As stated by the World Health Organization (WHO), the ‘nutritional status of children provides an indirect measurement of the quality of life of an entire population’1. Anthropometric indicators of pre-school undernutrition (stunting, wasting and underweight in children aged 0–5 years) are widely assessed, standardised, and used for diagnostic, operational and policy purposes. Prevalences of stunting, wasting and underweight among the under-fives are key outcome indicators of food insecurity and vulnerability information systems at national and international levels2, 3. They are also used to monitor and evaluate rural development programmes4 and to track achievements of poverty reduction strategies5. The proportion of underweight children features in the first Millennium Development Goal (MDG) to ‘eradicate extreme poverty and hunger’ and is used to assess progress in reducing child malnutrition6. The prevalence of wasting, in conjunction with oedema and mortality rates, is a key criterion for deciding food-aid and feeding programmes in emergency situations7.
Despite its widespread use, the age category ‘under-fives’ is often imprecisely defined. Nutrition surveys conventionally include pre-school children from birth to 59 months of age; however, many start at 1, 3 or 6 months of age and stop at 35 or 36 months. In emergency situations, standard survey guidelines exclude infants aged 0–5 months from assessments8, 9. They actually stipulate that ‘6–59 month olds are the most vulnerable to nutritional deficiency’10. Consequently, comparisons across surveys and analyses of time trends based on ‘undernutrition in under-fives’ are limited by the possible differences in the age ranges surveyed.
There are several reasons why 0–5-month-old infants are excluded from nutritional surveys. Their measurement presents practical and technical difficulties. Weight is easy to measure, but in this age group baby-weighing scales accurate to 10 g should be used and not scales that measure with an accuracy of 100 g such as the Salter scale or bathroom scalesReference Golden11. Measuring length is more difficult, because survey personnel may not be used to measuring very young infants and fear hurting them. Infants are not easy to reach as their parents may be reluctant to let them be measured. The adequacy of the current 1977 National Center for Health Statistics (NCHS)/WHO reference for infants aged less than 6 months has been questioned12, Reference Victora, Morris, Barros, de Onis and Yip13. Moreover, it is still commonly assumed that breast-feeding protects against early malnutrition until approximately 6 months of age. This belief was conveyed by the nutritional literature during the 1980s based on observations of higher mortality and morbidity rates among bottle-fed infantsReference Martorell, Habicht, Falkner and Tanner14. Finally, as shown by Shrimpton et al. Reference Shrimpton, Victora, de Onis, Lima, Blössner and Clugston15, on a global level, the mean weight-for-length Z-scores only become negative at about 6 months of age. Thus wasting is perceived as being rare before the age of 6 months, implying that in emergency situations, infants need not be surveyed before that age.
The aim of the present analysis is to quantify, from a public health perspective, the impact of excluding 0–5-month-old infants on the global prevalence of stunting, wasting and underweight in children under 5 years (U5) or under 3 years (U3) of age. Using prevalence estimates from the most recent national surveys conducted in developing countries and countries in transition, we compared these indicators when including or excluding infants aged 0–5 months. In this cross-sectional analysis we examined the magnitude of the impact of excluding 0–5-month-old infants (1) at global and regional level, (2) according to the severity of undernutrition as classified by WHO16 and (3) in a subgroup of countries considered to be in a situation of nutrition emergency. For countries with consecutive surveys, we examined the appropriateness of reported trend analyses with respect to comparability of age range. Implications of our findings for evaluation of nutrition interventions and design of programmes and policies are discussed.
Methods
Prevalence of stunting, wasting and underweight
Prevalence of stunting, wasting and underweight is defined as the percentage of infants/children with length/height-for-age, weight-for-length/height and weight-for-age respectively below − 2 standard deviations of the median of the 1977 NCHS/WHO reference12. The primary data source was the Demographic and Health Surveys (DHS), complemented with the Multiple Indicator Cluster Surveys (MICS) end-decade assessments, since both provided prevalence estimates of undernutrition as well as other indicators related to infant nutrition. DHS data were taken from the Macro International website17 using the STATcompiler tool and consistency was checked with published final reports. MICS data were taken from MICS2 national reports18.
Surveys were selected based on the following criteria:
• prevalence estimates and sample sizes for both age groups, 0–59 (or 0–35) months and 0–5 months, were available from nationally representative surveys from developing countries or countries in transition;
• for surveys that did not specify the earliest age included (e.g. age group defined as ‘ < 6 months’), we took either the availability of breast-feeding data for 0–3-month-old infants or sample sizes that reflected a realistic proportion of the total sample of U5 (not less than 10%) or U3 (not less than 17%) as evidence that infants aged 0–5 months were sampled;
• the sample size in the age group 0–5 months was at least 100 infants.
As of January 2006, prevalence estimates were available for 160 surveys conducted from 1986 onwards and covering 86 countries. One hundred and thirteen surveys met our criteria. In the cross-sectional analysis, we used only the most recent survey available for each country; thus the analysis comprises surveys from 76 countries.
We used the WHO classification16 of severity of prevalence among U5 (low, medium, high and very high corresponding to prevalences < 20%, 20–29%, 30–39% and ≥ 40% for stunting and < 10%, 10–19%, 20–29% and ≥ 30% for underweight).
A subgroup of countries in a situation of nutrition emergency was defined based on the United Nations Standing Committee for Nutrition (SCN) criterion19, i.e. a prevalence of wasting among U5 of more than 10%.
Appropriateness of trend analysis was examined using published DHS reports presenting results from consecutive surveys.
Other indicators
Infant mortality rate (IMR) and child mortality rate (CMR) are defined as the probability of dying before the first birthday and between the first and fifth birthdays, respectively. Rates are expressed per 1000 living infants and are estimated with reference to the five-year period preceding the survey. Only DHS estimates were used because of differences in methodology with MICS.
Prevalence of low maternal body mass index (BMI) is defined as the percentage of mothers 20–49 years of age with a BMI < 18.5 kg m− 2, excluding pregnant women and women with a birth in the two or three months preceding the survey16. In the absence of reliable data on low birth weight or intrauterine growth failure, low BMI of mothers was used as a proxy for poor prenatal nutritionReference Kramer20. Only one MICS survey provided the prevalence of low maternal BMI (Democratic Republic of Congo, 2000).
Prevalence of exclusive breast-feeding (EBF) is defined as the percentage of infants aged 0–3 months who received only breast milk, and no other foods or liquids, in the 24-h period preceding the survey.
Data analysis
For surveys providing prevalence of undernutrition in U5, the prevalence estimates were calculated for the age group 0–35 months using estimates and sample sizes of each age group.
For each of the three indicators (stunting, wasting and underweight), the following procedure was used to calculate prevalence in the age group 6–59 months. For example, the prevalence of stunting in the age group 6–59 months was obtained by subtracting the number of stunted 0–5-month-old infants from the number of stunted 0–59-month-old children, which was then divided by the sample size of children aged 6–59 months. The impact of excluding infants aged 0–5 months on prevalence of undernutrition is represented by the following formulas:
where Pi–j is the prevalence of undernutrition for infants or children aged i to j months.
Regional and global estimates were computed by weighting the national prevalences with the 0–59 month (or 0–35 month) and the 0–5 month populations of the countries. Population estimates for the year 2000 were used21. No population estimates were available for infants aged 0–5 months; we arbitrarily set them as equal to half the population of the age group 0–11 months. Countries were grouped according to the FAO regional classification3.
For each country, we computed a chi-square test to compare prevalences among infants aged 0–5 months and children aged 6–59 months (or 6–35 months). The Yates correction was applied where necessary. A value χ2 ≥ 3.84 indicated that prevalence estimates were different at the P = 0.05 error level, and consequently that the impact of excluding 0–5-month-old infants was significant. Spearman rank correlations were used to test associations of prevalence with other indicators. Statistical analyses were performed using the STATISTICA™ software (StatSoft Inc., Tulsa, OK, USA, 1995).
Results
Age ranges in nutrition surveys
Of 160 surveys (119 DHS, 41 MICS) providing prevalence estimates for 0–5-month-old infants, 47 did not meet our selection criteria (18 started at age 1 month, 22 started at age 3 months, two did not provide sample sizes for infants, five had sample sizes below 100 infants). Among the remaining 113 eligible surveys, the majority (84) did not specify exactly the earliest age included, i.e. the youngest age group was defined as ‘ < 6 months’; however, all complied with our selection criteria. The eldest age group included in surveys varied: 59 months (91 surveys), 35 months (18 surveys), 36 months (2 surveys), 47 and 60 months (one survey each).
Prevalence of undernutrition among U5, U3 and 0–5-month-old infants
Prevalence estimates of undernutrition, sample sizes and P-values are shown for each country in the Appendix. Regional and global prevalences of stunting, wasting and underweight including and excluding the age group 0–5 months, and the differences in prevalence, are shown in Table 1.
Table 1 Regional and global prevalences of stunting, wasting and underweight in infants aged 0–5 months, U3 and U5, and impact of excluding infants aged 0–5 months on prevalence

U5 – children under 5 years of age; U3 – children under 3 years of age; n – number of countries; pp – percentage points.
Regional and global prevalence estimates are weighted by country child populations21. The impact of excluding infants aged 0–5 months is the difference [b–a] in U3 and [d–c] in U5.
The global prevalence of stunting, wasting and underweight was 34.2, 7.8 and 27.1%, respectively, in U5 and 34.5, 11.3 and 33.1%, respectively, in U3.
The global prevalence of stunting, wasting and underweight in infants aged 0–5 months was 10.8, 6.7 and 7.3%, respectively. One-third of the countries showed prevalences of stunting above 10%, and two of them, Comoros and Korea DPR, had prevalences of 24.8 and 21.9%, respectively. Wasting was above 5% in almost half the countries and exceptionally high in Somalia (19.6%). Stunting, wasting and underweight were prevalent in 0–5-month-old infants in all regions, but more so in Asia. The lowest prevalences were found in Latin American and Caribbean countries for all three indicators in this age group.
Impact of excluding 0–5-month-old infants
Chi-square tests were significant (P ≤ 0.05), indicating that there was a significant impact of excluding 0–5-month-old infants on prevalence of stunting among U3 in 71 countries out of 76 (among U5 in 65 countries out of 72), on prevalence of wasting among U3 in 45 countries (among U5 in 30) and on prevalence of underweight among U3 in 71 countries (among U5 in 65). At global level, excluding infants aged 0–5 months produced an overestimation of the prevalence of stunting, wasting and underweight by 3.0, 0.3 and 2.6 percentage points, respectively, in U5 and by 4.8, 1.0 and 5.2 percentage points, respectively, in U3. Results regarding wasting will not be discussed further because of the minimal impact on this indicator.
As shown in Table 1, the level of impact varied by geographical region with the same pattern as that of prevalence estimates of stunting and underweight in pre-school children. The highest impact for stunting was seen in Asian and Eastern & Southern African countries (5.8 percentage points in U3 in both regions). For underweight, the highest impact was observed in Asia followed by Eastern & Southern African countries (6.9 and 5.6 percentage points in U3, respectively). Transition countries and countries of Latin America and the Caribbean performed differently to other countries, with an impact on stunting in U3 of less than 2.5 percentage points on average. Among U5, impact was lower.
Table 2 shows the impact of excluding 0–5-month-old infants on prevalence of stunting and underweight according to the WHO classification of severity of undernutrition. Countries with the highest levels of stunting and underweight in this age group were those with the largest overestimations of prevalence in U3 and U5.
Table 2 Global prevalences of stunting and underweight in infants aged 0–5 months, the impact of excluding them and related indicators, by severity of prevalence of undernutrition in U5 (WHO classification16)
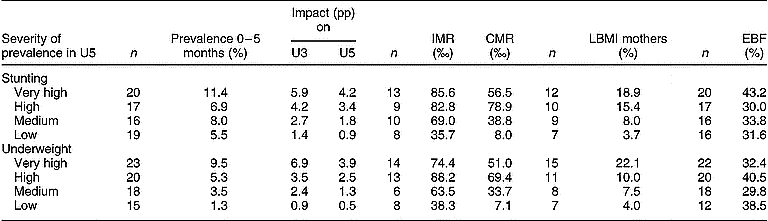
U5 – children under 5 years of age; WHO – World Health Organization; U3 – children under 3 years of age; n – number of countries; pp – percentage points; IMR – infant mortality rate; CMR– child mortality rate; LBMI – low body mass index; EBF – exclusive breast-feeding in infants aged 0–3 months.
Prevalence estimates are weighted by country child populations21. Impact of excluding infants aged 0–5 months is the difference in prevalence of stunting and underweight between the 6–59 month and 0–59 month age groups in U5, and between the 6–35 month and 0–35 month age groups in U3.
Countries in a situation of nutrition emergency
Among 17 countries considered to be in a situation of nutrition emergency, the prevalence of underweight for 0–5-month-old infants ranged from 3 to 15% and was especially high in Comoros and Somalia (14.6 and 14.8%, respectively). The prevalence of stunting for the same age group ranged from 3 to 25% and exceeded 15% in five countries (Yemen, Madagascar, Somalia, Korea DPR and Comoros). In all countries except one (Ethiopia), the prevalence of wasting was higher than 5% for infants aged 0–5 months.
The chi-square tests were significant (P ≤ 0.05) in all countries for underweight and for stunting, in all except one (Guyana, where the impact on stunting was very low). Thus excluding 0–5-month-old infants has a significant impact on prevalence estimates of stunting and underweight in most countries in a situation of nutrition emergency.
Correlation between prevalence of undernutrition in infants and other indicators
Prevalence of underweight in 0–5-month-old infants was highly correlated with prevalence of low maternal BMI (r = 0.78, n = 41 countries) and with IMR (r = 0.51, n = 43 countries). Significant but weaker correlations were also observed with stunting (data not shown). No correlation was observed with EBF prevalence.
Appropriateness of reported trends in pre-school undernutrition
There were 39 countries with two or more consecutive DHS. Trends and percentage change in the prevalence of undernutrition were presented in 50 DHS reports. The magnitude of the reported change in prevalence of stunting and underweight was on average 2 percentage points, ranging from − 12 to +12 percentage points. In 26 reports, prevalence estimates were compared using similar age groups (either the survey samples included the same age groups or, if different, the prevalence estimates were recalculated to standardise the age range); in 19 reports, prevalences were compared among age groups that were not similar; and in four reports it was not clear how the comparison was done. Thus in nearly half of the reports, trends were analysed by comparing the prevalence of undernutrition in age groups that differed by 1 to 3 months. For instance, in U5 children, comparisons were actually made between the < 6–59 month age group and the 1–59 or 3–59 month age group. In three reports, trends were assessed by comparing the prevalence in U5 with that in U3.
Discussion
The main purpose of our analysis was to quantify the impact of excluding 0–5-month-old infants from nutrition surveys on prevalence of stunting, wasting and underweight in U5 and U3. This is the first attempt, to the best of our knowledge, to examine this issue. One important observation is that prevalence estimates of ‘undernutrition in under-fives’ reported in the literature mask variation in age ranges and that often infants aged 0–5 months are not included.
One drawback to our analysis relates to the assessment of infant growth using the 1977 NCHS/WHO growth reference12. Two limitations of the infancy portion of this growth curve have been acknowledged, i.e. the characteristics of the original sample (mostly bottle-fed babies) and the inadequate curve-fitting proceduresReference Garza and de Onis22. In our study, it was not possible to recalculate prevalence estimates against the 2000 Centers for Disease Control and Prevention referenceReference Kuczmarski, Ogden, Guo, Grummer-Strawn, Flegal and Mei23. Nevertheless, the latter differs only minimally from the 1977 NCHS/WHO reference in the age group 0–5 months; thus using the more recent reference would not have modified our observations.
Our study shows that excluding 0–5-month-old infants causes an overestimation of the prevalence of stunting and underweight in U3 (by approximately 5 percentage points) and to a lesser extent in U5 (by approximately 3 percentage points). Wasting was affected only minimally ( ≤ 1 percentage point in U5 and U3). The overestimations of stunting and underweight are especially large in countries with high prevalences in U5 (some Asian and African countries). Moreover, in these countries, prevalences of stunting and underweight in early infancy are also found to be high. It would have been desirable to have more countries from Asia as it is the region where pre-school undernutrition is most prevalent. The geographical patterns found in our study are consistent with other worldwide analyses of undernutritionReference De Onis, Monteiro, Akre and Clugston24, Reference Ruel, Martorell and Haschke25.
We argue that it is important to include infants from birth in order to standardise the collection, analysis and use of nutritional indicators among organisations. As emphasised by SCN26, it is necessary to have consistent age ranges when assessing trends in pre-school undernutrition.
If consecutive surveys do not consistently include infants aged 0–5 months, the subsequent bias, although not large, up to 7 percentage points, can lead to erroneous interpretation of trends in the nutrition situation. Moreover, when evaluating the effect of an intervention or a programme on prevalence of undernutrition by comparing consecutive assessments, the bias caused by inconsistent inclusion of 0–5-month-old infants can underestimate, cancel or overestimate the effect. For example, if a programme produced a true decrease in prevalence of undernutrition of approximately 5 percentage points, including infants aged 0–5 months in the baseline assessment but excluding them at follow-up would mask the effect. It could be wrongly concluded that the programme failed to reach its objective.
PrudhonReference Prudhon27 and GoldenReference Golden11 have reported nutritional problems affecting 0–5-month-old infants and have emphasised the lack of data on this age group. The first six months of life, when rapid growth occurs, are the most vulnerable to nutritional insultsReference Shrimpton, Victora, de Onis, Lima, Blössner and Clugston15. Failure to grow at this critical time has an important influence on a child's future developmentReference Mendez and Adair28. Yet very little is known at a population level about the postnatal period of infant growth and there have been few attempts to explain regional differences in the patterns of growth falteringReference Dewey29. RuelReference Ruel, Martorell and Haschke25 stated that part of the excessive levels of stunting and wasting in the early postnatal period found in some countries was explained by greater levels of intrauterine growth retardation. Stunting in the first months of life was found to be more associated with mother's nutritional status than with infant feeding in some studiesReference Dewey29, Reference Schmidt, Muslimatun, West, Schultink, Gross and Hautvast30. Our results suggest that maternal nutrition might be more important than early feeding practices to infant growth during the first semester; however, caution is needed when inferring causality with this type of cross-sectional analysis.
In developing countries, one child in 10 dies before the fifth birthday, an unacceptably high death rate that affects sub-Saharan Africa most severely6. A major contributing factor is undernutritionReference Caulfield, De Onis, Blössner and Black31. A large share of these deaths occur in the neonatal period (38% in 2000)Reference Lawn, Cousens and Zupan32. Early undernutrition is an important factor, as shown by the strong correlation between IMR and the prevalence of underweight among 0–5-month-old infants. Because of their higher risk of dying when they are malnourished, infants of this age group must not be overlooked in nutrition surveysReference Golden11. Furthermore, if the MDG of a two-thirds reduction in U5 mortality rates by 2015 is to be met, there is an urgent need also to consider undernutrition in infants aged 0–5 months.
Presently, the majority of emergency nutrition guides recommend measuring infants from 6 months and/or longer than 65 cm7, 8, 10. Interestingly, the effect of excluding 0–5-month-old infants changed the prevalence of wasting only minimally, a finding that could justify their exclusion from surveys in emergencies. However, we argue that including infants from birth in nutrition surveys is important in the context of chronic food insecurity as well as in emergencies for the following reasons. There is evidence that infants aged 0–5 months are affected by undernutrition and thus also represent a group particularly ‘vulnerable to nutritional deficiency10 which must not be excluded from nutrition surveys in emergencies. Furthermore, the prevalence of stunting in 0–5-month-old infants can be used as a proxy for pre-crisis maternal nutritional status, which is usually not assessed in emergencies. Assessing anthropometry of infants aged 0–5 months is therefore crucial not only because of the long-term consequences of early undernutrition but also because it can shed light on prenatal undernutrition. This in turn is important, when designing programmes, for prioritising efforts on pre- or postnatal causes of undernutrition. This information could be useful for designing more relevant interventions once the situation is stabilised. As surveys conducted in emergencies often serve as baselines to judge achievements of interventions after the initial crisis, comparable age ranges are especially needed.
The implications for nutrition surveys include: overcoming practical difficulties in taking anthropometric measurements on infants by training personnel, using more accurate scales, and increasing efforts to obtain a precise determination of age through the systematic use of calendars of local events. The cost of including 0–5-month-old infants could be outweighed by the advantage of early detection of undernutrition. The high prevalence of undernutrition in early infancy in the developing world urgently calls for more systematic assessments. There is also a great need to focus the attention of policy-makers on prenatal and early child nutritional status as one of the key indicators of long-term development.
We recommend that organisations working in the development as well as the humanitarian field revise the age selection guidelines of their nutrition surveys to include infants from birth.
Acknowledgements
This study was funded by FAO and FIVIMS (Food Insecurity and Vulnerability Information and Mapping Systems). We thank Pierre Traissac and Davide Arcella for their valuable statistical advice.
Appendix Impact of excluding 0–5-month-old infants from nutrition surveys: data by country

pp – percentage points; U3 – children under 3 years of age; U5 – children under 5 years of age; MICS – Multiple Indicator Cluster Survey; DHS – Demographic and Health Survey; N/A – not available.
Impact of excluding 0–5-month-old infants is the difference in prevalence of stunting, wasting and underweight between the 6–59 month and 0–59 month age groups in U5 and between the 6–35 month and 0–35 month age groups in U3.
*P < 0.05, **P < 0.01, ***P < 0.001 for χ2 tests comparing prevalence in infants aged 0–5 months with prevalence in children aged 6–59 months or 6–35 months.





