bodnar and Patrick 14 – Reference Jaskolka, Retnakaran and Zinman 16 ). Physical activity before and early in pregnancy, as well as smoking during pregnancy was shown to reduce the risk of pre-eclampsia( Reference Alpoim, Godoi and Pinheiro Mde 17 , Reference Wikstrom, Stephansson and Cnattingius 18 ). Sufficient vitamin D intake during pregnancy has also been suggested to reduce the risk of pre-eclampsia( Reference De-Regil, Palacios and Lombardo 19 , Reference Hypponen, Cavadino and Williams 20 ).
Vitamin D is a complex of fat-soluble secosteroids involved in Ca and P homeostasis and in the development of the skeleton. In addition, vitamin D also has non-skeletal functions that include immunomodulatory and anti-inflammatory properties, and has also been found to affect hormone secretion, cardiovascular function and blood pressure( Reference Autier, Boniol and Pizot 13 ).
It is well established that the fetal environment plays an important role in later health and disease risk( Reference Barker, Eriksson and Forsen 21 ). During pregnancy, growth and development of the fetus depend on the nutritional status of the pregnant mother and on the capability of the placenta to exchange nutrients, such as vitamin D, between the pregnant woman and fetus( Reference Hart, Lucas and Walsh 22 ). Vitamin D insufficiency is common among otherwise healthy pregnant women( Reference Petersen, Olsen and Molgaard 23 , Reference Milman, Hvas and Bergholt 24 ) and, as maternal and fetal vitamin D statuses are strongly correlated, there is increasing interest in maternal vitamin D insufficiency during pregnancy and its potential long-term health consequences for the offspring( Reference Perni, Wikstrom and Cnattingius 25 ).
So far, only one previous study has examined if extra vitamin D from supplementation early in life influences the risk of developing pre-eclampsia later in life( Reference Hypponen, Hartikainen and Sovio 26 ). The study followed 2969 women in Northern Finland born in 1966 who, in their first year of life, regularly or irregularly, received up to 50 µg of vitamin D daily, as well as women who did not receive any supplementation. After adjusting for biological, social and medical characteristics, a 50 % risk reduction of pre-eclampsia was found among women who had regularly received vitamin D supplementation during infancy, compared with those who received irregular or no supplementation( Reference Hypponen, Hartikainen and Sovio 26 ).
Studies examining the importance of fetal nutrition on health later in life are time-consuming and may involve substantial costs and logistic difficulties. Conducting ‘societal experiments’ (e.g. fetal undernutrition during famines) has been widely used in exploring early origins of adult diseases in a cost-effective manner( Reference Thurner, Klimek and Szell 27 , Reference Lumey, Stein and Susser 28 ). The present study is based on a societal experiment around margarine fortification with vitamin D in Denmark. In June 1985, mandatory fortification of margarine with vitamin D, which started in the 1930s and supplied on average 13 % (3–29 %) of all dietary intake of vitamin D to adult Danes( Reference Haraldsdóttir, Holm and Jensen 29 ), was abandoned( 30 – 33 ). Consequently, individuals born before 1985 were exposed to vitamin D from fortified margarine during fetal development and individuals born after the cancellation were not.
The purpose of the present study was to examine if fetal exposure to extra vitamin D from fortified margarine lowered the risk of pre-eclampsia later in life.
Methods and materials
Data sources
Information about the study population was retrieved from the Danish Civil Registration System (CSR); information on the pregnant women and their newborns was retrieved from the Danish Medical Birth Registry (MBR); and the presence of pre-eclampsia in the pregnant women was identified from the Danish National Patient Registry (DNPR). Age of delivery was calculated based on information on maternal day of birth retrieved from the CSR and information on date of delivery retrieved from the MBR.
The CSR was established in April 1968 and has since registered all people alive and living in Denmark with a 10-digit civil person register (CPR) number. The CPR number enables linkage of individual information from different nationwide registers and large clinical databases( Reference Pedersen 34 ). The MBR was established in 1973 and contains information obtained during the antenatal care visits for all women with permanent residence in Denmark( Reference Knudsen and Olsen 35 ). The DNPR was established in 1977, and records information on all patients discharged from Danish non-psychiatric hospitals since 1977 and emergency and outpatient departments since 1995( Reference Lynge, Sandegaard and Rebolj 36 ).
Definition of outcome
Since 1994, the diseases collected into the DNPR have been coded according to the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10). The present study included the following ICD-10 codes: gestational hypertension (code O13.9); mild to moderate pre-eclampsia (code O14.0); severe pre-eclampsia (code O14.1); HELLP syndrome (code O14.2); pre-eclampsia, unspecified (code O14.9); eclampsia in pregnancy (code O15.0); eclampsia during delivery (code O15.2); and eclampsia, unspecified as to time period (O15.9). The included diagnoses were categorized into three groups (Table 1). In cases where a woman had been diagnosed with more than one of the included diagnoses during the included pregnancy, only the diagnosis indicating the most severe form of pre-eclampsia was assigned.
Table 1 Description of gestational hypertension, pre-eclampsia and eclampsia by International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD10) code and grouping

HELLP, haemolysis, elevated liver enzymes, low platelets.
Study population
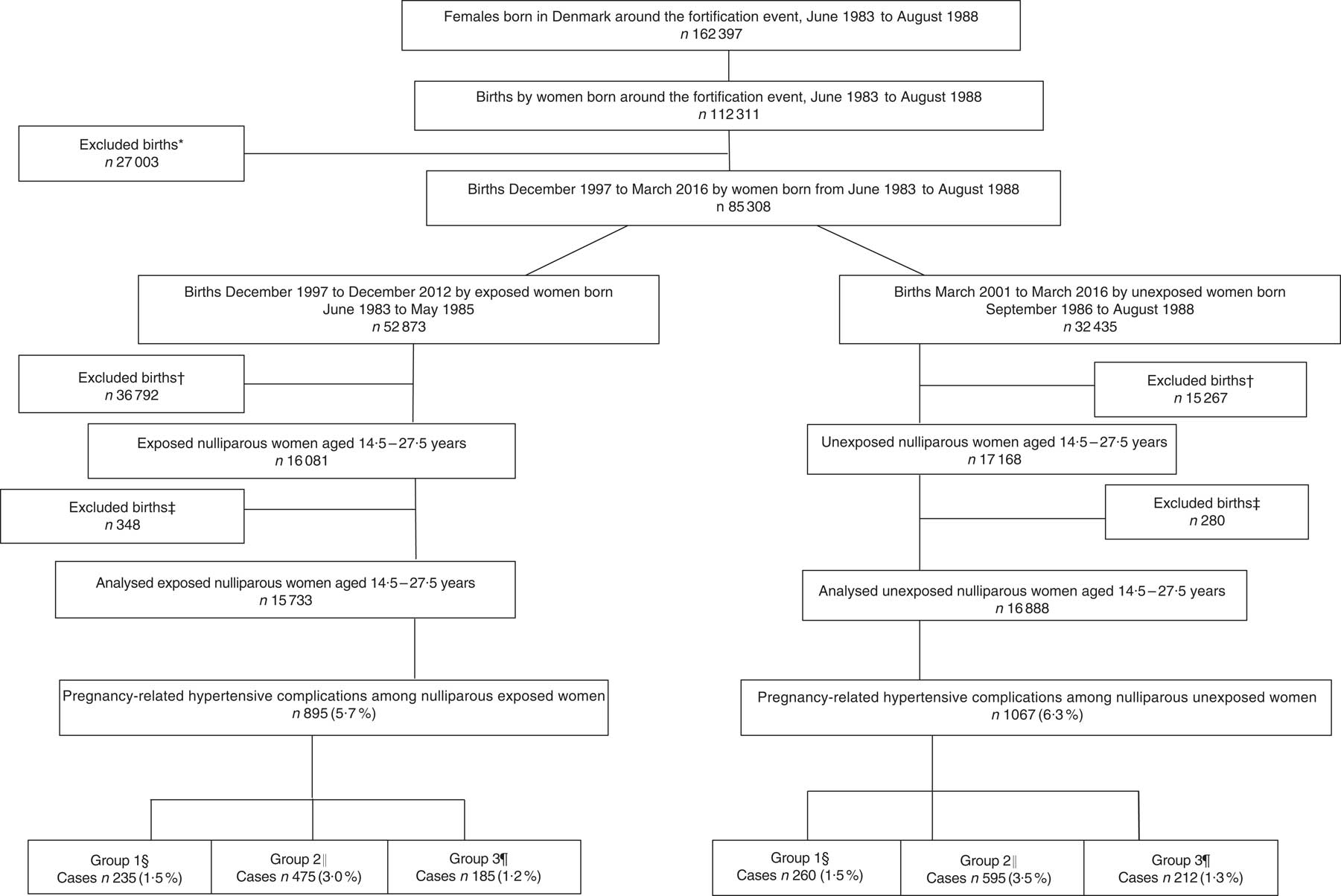
In Denmark, it was mandatory to fortify margarine with vitamin D (1·25 µg/100 g) until June 1985. A 15-month ‘wash-out period’, including the full 9 months of pregnancy and additional 6 months to secure that the fortified margarine was no longer available at home or in stores, was introduced from June 1985 to September 1986. Therefore, the cohort of women born in the two years immediately before June 1985, born from June 1983 to May 1985, was defined as exposed to extra vitamin D during fetal development and the cohort of women born after the wash-out period, born from September 1986 to August 1988, was defined as unexposed. From June 1983 to August 1988, 162397 females were born in Denmark; among these females, 112 311 gave birth. Those born during the ‘wash-out period’ were excluded from the study (n 27 003), reducing the number of births to 85 308. In the present study, the number of births by women who, due to the mandatory margarine fortification, were exposed to additional vitamin D during fetal life was 52 873. The number of births by women unexposed to the extra vitamin D during fetal development was 32 435. Women in the exposed cohort were frequently older and more often multiparous; therefore, the number of births among the exposed women was higher compared with the unexposed. As incidence of pre-eclampsia is higher in first-time pregnancies, the study population was restricted to nulliparous women( Reference Mol, Roberts and Thangaratinam 3 – Reference Skjaerven, Wilcox and Lie 5 ). Based on maternal day of birth and date of delivery, the study population was restricted to women giving birth at age 14·5 to 27·5 years and delivering their offspring after gestational week 22 to ensure similar age in both cohorts. Newborns with birth weight of 0 g and very young women, as well as very young women with pre-pregnancy BMI below the established cut-offs for this age group( Reference Cole, Bellizzi and Flegal 37 ), were also excluded, resulting in 16 081 births in the exposed group and 17 168 births in the unexposed group. Finally, those with missing information on singleton and multiple births, offspring birth weight or gender, smoking status or gestational age were also excluded. The final groups consisted of 15 733 exposed nulliparous women and 16 888 unexposed nulliparous women. A flowchart of the study population is illustrated in Fig. 1. The sampling is illustrated in Fig. 2. The nulliparous women exposed to the vitamin D fortification in utero gave birth between December 1997 and December 2012; nulliparous women unexposed to the vitamin D fortification in utero gave birth between March 2001 and March 2016.
Fig. 1 Flowchart of the study population of women born between June 1983 and August 1988, who gave birth to their first child at age 14·5 to 27·5 years. *Women excluded because they gave birth during the 15-month wash-out period from June 1985 to September 1986. †Births where the woman was either below 14·5 or above 27·5 years of age, the offspring’s birth weight was misclassified, gestational weeks was <22 weeks or pre-pregnancy BMI was <15·46 kg/m2. ‡Excluded due to missing information on age at delivery, smoking habits, singleton and multiple births, gestational age at delivery or offspring gender. §Group 1, gestational hypertension; ICD-10 code: O13.9. ║Group 2, mild to moderate and unspecified pre-eclampsia; ICD-10 codes: O14.0 and O14.9. ¶Group 3, severe pre-eclampsia, HELLP syndrome and eclampsia; ICD-10 codes: O14.1, O14.2 and O15.0 (ICD-10, International Statistical Classification of Diseases and Related Health Problems 10th Revision; HELLP, haemolysis, elevated liver enzymes, low platelets)
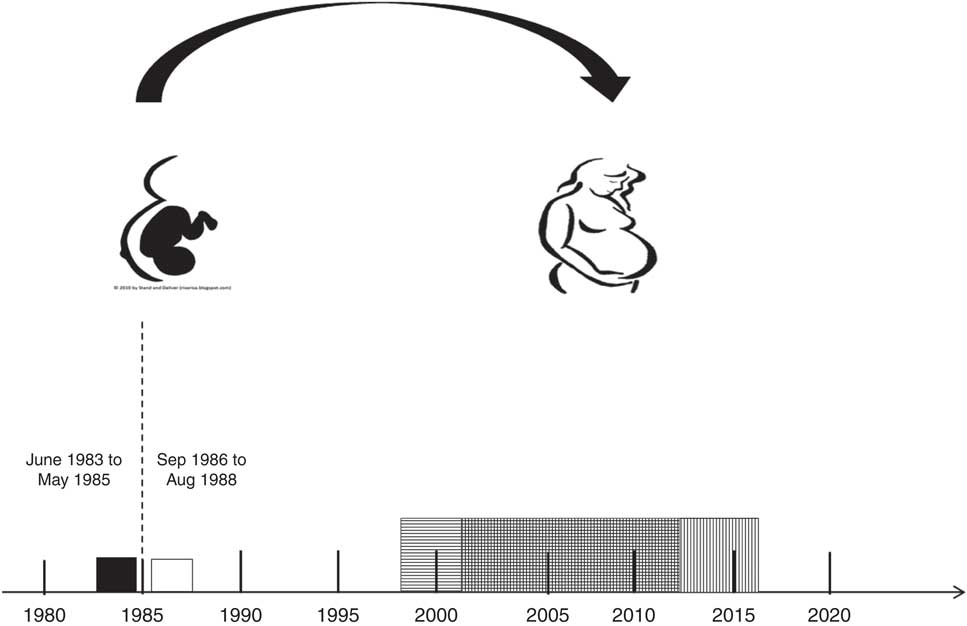
Fig. 2 Sampling of the study population of women born between June 1983 and August 1988, who gave birth to their first child at age 14·5 to 27·5 years. (![]() ), exposed women born between June 1983 and May 1985; (
), exposed women born between June 1983 and May 1985; (![]() ), unexposed women born between September 1986 to August 1988; (– – – – –), the ‘wash-out’ period from June 1985 to September 1986. The exposed women gave birth between December 1997 and December 2012 (
), unexposed women born between September 1986 to August 1988; (– – – – –), the ‘wash-out’ period from June 1985 to September 1986. The exposed women gave birth between December 1997 and December 2012 (![]() ); the unexposed women gave birth from March 2001 to March 2016 (
); the unexposed women gave birth from March 2001 to March 2016 (![]() )
)
Variables
Information on pre-pregnancy BMI, smoking status, singleton and multiple births and gender of the newborn was retrieved from the MBR.
Pre-pregnancy BMI has been included in the MBR since 2004. At the first antenatal visit, normally taking place during the first trimester, the woman’s BMI is calculated based on self-reported information. Applying International Obesity Task Force standards for adolescent BMI( Reference Cole, Bellizzi and Flegal 37 , Reference Cole, Flegal and Nicholls 38 ), women with pre-pregnancy BMI below 15·46 kg/m2 were excluded( Reference Cole, Bellizzi and Flegal 37 ). Obesity defined as BMI≥30·0 kg/m2 is an established risk factor for pre-eclampsia( Reference Jeyabalan 39 , Reference Uzan, Carbonnel and Piconne 40 ) and the association between pre-pregnancy BMI and pre-eclampsia may not be linear( Reference Bartsch, Medcalf and Park 41 ). Therefore, we categorized pre-pregnancy BMI into the four following categories: <18·5 kg/m2, 18·5–24·9 kg/m2, 25·0–29·9 kg/m2 and ≥30·0 kg/m2.
Information on smoking habits of the pregnant women has been collected into the MBR since 1997. This information was grouped into three categories: current smokers, former smokers (women who stopped smoking during or after the first trimester in the current pregnancy) and non-smokers.
The Danish national guidelines regarding late term definition were changed in 2009( Reference Hedegaard, Lidegaard and Skovlund 42 ). Therefore, to make cohorts comparable with regard to term of birth, we categorized gestational age at birth by weeks+days, rather than pre-term, full-term and post-term categories. The following seven categories were constructed: (i) <37, (ii) 37+0 to 37+6, (iii) 38+0 to 38+6, (iv) 39+0 to 39+6, (v) 40+0 to 40+6, (vi) 41+0 to 41+6 and (vii) ≥42 complete weeks of gestation.
Statistical analyses
Differences between exposed and unexposed women were tested by the χ 2 test for categorical data and the Mann–Whitney rank-sum test for skewed continuous data. The age restriction caused the age distribution to be skewed a priori; therefore, a formal statistical test of normality was unnecessary.
The association between exposure status and risk of pre-eclampsia later in life was examined by logistic regression, generating OR with 95 % CI. The following confounders were included into the multivariate regression models: woman’s age at delivery, smoking habits, and singleton and multiple pregnancies. These confounders were hypothesized to be related to pre-eclampsia risk( Reference Mol, Roberts and Thangaratinam 3 , Reference Jaskolka, Retnakaran and Zinman 16 , Reference Alpoim, Godoi and Pinheiro Mde 17 , Reference Sibai, Dekker and Kupferminc 43 ) or to vitamin D levels during gestation( Reference Hypponen, Cavadino and Williams 20 , Reference Hypponen, Hartikainen and Sovio 26 , Reference Hypponen, Laara and Reunanen 44 ). The main analyses were not adjusted for pre-pregnancy BMI; this information is available only from 2004 and forwards, thus adjustment would have made exposed and unexposed women incomparable in terms of age. To explore the impact of pre-pregnancy BMI, sensitivity analyses were conducted as sub-analyses including only those women who had information on pre-pregnancy BMI; the analyses in this subgroup were adjusted for pre-pregnancy BMI.
Earlier studies have found that smoking during pregnancy may protect against development of pre-eclampsia. Furthermore, smoking has previously been associated with vitamin D levels( Reference Jaaskelainen, Knekt and Marniemi 45 , Reference Thuesen, Husemoen and Fenger 46 ). Interactions between smoking and vitamin D exposure status in relation to pre-eclampsia risk were analysed by conducting the models stratified by women’s smoking status. Additionally, interactions between exposure status and women’s age at delivery( Reference Mol, Roberts and Thangaratinam 3 , Reference Cavazos-Rehg, Krauss and Spitznagel 15 ), as well as between exposure status and pre-pregnancy BMI( Reference Roberts, Bodnar and Patrick 14 ), were tested using likelihood ratio tests.
Data were analysed using the statistical software package Stata version 13; P<0·05 was considered statistically significant.
Ethics
The study was conducted in accordance with Danish law and approved by the Danish Data Protection Agency (journal number 2012-41-1156) providing permission to access the relevant registries. The study was based on already collected data, the use of which does not require ethical approval.
Results
The proportion of births with pre-eclampsia, including cases of eclampsia (group 2 and 3; Table 1), among the nulliparous women exposed or unexposed to vitamin D fortification during fetal life was 4·2 % (660/15 733) and 4·8 % (807/16 888), respectively. Despite the age restrictions, the age at delivery was slightly higher for the exposed women compared with unexposed; the median (5th–95th percentile) age being 24·9 (19·5–27·3) and 24·7 (19·4–27·3) years, respectively (P<0·0001). Furthermore, exposed compared with unexposed women were more often current smokers (18·9 v. 15·8 %, respectively) and more often gave birth at late gestational age (6·1 v. 3·6 %, respectively). Moreover, exposed compared with unexposed women more often had a BMI<18·5 kg/m2 (4·4 v. 5·2 %, respectively) and less often had a BMI≥30·0 kg/m2 (13·6 v. 13·9 %, respectively). Other characteristics were not different between exposed and unexposed women (Table 2).
Table 2 Characteristics of women and their offspring, according to whether the woman was exposed to extra vitamin D from food fortification in fetal life or not; women born between June 1983 and August 1988, who gave birth to their first child at age 14·5 to 27·5 years

HELLP, haemolysis, elevated liver enzymes, low platelets; ICD-10, International Statistical Classification of Diseases and Related Health Problems 10th Revision.
* Tested by the Mann–Whitney rank-sum test.
† Tested by the χ 2 test.
‡ ICD-10 code: O13.9.
§ ICD-10 codes: O14.0 and O14.9.
¶ ICD-10 codes: O14.1, O14.2 and O15.0.
║ Information available only from 2004 and forwards.
Overall, there was a lower risk of pregnancy-related hypertensive complications among women who were exposed to extra vitamin D in fetal development compared with the unexposed women (Table 3). However, in the analyses stratified by outcome severity, the exposure effect was observed only among women diagnosed with mild to moderate and unspecified pre-eclampsia. No significant associations were found for the diagnoses of gestational hypertension, severe pre-eclampsia, HELLP syndrome and eclampsia. Adjusting for women’s age at delivery, smoking habits, singleton and multiple births and offspring gender gave essentially similar results (Table 3). The estimates for the covariates are listed in the online supplementary material, Supplemental Table 1.
Table 3 Crude and adjusted odds for pre-eclampsia among women exposed to extra vitamin D in fetal life; women born between June 1983 and August 1988, who gave birth to their first child at age 14·5 to 27·5 years

HELLP, haemolysis, elevated liver enzymes, low platelets; ICD-10, International Statistical Classification of Diseases and Related Health Problems 10th Revision.
* Adjusted for age at delivery, maternal smoking status, and singleton or multiple births.
† ICD-10 code: O13.9.
‡ ICD-10 codes: O14.0 and O14.9.
§ ICD-10 codes: O14.1, O14.2 and O15.0.
The interaction between exposure to vitamin D and smoking status was significant (χ 2=11·82; df=2; P=0·003) and stratifying analyses on smoking status revealed that the strongest association between in utero vitamin D exposure status and pre-eclampsia risk was seen among current smokers, where the risk of all types of pre-eclampsia was halved or more for the exposed women compared with unexposed ones (Table 4).
Table 4 Crude and adjusted odds for pre-eclampsia among smoking and non-smoking women exposed to extra vitamin D from fortification during fetal life; women born between June 1983 and August 1988, who gave birth to their first child at age 14·5 to 27·5 years

HELLP, haemolysis, elevated liver enzymes, low platelets; ICD-10, International Statistical Classification of Diseases and Related Health Problems 10th Revision.
* Adjusted for age at delivery, and singleton and multiple births.
† ICD-10 code: O13.9.
‡ ICD-10 codes: O14.0 and O14.9.
§ ICD-10 codes: O14.1, O14.2 and O15.0.
Pre-pregnancy BMI measurements were available only for a sub-population of 13 640 in the exposed group and a sub-population of 14 871 in the unexposed group. Sensitivity analyses in these sub-populations were restricted to women of similar age (age 20–27 years) and were adjusted for pre-pregnancy BMI; the results of these analyses (Table 5) were similar to the main findings.
Table 5 Crude and adjusted odds for pre-eclampsia among women exposed v. unexposed to extra vitamin D from fortification during fetal life, restricted to include only those women with information on pre-pregnancy BMI; women born between June 1983 and August 1988, who gave birth to their first child at age 14·5 to 27·5 years

HELLP, haemolysis, elevated liver enzymes, low platelets; ICD-10, International Statistical Classification of Diseases and Related Health Problems 10th Revision.
* Adjusted for age at delivery, maternal smoking status, pre-pregnancy BMI, and singleton or multiple births.
† ICD-10 code: O13.9.
‡ ICD-10 codes: O14.0 and O14.9.
§ ICD-10 codes: O14.1, O14.2 and O15.0.
Interactions between exposure status and women’s age at delivery (χ 2=3·58; df=4; P=0·47), as well as between exposure status and pre-pregnancy BMI, were not significant (χ 2=0·77; df=3; P=0·86).
Discussion
In accordance with the incidence of pre-eclampsia worldwide, approximately 4·5 % of the women in our sample were diagnosed with pre-eclampsia of different severity( Reference Mol, Roberts and Thangaratinam 3 ). The present study found that the risk of mild to moderate and unspecified pre-eclampsia was lower in those nulliparous women who were exposed to small amounts of additional vitamin D from food fortification during their fetal development, suggesting that vitamin D may have exerted programming effects on the risk of pregnancy complications later in life. Our results also indicate that the effects of vitamin D on the risk of pre-eclampsia were most obvious among women who smoked during pregnancy.
Our findings are in accordance with those of Hypponen et al.( Reference Hypponen, Hartikainen and Sovio 26 ), who found that the risk of pre-eclampsia was halved among the women who regularly received vitamin D supplementation in their first year of life. The supplemented dose in the study by Hypponen et al.( Reference Hypponen, Hartikainen and Sovio 26 ) corresponded to 50 µg vitamin D/d. In our study, the extra daily vitamin D doses delivered with fortification were much smaller. Based on our calculations, the dosage could have been about 0·5–0·6 µg of extra vitamin D daily for the women; for the fetus, the dosages probably were even lower( Reference Haraldsdóttir, Holm and Jensen 29 , Reference Fagt 47 ). It is important to note that while we examined the influence of vitamin D exposure during fetal life, Hypponen et al.( Reference Hypponen, Hartikainen and Sovio 26 ) examined the influence of vitamin D supplementation during the child’s first year of life. Other previously conducted studies, including one from our group, showed that vitamin D supplementation dosage and timing were important to prevent type 1 diabetes, where supplementation in infancy with high dosages was shown to have a protective effect( Reference Hypponen, Laara and Reunanen 44 ) while exposure to low-extra dosages from fortification throughout fetal development was not( Reference Jacobsen, Hypponen and Sorensen 48 ). The dose and timing of the extra vitamin D seem to be of less importance in relation to prevention of pre-eclampsia( Reference Hypponen, Hartikainen and Sovio 26 ).
Notably, our study and the study by Hypponen et al.( Reference Hypponen, Hartikainen and Sovio 26 ) utilized different study designs. Hypponen et al.( Reference Hypponen, Hartikainen and Sovio 26 ) conducted a classical cohort study, where information on vitamin D supplementation during the child’s first years of life was collected. The female offspring were followed for pre-eclampsia outcome in their own pregnancies. Such a study takes a long time, many resources and is difficult logistically. The societal experiment introducing a termination of the mandatory vitamin D fortification programme in Denmark provided us with a unique opportunity to investigate research questions similar to those of Hypponen et al.( Reference Hypponen, Hartikainen and Sovio 26 ) quickly and cost-effectively, and without the necessity to address each woman’s individual vitamin D intake. However, the societal experiment design is prone to various biases, which are outlined below. On the other hand, in the study by Hypponen et al.( Reference Hypponen, Hartikainen and Sovio 26 ), vitamin D intake is prone to recall bias. Further research confirming our and Hypponen et al.’s( Reference Hypponen, Hartikainen and Sovio 26 ) findings is needed. Specifically, a randomized controlled trial would be needed to firmly conclude on causal relationships between fetal vitamin D exposure and pre-eclampsia risk later in life. Such a trial, however, would have to track offspring from quite large groups of in utero vitamin D-supplemented and non-supplemented women for two or three decades after the supplementation actually took place. We would recommend that, before conducting a randomized controlled trial, an effort is made to identify already existing cohorts, similar to the one used in the study by Hypponen et al.( Reference Hypponen, Hartikainen and Sovio 26 ), with information on fetal exposure to extra vitamin D. An alternative solution could be an observational study, with objective vitamin D biomarker measurement in fetal or neonatal blood, coupled with register information on pre-eclampsia incidence later in life. In fact, such a study is currently being conducted by our research group.
In Denmark, since 1981 neonatal blood has been routinely collected from all newborns 48–72 h after birth to screen for phenylketonuria. The remaining blood left after screening is stored on dried blood spots (DBS charts) in the Danish Neonatal Screening Biobank. Contemporary vitamin D analyses techniques can detect vitamin D concentration on the DBS charts( Reference Norgaard-Pedersen and Hougaard 49 ). Currently, our research group is conducting a case–control study which measures vitamin D levels in the blood on the DBS charts in a large population-based sample of women diagnosed with pre-eclampsia, and randomly selected control women. Results of this study will be used to validate the findings of the present study. If our findings are confirmed by more robust study designs, this will have important public health implications because it will demonstrate a possibility of pre-eclampsia prevention by simple and affordable food fortification. Efforts to prevent pre-eclampsia are important, as the disease bears health risks for the mother and the offspring, and the only definite cure known so far is delivery( Reference Mol, Roberts and Thangaratinam 3 ).
The biological mechanisms linking fetal vitamin D and later pre-eclampsia risk are most probably related to the maturity of the immune system. T-cell development and maintenance may be of importance in forming the adult immune system. A balance between helper T1 cytokines (Th1) and helper T2 cytokines (Th2) is necessary for an optimal immune system( Reference Mora, Iwata and von Andrian 50 ). Vitamin D receptors are found on most immune cells, making hormonal vitamin D an important immune regulator stimulating Th2 expression and inhibiting Th1 expression( Reference Hypponen, Hartikainen and Sovio 26 , Reference Jonsson, Matthiesen and Berg 51 ). An overexpression of Th1 response may be involved in the development of pre-eclampsia( Reference Sibai, Dekker and Kupferminc 43 , Reference Hypponen 52 ).
Several studies have reported that smoking in pregnancy reduces the risk of pre-eclampsia( Reference Conde-Agudelo, Althabe and Belizan 53 , Reference England and Zhang 54 ). Smoking may interact with other risk and/or protective factors, including prenatal vitamin D level. Therefore, analyses differentiated by smoking status were used to investigate if small doses of extra vitamin D from food fortification affected pre-eclampsia risk. Indeed, we found that prenatal vitamin D intake seemed to be a protective factor in currently smoking pregnant women (i.e. women who smoked during pregnancy benefited most from extra vitamin D intake during their own gestation). The fact that the women exposed to extra vitamin D during fetal life were more often smokers should not bias our results, as they smoked while being pregnant, and exposure to vitamin D occurred during the pregnant women’s own fetal development. Exposure to smoking during fetal life (i.e. smoking status of the mothers of the studied women) was not recorded. Importantly, although smoking is associated with a lower risk of pre-eclampsia( Reference Jaaskelainen, Knekt and Marniemi 45 , Reference Thuesen, Husemoen and Fenger 46 , Reference Brot, Jorgensen and Sorensen 55 ), smoking is also associated with several adverse perinatal outcomes and should never be promoted to reduce the risk of pre-eclampsia.
Strengths and limitations
The use of a societal experiment design to study an association between exposure to extra vitamin D during fetal life and later risk of pre-eclampsia has both strengths and limitations. The design determined the sampling, exposure measurement and possible confounding. In our design, period of birth approximated prenatal exposure to extra vitamin D from the fortified margarine. As the fortification programme was cancelled in 1985, we analysed women from adjacent birth cohorts born around 1985. We knew the exact date of the cancellation of the fortification programme and therefore we could define precise exposure and non-exposure periods for the adjacent national birth cohorts. As the birth cohorts were very close to each other and covered a narrow time period, we considered all the characteristics of our study population, except for prenatal exposure to vitamin D fortification, to be randomly distributed between exposed and unexposed individuals; thus, confounding was not expected. To exemplify our reasoning in regard to a low social status as a potential confounder for pre-eclampsia( Reference Silva, Coolman and Steegers 56 ), the following arguments can be provided. The study population covers all births, i.e. from all social strata, retrieved from the national Danish medical registries including all Danes. Maternity care in Denmark is free of charge and all pregnant women, i.e. irrespective of social status, are followed by a standardized protocol of prenatal care and are consequently registered into the national Danish medical registries. Thus, we assume that all social strata are equally represented in both groups.
The confounding in our design, however, could occur if secular changes in alternative exposure sources (i.e. vitamin D intake via food or exposure to bright sunshine), outcomes (i.e. pre-eclampsia incidence) and potential confounders (e.g. mother’s socio-economic status and smoking, mother’s exposure to infections and physical activity) were taking place in Denmark during 1983–1988, a narrow time period covering our exposed and unexposed birth cohorts. Consequently, the limitation of the societal experiment design is that it assumes that in the period analysed, there were no other changes except the one of interest. We could not identify such secular changes in our study. Nevertheless, we acknowledge that some secular changes where evidence is difficult or impossible to document may have occurred. For example, women born in 1983–1985 may have been slightly less exposed to maternal smoking (or exposure to smoking in fetal life), compared with women born in 1986–1988, as since the 1970s all pregnant women in Denmark were advised to stop smoking( 57 ) and studies suggest that smoking during pregnancy is declining( Reference Ekblad, Gissler and Korkeila 58 , Reference Wisborg, Henriksen and Hedegaard 59 ). We were unable to get information on smoking habits of the mothers of our study population, as smoking habits were not recorded into the MBR until 1997( Reference Mattsson, Kallen and Rignell-Hydbom 60 ). Moreover, in the period 1987–1988 changes occurred in the Danish fiscal policy aiming at reducing household expenditure( 61 ). A change towards a healthier self-reported diet in the Danish population during 1985–2001 was also noted( Reference Fagt, Matthiesen and Biltoft-Jensen 62 , Reference Osler, Heitmann and Schroll 63 ). Thus, there could have been confounding which we did not consider.
We did, however, consider alternative exposures to vitamin D around 1985: changes in fortified margarine intake in 1983–1988; changes in vitamin D supplement recommendations for pregnant women; and women’s exposure to bright sunshine hours during the same period. Food disappearance statistics showed that the change in margarine consumption in the period analysed was minimal: from 16·9 kg/capita in 1983 to 16·2 kg/capita in 1985 and 17·0 kg/capita in 1988( Reference Fagt 47 ). Vitamin D supplementation during pregnancy recommendations did not change during 1983–1988. Therefore, we assume that the overall alternative fetal exposure to vitamin D from foods was comparable in both groups. Further, differences in monthly bright sunshine hours per year during 1983–1988 were not significant. Amounts of bright sunshine hours during gestation, compared between the individuals in exposed and unexposed cohorts, were however significantly different, and ideally should have been adjusted for in our analyses( Reference Jacobsen, Moldovan and Vaag 64 , Reference Jacobsen, Frederiksen and Heitmann 65 ).
The main strength of the present large-scale study is the use of data from the Danish nationwide medical registries. The Danish national administrative and medical registers are considered to have high completeness and good validity. A high degree of validity of the ICD-10 pre-eclampsia diagnoses in the DNPR has been confirmed by comparing diagnoses of pre-eclampsia from the registry with standardized phone interviews in a study carried out between 1998 and 2002 that included a total of 3039 pregnancies( Reference Klemmensen, Olsen and Osterdal 66 ). However, the coding of the diagnoses gestational hypertension and pre-eclampsia included other serious diseases and may have introduced non-differential misclassification errors causing a disappearance of the observed association( Reference Grimes and Schulz 67 ). The lower gestational age at delivery observed in older women supports the validity of the data from the MBR, as it reflects national guidelines for earlier induction of labour in post-term pregnancies in Denmark in 2009( Reference Hedegaard, Lidegaard and Skovlund 42 ). As the present study is based on information retrieved from the Danish national health registries collected independently of the study, selection and information bias are considered to be negligible. The registers, however, contain no information on the smoking status of the mothers of the women in our study population, which may be considered a potential limitation in our analyses.
Conclusion
The results of our study suggest that small extra doses of vitamin D from food fortification during fetal development may decrease the risk of developing pre-eclampsia during first pregnancy in adulthood. The beneficial effect of the extra vitamin D during fetal development appears to be particularly protective against pre-eclampsia for women who smoke during pregnancy.
Acknowledgements
Financial support: This study was funded by the Danish Agency for Science, Technology and Innovation, the Ministry of Science, Higher Education, under the instrument ‘Strategic Research Projects’ (grant previous 11-116213 now 0603-00453B) and by the PhD School of Faculty of Health Sciences the University of Southern Denmark. The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: B.L.H. conceived the research idea; M.S. and B.L.H. designed the research; M.S. performed the statistical analysis; M.S., P.D., P.F., R.H. and B.L.H. interpreted the results; M.S. drafted the manuscript and P.D., P.F., R.J. and B.L.H. commented on it; M.S. and B.L.H. had primary responsibility for the final content. All authors read and approved the final manuscript. Ethics of human subject participation: Access and linkage permission was obtained from the Danish Data Protection Agency (journal number 2012-41-1156). The study was based on already collected data which by Danish law do not need ethical approval.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980017003135