Obesity is considered a major public health problem globally. In 2016, almost two billion (39 %) adults 18 years and above were overweight, and 650 (13 %) million had obesity(1). In Saudi Arabia, the percentage of overweight among the Saudi population is 38 %, and the percentage of obesity is 20 %(2). Remarkably, there has been a significant decline of more than 40 % in the occurrence of obesity and overweight among young Saudi individuals from 2012 to 2021. Despite this observable decline, obesity remains prevalent across several demographic factors, including age, sex and geographical distribution, throughout Saudi Arabia(Reference Wahabi, Fayed and Shata3). Obesity is categorised as a low-grade chronic and systemic inflammatory condition. Extensive research has been conducted to develop treatment strategies and preventive measures for this condition(Reference Cani, Osto and Geurts4). It has been documented that there is an association between obesity and the composition of gut microbiota (GM) in human subjects(Reference Jumpertz, Le and Turnbaugh5). Many studies reported that the relative proportion of microbiota varies between individuals with obesity and lean people(Reference Yuan, Yang and Liang6). In addition to this, Bombani et al. found that the GM population differs considerably depending on the degree of obesity(Reference Bombin, Yan and Bombin7).
GM is considered a contributory factor in maintaining energy metabolism and fat storage through many mechanisms(Reference Fontané, Benaiges and Goday8). Indeed, some probiotics have demonstrated anti-obesity properties and can be used as a complementary technique for obesity management(Reference Pedret, Valls and Calderón-Pérez9). Furthermore, probiotics and synbiotics, whether single strain or multi-strain, may have a positive impact on weight loss and other related anthropometric indices in individuals with overweight or obesity(Reference Álvarez-Arraño and Martín-Peláez10,Reference Zou and Chen11) . Furthermore, some studies observed the effects of probiotics/synbiotics in lowering obesity biomarkers such as oxidative stress(Reference Pourrajab, Fatahi and Dehnad12). However, GM diversity and composition are profoundly influenced by the individual host’s diet, lifestyle and environmental factors(Reference Álvarez-Arraño and Martín-Peláez10,Reference Graf, Di Cagno and Fåk13) . The ratio of Firmicutes to Bacteroidetes has also been linked to obesity and sex differences. For example, women exhibited a higher proportion of Firmicutes independent of BMI, while males exhibited a greater percentage of Firmicutes when their BMI was 33 kg/m2 and a lower percentage when their BMI > 33 kg/m2. Notable differences between men and women have also been observed in some microbial strains such as the Bacteroides genus, with lower counts seen in men with morbid obesity than their leaner counterparts, a finding not seen in women(Reference Haro, Rangel-Zúñiga and Alcalá-Díaz14).
Dietary supplementation of probiotics for the purpose of altering GM composition is potentially effective in achieving favourable metabolic outcomes. A recent systematic review indicated that certain strains of probiotics, such as Streptococcus thermophilus, Lactobacillus bulgaricus and Lactobacillus acidophilus, are potentially effective for combating obesity and overweight, particularly when multiple strains are used instead of a single strain. The majority of the studies indicated in the review however were done in Western(Reference Tomé-Castro, Rodriguez-Arrastia and Cardona15) and Southeast Asian populations(Reference Ayob, Muhammad Nawawi and Mohamad Nor16–Reference Ghafar, Yaakup and Ali19). In fact, there is a scarcity of evidence with respect to Arab ethnic groups where cardiometabolic disorders are common. Hence, the present study aimed to examine the potential anti-obesity effects of multi-strain probiotic supplementation in Arab individuals suffering from overweight or obesity. The use of multi-strain probiotics that contain Bifidobacterium strains is of particular interest as it has been shown to affect visceral fat distribution, at least in animal models(Reference Yin20). Consequently, the present study aims to determine whether such strains in combination with others will elicit the same favourable effects in overweight and obese humans.
Methods
Study design
This study is a 12-week, single centre, double-blind, randomised, placebo-controlled trial conducted at the occupational health clinics of King Saud University Medical City, Riyadh, Saudi Arabia.
Subjects
Recruitment was done for students and employees of King Saud University, who received a study invitation and registration link via e-mail. Power calculation was done using G-Power software (version 3·0·10) following the probiotic intervention effect reported by Gomes et al., using waist circumference (WC) as a primary outcome. The obtained power calculation required n 17 participants/group (95 % power, 5 % type 1 error) to detect a difference in WC(Reference Gomes, de Sousa and Botelho21). Enrolment of participants was substantially increased taking into consideration large dropouts.
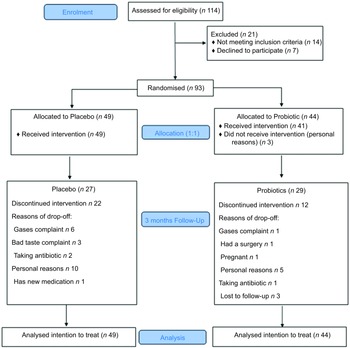
The inclusion criteria included adult Saudi healthy volunteer adults, males and females who were overweight (BMI, BMI 25–29·9 kg/m2), obese (BMI 30–34·9 kg/m2) and/or abdominally obese (defined as having WC >88 cm in females and >102 cm in males), aged 19–40 years, with relatively stable body weight in the last 3 months before the trial. Subjects who suffer from diseases that may affect weight, such as immune system diseases, thyroid disorders, diabetes type 1, diabetes type 2, any type of cancer, neurological disorders, psychiatric disorders and kidney failure, were excluded. Additionally, lactating or pregnant women, those who had gastrointestinal conditions or surgery, on hormone replacement therapy and on antibiotics or probiotic/prebiotic supplements 2 weeks before the trial, were excluded. Eligible subjects were asked to sign consent prior to enrolment and were blinded to the allocation of treatment. Figure 1 shows the study flowchart.

Fig. 1 Flowchart of the study subjects describing their participation and allocation
Blinding
Allocation of treatment was blinded for all participants in this study, including investigators, research staff or subjects. The study product, HEXBIO, MCP® BCMC® strains, was supplied by B-Crobes Laboratory Sdn. Bhd (Ipoh, Malaysia) in sachets packed and coded as numbers. Unblinding was done at the end of the intervention proper, with a formal request letter sent to the company to unblind the study product.
Randomisation
Subjects were allocated in blocks based on their sex, age and BMI. From those blocks, a list of pairs was generated and coded as ‘1’ or ‘2’. The list was sent to the inpatient pharmacy for allocation. The randomisation scheme was computer-generated using MS Excel, in which one subject was assigned either code ‘1’ or ‘2’.
Treatment
A hypoenergetic diet was applied to subjects in both groups, and they were asked to stabilise their physical activity during the entire intervention period, which started 1 week after the first visit and extended until the 12th week. Each subject received three boxes containing either probiotics or placebo, each weighed 3 g and were supplied by HEXBIO® B-Crobes Laboratory Sdn Bhd, Ipoh, Malaysia, which were indistinguishable in terms of colour, weight and shape. The probiotic sachet comprises a granular powder consisting of six strains of microorganisms (30 × 109 CFU). Both the placebo and the probiotic contained the same excipients, with the key difference being that the placebo did not include the live bacteria present in the probiotic. Two sachets should be ingested daily by dissolving their contents in approximately 50 ml of room-temperature water: the first sachet used 10 min prior to the first meal, and the second sachet consumed 10 m prior to the last meal. To promote compliance among subjects with the study instructions, regular contact was used via WhatsApp or phone calls, with weekly communication during the first month and then monthly for the remainder of the intervention period.
Energy-restricted diet and intake
Energy requirements were assessed using the Saudi Ministry of Health https://www.moh.gov.sa/en/HealthAwareness/MedicalTools/Pages/CalorieCalculate.aspx, taking into consideration sex, age and the type of daily physical activity for each of the study subjects. Given that the study’s target population consisted of overweight and obese adults, a hypoenergetic diet was prescribed to all participants, with a reduction of 300–500 calories tailored to each participant’s needs. Each participant received a personalised calorie calculation guide and a healthy eating guideline developed by the Saudi Ministry of Health. According to the Food Calorie Calculator for Weight Loss on the Saudi Ministry of Health, the recommended distribution of energy is as follows: 45–60 % carbohydrates, 20 % protein and 15–35 % fat. To ensure adherence to the study protocols, regular follow-up was maintained through WhatsApp or phone calls, with weekly check-ins during the first month and monthly follow-ups for the remainder of the intervention period. Dietary intake was evaluated using the 24-h dietary recall questionnaire during the 3 d of the week with 1 d during the weekend. All macronutrients were analysed using a validated food processor nutrition analysis software (ESHA Research).
Physical activity and anthropometric measurements
The physical activity was assessed at baseline and follow-up using the International Physical Activity Questionnaire (IPAQ), short form, self-administered format(Reference Craig, Marshall and Sjostrom22). Anthropometrics included weight, WC, hip circumferences (HC) and height; they were assessed with the subject wearing lightweight clothing. Weight was measured before breakfast, using a calibrated column scale to the nearest 0·1 kg. WC and HC were measured to the nearest 0·5 cm using a standard tape measure. Height was taken using stadiometer to the nearest 0·5 cm. Based on anthropometric data, the body adiposity markers were estimated depending on the following equations:
-
BMI = weight (kg)/height2 (m).
-
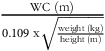
Conicity Index (CI) =
 ${{{\rm{WC\;}}\left( {\rm{m}} \right)}} \over {{0.109{\rm{\;x}}\sqrt {{{{{\rm{weight\;}}\left( {{\rm{kg}}} \right)}} \over {{{\rm{height\;}}\left( {\rm{m}} \right)}}}} }}$
(Reference Valdez, Seidell and Ahn23).
${{{\rm{WC\;}}\left( {\rm{m}} \right)}} \over {{0.109{\rm{\;x}}\sqrt {{{{{\rm{weight\;}}\left( {{\rm{kg}}} \right)}} \over {{{\rm{height\;}}\left( {\rm{m}} \right)}}}} }}$
(Reference Valdez, Seidell and Ahn23).
Biochemical analyses
All analyses were done at the Chair for Biomarkers of Chronic Diseases, King Saud University, using the colorimetric method. Fasting blood samples were taken twice, at baseline and at the end of the intervention. The Konelab routine analyzer (Konelab) was used for routine analysis of fasting blood glucose (FBG), lipid profiles including total cholesterol (TC), HDL-cholesterol and TAG. LDL-cholesterol was calculated using the Friedewald equation, according to Whelton et al. (Reference Whelton, Meeusen and Donato24). The D-10 device (BIO-RAD) was used to determine HbA1c levels.
Data analysis
Data were analysed SPSS version 23·0 (IBM SPSS). Categorical data were presented as frequencies (N) and percentages (%). Independent t test and Mann–Whitney U tests were used to compare baseline differences between groups. Repeated measure ANOVA was used to assess main and interaction effects. Bonferroni corrections were applied to adjust for multiplicity. Intent-to-treat analysis was done, and the last observation carried forward method was applied in case of missing values in all variables. Per-protocol analysis was applied only to the primary outcome (WC). A P-value <0·05 was considered statistically significant.
Results
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results and their interpretation, as well as the experimental conclusions that can be drawn.
Baseline characteristics of subjects
Baseline characteristics of subjects are summarised in Table 1. No significant differences between groups were observed in all parameters. Majority of the subjects were females (sixty-five females and twenty-eight males). The number of dropouts was n 34 (36 %), and this created an uneven allocation in the final analysis (Fig. 1). No serious adverse effects or symptoms were reported with the study product; however, there was a presence of the normal expected temporary side effects, including bloating, diarrhoea and colic sometimes.
Table 1 Baseline characteristics of subjects

Note: Data presented as mean ± sd for normal and median (Quartile 1–Quartile 3) for non-normal variables. WC, waist circumference; HC, hip circumference; WHR, waist-hip ratio; CI, Conicity Index; BP, blood pressure; HbA1c, glycated Hb; FBG, fasting blood glucose; TC, total cholesterol; ST, sitting time; PA, physical activities; MET, metabolic equivalent tasks. P-values obtained from independent t test and Mann–Whitney U test for normal and non-normal variables, respectively; P < 0.05 considered significant.
Primary outcome and anthropometric results
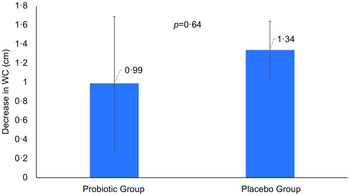
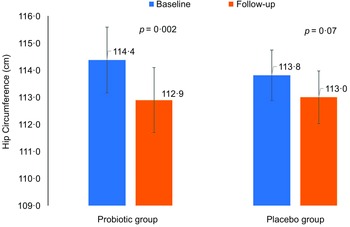
Differences in anthropometrics using intent-to-treat analysis were summarised in Table 2. The primary outcome was the difference in WC between groups. Between-group comparisons showed no significant differences in primary outcome and other indices. Per-protocol analysis of the primary outcome revealed no clinically significant difference in WC as indicated by insignificant main (P = 0·23) and interaction effects (P = 0·41) (not included in the table). Mean changes in WC were also NS (Fig. 2). However, many body measurements were significant at 12 weeks favouring the probiotic group, including WC, which modestly decreased over time (P = 0·07), and body weight (–0·9 kg, P = 0·02), with no significant change noticed in the placebo group. A significant reduction in BMI (P = 0·04) and HC (P = 0·002) was also observed in the probiotic group post-intervention (Figs. 3 and 4). Significant changes were also noticed in placebo group, including lower WC (P = 0·01) and CI (P = 0·03).
Table 2 Baseline and post-intervention changes in anthropometrics

Note: Data presented as mean ± sd for normal and median (Quartile 1–Quartile 3) for non-normal variables; significant at P < 0.05. WC, waist circumference; HC, hip circumference; WHR, waist-hip ratio; CI, Conicity Index; BP, blood pressure. P-values obtained from repeated measures ANOVA; P < 0.05 considered significant.

Fig. 2 Mean changes in waist circumference overtime in both groups

Fig. 3 Mean changes in BMI overtime in both groups

Fig. 4 Mean changes in hip circumference overtime in both groups
A total of sixty-three participants were obese (thirty probiotics, thirty-three placebo), and thirty (fourteen probiotic, sixteen placebo) were overweight. Six (9·5 %) participants became overweight from obese (three (10·0 %) probiotic, three (9·1 %) placebo, P = 0·90). Four (13·3 %) participants became normal from overweight (two (14·3 %) probiotic, two (12·5 %) placebo, P = 0·89), whereas two became obese from overweight (two probiotic). A total of forty participants (nineteen probiotic, twenty-one placebo) had higher WC (WC > 102 for men and > 88 for women). Eight (20·0 %) participants with higher WC became normal (WC ≤ 102 for men and ≤ 88 for women) (four (21·1 %) probiotic, four (19·0) placebo, P = 0·87). Only one participant increased their WC to abnormal level (WC > 102 for men and > 88 for women) who belonged to probiotic group. A total of eighteen participants (eleven probiotic, seven placebo) were above the threshold of abdominal obesity (in men as a waist-to-hip ratio of at least 0·90; for women, it’s a ratio of 0·85 or more). Out of eleven, three (27·3 %) became normal in probiotic group, while no one in the placebo group reduced their WHR to normal level (P = 0·130). The suggested CI cut-off points to diagnose obesity and metabolic abnormalities are 1·200 for males and 1·180 for females. A total of thirty-seven participants (eighteen probiotic, nineteen placebo) were above the threshold. Four (10·8 %) participants became normal (two (11·1 %) probiotic, two (10·5 %) placebo, P = 0·95).
Changes in dietary intake and physical activities post-intervention
Changes in dietary intake post-intervention are shown in Table 3. Between-group analysis showed no differences, but significant group*time interaction showed that fat intake decreased significantly in favour of the probiotic group (P = 0·02), while within-group analysis showed that there was a significant reduction in the energy (P = 0·002), protein (P = 0·007) and fat intake (P = 0·001) post-intervention, but only in the probiotic group. On the other hand, intake of carbohydrate and fibre was significantly reduced in the placebo group (P-values 0·04 and 0·01, respectively). No significant differences were observed in physical activity and sitting hours post-intervention (Table 3).
Table 3 Baseline and post-intervention changes in dietary intake and physical activity

Note: Data presented as median (Quartile 1–Quartile 3); significant at P < 0.05. CHO, carbohydrates; ST, sitting time; PA, physical activities; MET, metabolic equivalent tasks. P-values obtained from repeated measures ANOVA; P < 0.05 considered significant.
Changes in biochemical parameters post-intervention
Table 4 shows changes in the glycaemic and lipid profiles during the intervention. The between-group analysis was NS; however, within-group analysis showed that there was a significant reduction in HbA1c in both probiotic and placebo groups (P = 0·001), while the FBG reduced only in the probiotic group (P = 0·002). The lipid profile results changed by the end of the intervention; both TC and LDL-cholesterol reduced within the probiotic group, but this reduction was significant in LDL-cholesterol only (P = 0·02); however, TAG increased within both the probiotic and placebo groups (P-values 0·001 and 0·01, respectively) (Table 4).
Table 4 Baseline and post-intervention changes in glycaemic and lipid profiles

Note: Data presented as mean ± sd for normal variables, while non-normal variables are presented as median (interquartile range); significant at P < 0.05. HbA1c, glycated Hb; FBG, fasting blood glucose; TC, total cholesterol. P-values obtained from repeated measures ANOVA; P < 0.05 considered significant.
Discussion
The main findings of the present study demonstrated that although multi-strain probiotic supplementation for 12 weeks among Arab adults with overweight or obesity had statistically significant effects on many anthropometric measurements, including body weight, BMI and HC, these effects were not clinically meaningful when compared with placebo, with the exception of fat intake. These findings are in opposition with Michael et al., who used a multi-strain of lactobacilli and bifidobacteria (5 × 1010 CFU). They found a significant weight reduction favouring the probiotic group after 6 months of treatment (–1·30 kg, P = 0·0001). Also, the reduction was significant in BMI (−1·5 %, P < 0·0001), WC (−0·9 %, P < 0·0001) and WHR (−1·2 %, P < 0·0001). Interestingly, the reduction was greater in individuals who were overweight (−1·9 %, −1·5 kg) than individuals with obesity (−1·2 %, −1·06 kg)(Reference Michael, Jack and Masetti25). Additionally, in a 3-week randomised controlled trial, consumption of probiotic-fortified cheese led to a number of advantageous changes in health indicators among individuals with overweight or obesity; the reduction was larger in the probiotic group than the control group in body weight (−5·7 v. –4·4 kg, P = 0·08) and BMI (−2 v. –1·6 kg/m2, P = 0·03), suggesting probiotic positive effects on metabolic disorders(Reference Sharafedtinov, Plotnikova and Alexeeva26). Likewise, greater differences in body measurements were noticed for subjects who took the multi-strain probiotic (1 × 109 CFU) combined with a diet than the group who only applied a diet. The decrease was in WC (P = 0·03), WHR (P = 0·02) and CI (P = 0·03)(Reference Gomes, de Sousa and Botelho21). Furthermore, the administration of Lactobacillus gasseri SBT2055 for healthy adults revealed a significant decrease in the visceral, subcutaneous, total fat areas, body weight, BMI, WC, HC and WHR within the probiotic group and between-group comparisons at baseline and week 12, demonstrating that the inhibition of lipid absorption is a possible mechanism underlying the observed effects(Reference Kadooka, Sato and Imaizumi27). In another randomised controlled trial, taking the probiotic Lactiplantibacillus plantarum IMC 510® (1·5 × 1010 CFU) for 3 months led to a significant drop in body weight (P = 0·03), BMI (P = 0·03), WC (P = 0·04) and WHR (P = 0·04)(Reference Pagliai, Coman and Baldi28). The favourable effects observed within the probiotic group in the present study may be attributed to probiotic supplementation, considering that a hypoenergetic diet was applied to both the probiotic and placebo groups. The lack of clinically significant effect as compared with previous findings can be attributed to ethnic groups, as different populations may exhibit varying lag time in biological response to probiotic supplementation. According to Gupta and colleagues, geographical variations in microbiome structure can significantly affect how a population responds to microbiome-based therapeutics, including probiotics(Reference Gupta, Paul and Dutta29). Additionally, based on the existing understanding of the effectiveness of probiotic supplementation in mitigating overweight and obesity, current systematic review emphasised that probiotic genus, strain, dosage, duration of supplementation and delivery matrix were known as significant factors on the anti-obesity effects of probiotics(Reference DiMattia, Damani and Van Syoc30).
Similar to the present findings, one study assessed the effects of probiotic supplementation on weight reduction in healthy, young adult females using a 6-week supplementation with Bifidobacterium lactis BS01 and Lactobacillus acidophilus LA0 (2 × 109 CFU) without involving dietary restrictions. The findings showed that the BMI decreased higher in the supplemented group after treatment (by 4·1 % compared with 0·81 % in the placebo group); however, the differences were NS. WC was elevated by 0·67 % in the supplemented group and reduced by 1·33 % in the placebo group. Changes in either group were not statistically significant. Similarly, WHR increased by 1·195 % in the supplemented group and decreased by 1·36 % in the placebo group. The effect sizes were all modest and insignificant(Reference Czajeczny, Kabzińska and Wójciak31). Among treatment naïve subjects with type 2 diabetes, a significant improvement in WHR (P = 0·02) was observed favouring the probiotics group when compared with the placebo group, and no differences were noted in weight or BMI. However, within the group, this significant alteration was absent in weight, BMI and WHR following the administration of multi-strain probiotics over a period of 3 months(Reference Sabico, Al-Mashharawi and Al-Daghri32). Moreover, Zarrati and colleagues used three different groups for the intervention: probiotic yogurt enriched in multi-strain Lactobacillus and Bifidobacterium with a low caloric diet (PLCD), probiotic yogurt without a low caloric diet and regular yogurt with a low caloric diet (RLCD). The results revealed that the RLCD group had a greater reduction in body weight, BMI and HC (–24·87, –21·9 and –23·18, respectively) compared with the PLCD group (–24·23, –21·55 and 21·84, respectively) after the intervention. However, all changes were NS. In contrast, among all assessed variables, only WC changes were larger in the PLCD group compared with the RLCD group (–2·78 and –2·3, respectively), although this difference was still statistically insignificant (P = 0·7)(Reference Zarrati, Salehi and Nourijelyani33). Similarly, Omar and others reported no significant differences in body weight or fat mass at the conclusion of the study; body weight fluctuations were less than 5 %(Reference Omar, Chan and Jones34).
Regarding dietary intake, the probiotic group showed a significant reduction in calories compared with the baseline (–387·3 kcal/d, P = 0·002), but this reduction was not statistically significant when compared with the placebo group (–63 kcal/d, P = 0·09). This reduction is suggested to be primarily due to the decrease in fat intake (–29 %, P = 0·001) in the probiotic group, while the placebo group exhibited a 6 % reduction in fat intake (P = 0·41) compared with the baseline. Consuming excessive dietary fat not only elevates the body’s exposure to potentially pro-inflammatory free fatty acids and their derivatives but also suppresses the expression of tight junction proteins, such as zonulin and occludin, thereby increasing intestinal permeability(Reference Sivamaruthi, Kesika and Suganthy35). This promotes the absorption of endotoxins leading to metabolic endotoxemia. Therefore, Saudi clinical practice guideline emphasises on adopting a low-calorie diet with a targeted reduction in fat intake to less than 30 %(Reference Aldubikhi36). Likewise, Mahadzir et al. reported that after a 4-week period of MCP (3·0 × 1010 CFU) consumption, the same product of this study, the subjects in the probiotic group exhibited a significant decrease (P = 0·04) in their energy consumption, roughly 300 kcal/d, in comparison with their initial intake levels(Reference Mahadzir, Shyam and Barua37). Additionally, the reduction in food intake was also noticed in the Canadian study, but it was non-significant; however, the energy intake seemed to be consistently lower in the women probiotic group when compared with women in the placebo group(Reference Sanchez, Darimont and Panahi38). In contrast with current results, the findings of a study carried out in Japan, there was no significant difference in the intake of energy or main nutrients in the groups at any of the three different time points (week 4, week 8 and week 12)(Reference Kadooka, Sato and Imaizumi27). Hence, the observed reductions in body measurements cannot be solely attributable to changes in calorie consumption; instead, they may be attributable to the impact of synbiotics on the GM of individuals, hence inducing alterations in energy metabolism and perhaps facilitating weight reduction irrespective of calorie limitation(Reference Oraphruek, Chusak and Ngamukote39).
The mechanisms behind the reduction in anthropometrics and food intake are intertwined. The alteration of host energy homeostasis is one mechanism to reduce weight(Reference Fontané, Benaiges and Goday8), which includes the harvesting, storing and expenditure of energy obtained from the diet. For example, a 20 % increase in Firmicutes and a 20 % decrease in Bacteroidetes were associated with an additional energy harvest of 150 kcal/d(Reference Jumpertz, Le and Turnbaugh5). Also, SCFA, which are by-products of microbial metabolism, may help regulate host homeostasis in different ways. For example, SCFA affect the production of serotonin (5-HT), which prolongs satiety and reduces food intake(Reference Ridaura and Belkaid40). SCFA can also induce satiety in other ways; for example, acetate and propionate stimulate leptin secretion(Reference Zaibi, Stocker and O’Dowd41); butyrate releases glucagon-like peptide-1(Reference Yadav, Lee and Lloyd42). Additionally, through the postprandial phase, both glucagon-like peptide-1 and peptide YY are also produced in the intestine under the effect of SCFA(Reference Kim, Keogh and Clifton43,Reference Carvalho and Saad44) . Also, Backhed and his colleagues said that the change in body composition caused by probiotics could be the result of fasting-induced adipose factor suppression in the gut, which would change the production of SCFA(Reference Bäckhed, Ding and Wang45).
Similar to the present study findings, applying a hypoenergetic diet and probiotic cheeses lowered the values of FBG by 18 % in the treatment and control groups; yet only the control group achieved statistical significance(Reference Sharafedtinov, Plotnikova and Alexeeva26). Administration of a multi-strain probiotic supplement over a period of 6 months also resulted in a significant reduction in circulating FBG (38 %) as compared with the baseline among treatment naïve subjects with T2DM; however, there were no statistically significant differences in FBG between the placebo and probiotic groups at both the 3-month and 6-month time points(Reference Sabico, Al-Mashharawi and Al-Daghri46). In a 3-month intervention using a multi-strain probiotic for athletes, both the treatment group and the control group of female participants saw a drop in their FBG and HbA1c levels. In addition, a beneficial decrease in FBG concentration was observed in the male participants who received the probiotic intervention, whereas there was an increase in the male participants who received the placebo(Reference Smarkusz-Zarzecka, Ostrowska and Leszczyńska47).
In terms of lipid profile, no significant changes between groups were observed in the present study. Other findings confirm that multispecies probiotics have a positive effect on the lipid profile of postmenopausal women with obesity(Reference Szulińska, Łoniewski and van Hemert48). Another randomised controlled trial, which lasted for 3 weeks, evaluated the effect of a hypoenergetic diet with 50 g/d of full-fat probiotic cheese containing L. plantarum TENSIA on the lipid profiles. The combination of diet and probiotics reduced the values of TC and LDL-cholesterol significantly in the treatment group and control groups, while HDL-cholesterol and TAG were significantly reduced in the treatment group only(Reference Sharafedtinov, Plotnikova and Alexeeva26). In the same context, a meta-analysis finding found that probiotic yogurt significantly lowers TC and LDL-cholesterol levels in subjects with mild to moderate hypercholesterolaemia, specially, studies lasting more than 4 weeks, but there was no significant effect on HDL-cholesterol or TAG levels(Reference Pourrajab, Fatahi and Dehnad12). Additionally, Sabico et al. reported the consumption of multi-strain probiotics having significant benefits in terms of reduced TAG (48 %), TC (19 %) and the total/HDL-cholesterol ratio (19 %) in the probiotic group(Reference Sabico, Al-Mashharawi and Al-Daghri46). In contrast, Michael et al. noticed that the TC, HDL-cholesterol and TAG levels remained unchanged through 6 months for the study population; moreover, LDL-cholesterol levels increased 2·7 % from baseline in both the probiotic group (0·087 mmol/l, P = 0·07) and the placebo group (0·088 mmol/l, P = 0·06)(Reference Michael, Jack and Masetti25). Similarly, Kadooka et al. also found no significant changes in both lipid metabolism-related parameters, such as TC, LDL-cholesterol and HDL-cholesterol, and physiological parameters, such as blood test, urine test, blood pressure or pulse rate(Reference Kadooka, Sato and Imaizumi27).
Several hypotheses have been suggested on the mechanisms by which probiotics can reduce cholesterol levels, based mostly on in vitro research, for instance, bile salt hydrolase activity, binding of cholesterol to the probiotic cellular surface and production of SCFA(Reference Ishimwe, Daliri and Lee49). Bile salt hydrolase, which is expressed in probiotic strains, deconjugates bile salt to become less efficiently reabsorbed than conjugated bile acids, leading to the excretion of significant amounts of free bile acids in human faeces; thus, more cholesterol is needed to replace excreted bile salt, which ultimately reduces TC in the blood(Reference Pavlović, Stankov and Mikov50).
The study’s limitations include its duration of 12 weeks. Despite observing significant changes within the probiotic group, the absence of clinically significant differences between groups suggests that extending the treatment period might be necessary to achieve noticeable clinical outcomes. Michael et al. (2020) demonstrated that a meaningful impact on individuals with obesity typically requires at least 6 months of supplementation with a multi-strain probiotic(Reference Michael, Jack and Masetti25). Nonetheless, this study holds significance as the first of its kind to investigate the anti-obesity effects of multi-strain probiotics in a homogeneous ethnic Arab Saudi adult population. Moreover, it maintains merit due to its implementation of adequate statistical power and rigorous blinding procedures. Furthermore, the intervention protocol allowed for an assessment of probiotics in conjunction with the benefits of a hypoenergetic diet intervention.
Conclusions
In conclusion, a 12-week supplementation of multi-strain probiotics among overweight or obese Saudi adults showed beneficial effects on anthropometric indices, FBG and LDL-cholesterol compared with baseline, with no such improvements observed in the placebo group. However, these changes did not reach clinical significance with the exception of dietary fat intake in favour of the probiotics group. Future research should consider longer trial durations to verify whether alterations in GM composition would lead to clinically meaningful outcomes following extended probiotic intake.
Acknowledgements
The authors thank B-Crobes Laboratory Sdn. Bhd., Malaysia, for providing the probiotics ‘MCP® BCMC® strains’ and placebo sachets to be used in the clinical trial. B-Crobes did not contribute to the study design, data collection, data analysis or interpretation, manuscript writing or the decision to publish the results. The authors are also thankful to the work team from inpatient pharmacy at King Khalid University Hospital: Doaa Abdulaziz Bintaleb, Sondus Issam Ata and Abeer Alshareef for performing subject randomisation and dispensation of the study product.
Financial support
This work was supported by Researchers Supporting Project number (RSP2024R21), King Saud University, Riyadh, Saudi Arabia.
Conflict of interest
All authors declare no conflict of interest.
Authorship
Conceived and designed the experiments and investigation: S.M.A., H.A.F. and N.M.D. Recruited the patients and performed the data collection: S.M.A., T.A.B. and S.A. Performed and validated the sample collection and analysis: S.M.A., S.S.S. and S.D.H. Supervision: H.A.F. and N.M.D. Writing – original draft: S.M.A. Writing – review and editing: H.A.F., N.M.D. and S.S. The published version of the manuscript has been reviewed and approved by all authors.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of King Saud University College of Medicine, Riyadh, Saudi Arabia (IRB Log No. E-20-5503, 12·07·2021). Written consent was obtained from all subjects involved in the study.
Data availability statement
Data that have been collected or analysed for the current study are accessible from the corresponding author upon reasonable request.