Vitamin D deficiency (defined as serum 25-hydroxyvitamin D (25(OH)D) concentrations <25 or <30 nmol/l(1,Reference Bresson, Burlingame and Dean2) ) is a global health concern, with dark-skinned ethnic groups found to exhibit a higher prevalence compared to populations of white European ancestry(Reference Schleicher, Sternberg and Looker3,Reference Vearing, Hart and Charlton4) . In the US, data from the 2007 to 2018 National Health and Nutrition Examination Survey showed that the prevalence of vitamin D deficiency (<25 nmol/l) was highest in non-Hispanic black populations (56·6 %) compared to non-Hispanic white (26·4 %), Other (10·1 %) and Mexican American populations (6·9 %)(Reference Hu, Chen and Shi5). In the UK, an analysis of data from the UK Biobank cohort reported the prevalence of vitamin D deficiency (<25 nmol/l) to be highest in South Asian populations at 61·4 %, followed by 34·5 % in African populations, and 11·5 % in white populations(Reference Vearing, Hart and Charlton4). Additionally, dark-skinned ethnic groups have been disproportionately affected by pandemics caused by respiratory viruses, such as the 2009 Influenza A (H1N1) pandemic(Reference Thompson, Jungk and Hancock6) and coronavirus disease 2019 (COVID-19) pandemic(Reference Williamson, Walker and Bhaskaran7,Reference Mackey, Ayers and Kondo8) . In the COVID-19 pandemic, these disparities were not fully explained by sociodemographic characteristics and comorbidities, suggesting alternative factors may be involved(Reference Williamson, Walker and Bhaskaran7). Lower vitamin D status in these populations has been suggested as a contributing factor, yet the evidence linking vitamin D and acute respiratory tract infections (ARTI) remains limited(Reference Getachew and Tizabi9).
While existing guidelines for vitamin D supplementation have been largely informed by evidence on musculoskeletal health requirements(Reference Pludowski, Holick and Grant10), recent evidence suggests that vitamin D plays a role in both immune modulation and infectious disease(Reference Hewison11). Two key observations support the scientific rationale for the immunomodulatory role of vitamin D. Firstly, the vitamin D receptor is expressed in cells of the innate and adaptive immune system, including B and T cells, monocytes, macrophages and dendritic cells. Secondly, immune cells express the enzyme 25(OH)D-1α-hydroxylase (CYP27B1) that acts to convert 25(OH)D3 to the active form, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3)(Reference Hewison11). Cohort, case-control and cross-sectional studies in adults and children have demonstrated associations between low 25(OH)D concentrations and increased risk of ARTI(Reference Pham, Rahman and Majidi12,Reference Li, Liu and Bao13) , but results from randomised controlled trials (RCT) of vitamin D supplementation have been less consistent(Reference Jolliffe, Griffiths and Martineau14,Reference Martineau, Hanifa and Witt15) . A meta-analysis of 25 RCT including 11 321 participants found that vitamin D supplementation reduced the risk of ARTI by 11 %, and among those receiving daily or weekly vitamin D, protective effects were found to be stronger in those with vitamin D deficiency (<25 nmol/l) (OR: 0·30, 95 % CI: 0·17, 0·95)(Reference Martineau, Jolliffe and Hooper16). A more recent meta-analysis of 43 RCT including 48 488 participants found similar results, but it did not find enhanced protection in participants with the lowest 25(OH)D concentrations at baseline(Reference Jolliffe, Martineau and Griffiths17). However, both meta-analyses reported considerable heterogeneity among trials.
Examining ethnic health disparities is crucial for setting public health priorities and maximising benefits for individuals. Of all published reviews on vitamin D and prevention of ARTI(Reference Pham, Rahman and Majidi12,Reference Jolliffe, Griffiths and Martineau14,Reference Martineau, Jolliffe and Hooper16–Reference Abioye, Bromage and Fawzi18) , none have included a subgroup analysis for ethnicity. Additionally, no systematic review has summarised the evidence for the association between vitamin D and immune function in at-risk ethnic groups. Therefore, this systematic review aimed to examine the evidence for the relationship between vitamin D status or vitamin D supplementation in dark-skinned ethnic groups and the concomitant effects on both immune function biomarkers and prevention of ARTI. Additionally, a meta-analysis was performed to quantify the effect of vitamin D supplementation on ARTI incidence in ethnic subgroups.
Methods
The review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines(Reference Moher, Liberati and Tetzlaff19). A protocol had not been published a priori, nor was this review registered.
Search strategy
Systematic literature searches were performed in six electronic databases (PubMed, Embase, Web of Science, ScienceDirect, Scopus and Cochrane Central) for published articles of all years of record up until the date of 15 December 2021. Search terms were determined by examining key words in the literature and were agreed by all authors. The search terms included ‘Vitamin D’ and ‘Immune Function’ and ‘Acute Respiratory Infection’ and ‘Ethnic Groups’. The complete search strategy can be found in the online supplementary material. Reference lists of included studies and relevant reviews were hand-searched for additional articles.
Study eligibility criteria
Studies identified from the literature search were included when the following criteria were satisfied:
(1) Population: As Fitzpatrick’s skin type is not always reported or measured within studies, participants from any age group belonging to an ethnic or racial group other than white, with or without a white comparator were included. If a white comparator was included, results were stratified by non-white ethnicity.
(2) Study design: RCT (vitamin D supplementation v. placebo or other control), or cross-sectional, case-control, or cohort studies conducted in human subjects and reported in English language. In vitro or animal studies were excluded, along with review articles, book chapters, letters to the editor, conference papers, theses, case reports, abstract-only articles and meta-analyses.
(3) Exposure: Examining the effect of serum concentrations of vitamin D metabolites, dietary intake of vitamin D (from food and/or supplementation) or vitamin D deficiency.
(4) Outcomes: Biomarkers of immune function such as differential cell counts, concentrations of leucocyte types (e.g. leucocytes, lymphocytes, monocytes, neutrophils, granulocytes, dendritic cells), lymphocyte subsets (e.g. T, B, natural killer cells), antimicrobial proteins (e.g. cathelicidin, defensins), antibodies or antibody responses, immune cell activity, cytokines and C-reactive protein (CRP); incidence of ARTI, including upper respiratory tract infections and lower respiratory tract infections. Studies investigating COVID-19 were included, while tuberculosis and other chronic respiratory infections were excluded.
Screening process
Retrieved articles were evaluated for relevancy using the software Rayyan (http://rayyan.qcri.org). Two reviewers (ARB and SALN) independently screened titles and abstracts of the identified articles. A third reviewer (KHH) provided clarification when required. Full-text articles were retrieved before excluding ethnic groups. Conflicts over included articles were resolved by consensus. Study authors of potentially eligible studies were contacted if clarification or more information was required.
Data extraction
A customised data collection template was used to extract data from all studies. Data extracted included study characteristics (i.e. author, publication year, country), participant characteristics (i.e. population description), ethnic/racial groups (i.e. author-defined terms and collection method), age, sex, sample size, serum 25(OH)D concentrations and assay) and outcomes of interest (i.e. immune biomarkers, incidence frequency of ARI). If serum 25(OH)D was reported in ng/ml, it was converted to nmol/l by multiplying by 2·496 (1 ng/ml is equivalent to 2·496 nmol/l). For RCT, a description of the intervention and/or placebo and follow-up duration was also extracted. If vitamin D supplement dose was reported in international units, it was converted to µg (1 µg is equivalent to 40 international units). Statistical analyses and results were extracted for each outcome of interest, including number of participants (n) or events, mean, sd, correlation coefficients, P values and OR with 95 % CI. Where applicable, results were extracted from the models with the most covariates adjusted for.
Quality assessment
The methodological quality evaluation of all studies was conducted by one researcher (ARB). The Jadad Scale assessment(Reference Jadad, Andrew Moore and Carroll20) was used for RCT, the Newcastle–Ottawa Scale assessment(Reference Wells, Shea and O’Connell21) was used for cohort studies, and a modified version of the Newcastle–Ottawa Scale by Herzog et al. (Reference Herzog, Álvarez-Pasquin and Díaz22) was used for cross-sectional studies. The Jadad Scale awarded up to five points per study based on randomisation and method adequacy, blinding and method adequacy, as well as the accounting of all participants. The Newcastle–Ottawa Scale awarded up to nine stars for cohort studies while the adapted version awarded up to eight stars for cross-sectional studies for participant selection, comparability and assessment of outcome. A list of quality assessment criteria specific to the study subject was developed to standardise the decision for assessing risk of bias. Additionally, cut-off points were determined to aid the interpretation of total quality scores. Further details regarding these criteria and cut-offs can be found in the online supplement material (see online supplementary material, Supplementary Tables S1–3).
Data synthesis
Results and statistical analyses of the included studies were presented in a tabular form, and a narrative synthesis was used to summarise the studies. A meta-analysis was conducted for studies reporting ARTI incidence in placebo-controlled trials with appropriate data and low risk of bias, and combined ethnic groups were described according to the most appropriate terms. Studies investigating immune biomarkers were not included in a quantitative meta-analysis as there was a large degree of heterogeneity in the methods for participant populations (e.g. disease characteristics, age categories), study design and outcomes measured. Moreover, of the three RCT investigating immune biomarkers, only two had usable data.
Statistical analysis
The meta-analysis was performed using Review Manager (RevMan, Version 5·3, Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) software. Random-effects meta-analysis was conducted using the proportion of participants experiencing one or more ARTI in each ethnic group in the trials to obtain pooled OR and 95 % CI to estimate the effect of vitamin D supplementation on the risk of at least one ARTI compared to placebo. Heterogeneity was investigated using the I 2 values of 25 %, 50 % and 75 % corresponding to low, moderate and high levels of heterogeneity, respectively. The number of studies was too small to perform a sensitivity analysis or to assess publication bias.
Results
Study selection
The details of the search strategy and selection process are presented in Fig. 1. The literature search identified 2077 articles for screening after duplicates were removed. After title and abstract screening, 2005 records were removed and full texts of 72 articles were assessed for inclusion. An additional article was identified from screened reference lists and included in the full-text screening. Of these studies, 18 articles fulfilled the eligibility criteria and were suitable for inclusion. Several articles were excluded after assessing full texts because ineligible ethnic groups were included in the analyses, or authors mentioned individuals were from a specific country but did not specify ethnicity or ethnic origin. Moreover, many studies were considered potentially eligible because ethnicity was mentioned in the baseline characteristics but were subsequently excluded because either no statistical analyses were reported for the effect in ethnic groups or ethnicity was only used as a covariate. Authors contacted did not respond to requests for data. A full breakdown of exclusion reasons is described in Fig. 1.

Fig. 1 PRISMA flow chart of systematic review on the effect of vitamin D on immune function and prevention of acute respiratory infections
Characteristics of included studies
Tables 1 and 2 present details of the characteristics of included studies. Seven RCT (39 %)(Reference Chandler, Scott and Drake23–Reference Hibbs, Ross and Ann Kerns29), seven cross-sectional studies (39 %)(Reference Antwi, Huffman and Sullivan30–Reference Yao, Tu and Chang36) and four cohort studies (18 %)(Reference Szeto, Zucker and LaSota37–Reference Meltzer, Best and Zhang40) were included in the present review. The studies were published between 2013 to 2021 and were from the US (n 11; 61 %), India (n 1; 6 %); Malaysia (n 1; 6 %), Taiwan (n 1; 6 %), New Zealand (n 1; 6 %), Australia (n 1; 6 %), Canada (n 1; 6 %) and France (n 1; 6 %).
Table 1 Characteristics and outcomes of included RCT examining the effect of vitamin D on immune function or acute respiratory infections

25(OH)D, 25 hydroxyvitamin D; ARTI, acute respiratory tract infection; CLIA, chemiluminescent immunoassay; CRP, C-reactive protein; DEQAS, vitamin D External Quality Assessment Scheme; EIA, enzyme immunoassay; EQA, external quality assessment; HR, hazard ratio; IQR, interquartile range; LC-MS/MS, liquid chromatography-tandem mass spectrometry; LRTI, lower respiratory tract infection; NIST, National Institute for Standards and Technology; NR, not reported; PBMC, peripheral blood mononuclear cell; RCT, randomised controlled trials; RR, risk ratio; TNF, tumour necrosis factor; US, United States; URTI, upper respiratory tract infection.
* Follow-up was 3 months for data contributing to meta-analysis.
Table 2 Characteristics and outcomes of included cross-sectional and cohort studies examining the effect of vitamin D on immune function and acute respiratory tract infections

25(OH)D, 25 hydroxyvitamin D; ARTI, acute respiratory tract infection; CLIA, chemiluminescence immunoassay; CRP, C-reactive protein; EIA, enzyme immunoassay; ECLIA, electrochemiluminescence assay; EQA, external quality assessment; HIV, human immunodeficiency virus; HR, hazard ratio; hs-CRP, high sensitivity C-reactive protein; ID-LC-MS, isotope dilution-liquid chromatography-tandem mass spectrometry; IQR, interquartile range; LC-MS, liquid chromatography-tandem mass spectrometry; LRTI, lower respiratory tract infection; NHANES, National Health and Nutrition Examination Survey; NIST, National Institute for Standards and Technology; NR, not reported; PBMC, peripheral blood mononuclear cell; RCT, randomised controlled trials; RR, risk ratio; SARS-CoV, severe acute respiratory syndrome coronavirus; T2D, type 2 diabetes; TNF, tumour necrosis factor; sTNF-R, soluble tumour necrosis factor receptor; US, United States; URTI, upper respiratory tract infection.
Most participants were of white ethnicity (65 %), followed by black/African (23 %), Asian/Pacific (7 %), Mexican (2 %), Other (1 %), Māori (<1 %), Hispanic (<1 %) and Indigenous (<1 %) ethnicities. Nine articles reported the method for collecting data on ethnicity(Reference Chandler, Scott and Drake23,Reference Aloia, Islam and Mikhail26,Reference Camargo, Sluyter and Stewart27,Reference Hibbs, Ross and Ann Kerns29,Reference Antwi, Huffman and Sullivan30,Reference Chen, Wang and Kao32,Reference Xiao, Wood and Swist35,Reference Yao, Tu and Chang36,Reference Meltzer, Best and Zhang40) . Self-identification was used in seven articles(Reference Chandler, Scott and Drake23,Reference Aloia, Islam and Mikhail26,Reference Camargo, Sluyter and Stewart27,Reference Antwi, Huffman and Sullivan30,Reference Chen, Wang and Kao32,Reference Xiao, Wood and Swist35,Reference Meltzer, Best and Zhang40) , one used identification by parents(Reference Hibbs, Ross and Ann Kerns29), and one identified children as Asian if born to parents of Asian descent(Reference Yao, Tu and Chang36).
The review included a total of 36 707 (range: 80–16 602) participants. Participants’ age ranged from birth to 84 years and most studies were conducted in adults (>18 years) (n 11; 61 %)(Reference Chandler, Scott and Drake23,Reference Sinha-Hikim, Duran and Shen24,Reference Aloia, Islam and Mikhail26–Reference Denlinger, King and Cardet28,Reference Antwi, Huffman and Sullivan30,Reference Bobbitt, Peters and Li31,Reference Legeai, Vigouroux and Souberbielle34,Reference Xiao, Wood and Swist35,Reference Szeto, Zucker and LaSota37,Reference Crandell, Rockwell and Whitehead39) . Seven studies reported the proportion of participants with vitamin D deficiency (25(OH)D < 25 nmol/l), which ranged from 2·0 % to 70·6 %(Reference Varshney, Khadgawat and Gahlot25,Reference Camargo, Sluyter and Stewart27,Reference Denlinger, King and Cardet28,Reference Legeai, Vigouroux and Souberbielle34,Reference Yao, Tu and Chang36–Reference Binks, Smith-Vaughan and Marsh38) .
Four RCT compared the effects of a vitamin D regimen with placebo(Reference Sinha-Hikim, Duran and Shen24,Reference Aloia, Islam and Mikhail26–Reference Denlinger, King and Cardet28) , two compared higher v. lower dose vitamin D regimens(Reference Varshney, Khadgawat and Gahlot25,Reference Hibbs, Ross and Ann Kerns29) and one compared the effects of three different doses of vitamin D regimens with placebo(Reference Chandler, Scott and Drake23). Immune function outcomes were reported in 11 studies(Reference Chandler, Scott and Drake23–Reference Varshney, Khadgawat and Gahlot25,Reference Antwi, Huffman and Sullivan30–Reference Szeto, Zucker and LaSota37) , while ARTI outcomes were reported in seven studies(Reference Aloia, Islam and Mikhail26–Reference Hibbs, Ross and Ann Kerns29,Reference Binks, Smith-Vaughan and Marsh38–Reference Meltzer, Best and Zhang40) .
Assay methods
Fifteen studies provided information about the methods used to measure serum 25(OH)D(Reference Chandler, Scott and Drake23–Reference Denlinger, King and Cardet28,Reference Antwi, Huffman and Sullivan30–Reference Binks, Smith-Vaughan and Marsh38) and five studies standardised these measurements(Reference Varshney, Khadgawat and Gahlot25–Reference Camargo, Sluyter and Stewart27,Reference Chen, Wang and Kao32,Reference Yao, Tu and Chang36) . These methods included chemiluminescent immunoassay (n 6)(Reference Varshney, Khadgawat and Gahlot25,Reference Denlinger, King and Cardet28,Reference Bobbitt, Peters and Li31,Reference Gopal, Thevarajah and Ng33,Reference Xiao, Wood and Swist35,Reference Szeto, Zucker and LaSota37) , RIA (n 3)(Reference Chandler, Scott and Drake23,Reference Chen, Wang and Kao32,Reference Legeai, Vigouroux and Souberbielle34) , liquid chromatography–tandem MS (LC-MS/MS; n 2)(Reference Aloia, Islam and Mikhail26,Reference Camargo, Sluyter and Stewart27) , HPLC-MS/MS (n 1)(Reference Sinha-Hikim, Duran and Shen24), electrochemiluminescence binding assay (n 1)(Reference Yao, Tu and Chang36), enzyme immunoassay (n 1)(Reference Antwi, Huffman and Sullivan30) and isotope dilution-liquid chromatography–tandem MS (ID-MS/MS; n 1)(Reference Binks, Smith-Vaughan and Marsh38). These measurements were standardised to the vitamin D External Quality Assessment Scheme (n 2) and National Institute of Standards and Technology (n 3).
Risk of bias in studies
Table 3 shows the quality of evidence for the RCT(Reference Chandler, Scott and Drake23,Reference Sinha-Hikim, Duran and Shen24,Reference Aloia, Islam and Mikhail26–Reference Hibbs, Ross and Ann Kerns29) using the Jadad Scale. Most trials reported an adequate method of randomisation (n 4)(Reference Chandler, Scott and Drake23,Reference Sinha-Hikim, Duran and Shen24,Reference Camargo, Sluyter and Stewart27,Reference Hibbs, Ross and Ann Kerns29) . While blinding methods were mentioned in all trials, only three studies reported an adequate method of blinding(Reference Chandler, Scott and Drake23,Reference Sinha-Hikim, Duran and Shen24,Reference Camargo, Sluyter and Stewart27) . An account of withdrawals and drop-outs was mentioned in most trials (n 6)(Reference Chandler, Scott and Drake23,Reference Varshney, Khadgawat and Gahlot25–Reference Hibbs, Ross and Ann Kerns29) . Overall, the mean score was 4·14 out of 5, and all studies considered good quality.
Table 3 Quality appraisal of randomised controlled trials using Jadad scale(Reference Jadad, Andrew Moore and Carroll20)

Tables 4 and 5 demonstrate the quality of evidence for the cross-sectional and cohort studies(Reference Antwi, Huffman and Sullivan30–Reference Meltzer, Best and Zhang40) using the Newcastle–Ottawa Scale. Eight studies included a sample representative of the target population(Reference Antwi, Huffman and Sullivan30–Reference Chen, Wang and Kao32,Reference Legeai, Vigouroux and Souberbielle34–Reference Yao, Tu and Chang36,Reference Crandell, Rockwell and Whitehead39,Reference Meltzer, Best and Zhang40) and seven studies adjusted for confounders(Reference Antwi, Huffman and Sullivan30–Reference Chen, Wang and Kao32,Reference Xiao, Wood and Swist35,Reference Yao, Tu and Chang36,Reference Crandell, Rockwell and Whitehead39,Reference Meltzer, Best and Zhang40) . Of the cross-sectional studies, five studies described non-respondents(Reference Antwi, Huffman and Sullivan30,Reference Chen, Wang and Kao32,Reference Gopal, Thevarajah and Ng33,Reference Xiao, Wood and Swist35,Reference Yao, Tu and Chang36) , one study provided sample size justification(Reference Chen, Wang and Kao32) and four reported an adequate measurement of association(Reference Bobbitt, Peters and Li31–Reference Gopal, Thevarajah and Ng33,Reference Yao, Tu and Chang36) . All studies described appropriate ascertainment of exposure and outcomes, and adequacy of follow-up was mentioned in all cohort studies. The mean score for cross-sectional studies was 5·57 out of 8, and for cohort studies, it was 7 stars out of 9, with most studies considered fair to good quality.
Table 4 Quality appraisal of cross-sectional studies using adapted Newcastle–Ottawa scale(Reference Herzog, Álvarez-Pasquin and Díaz22)

Table 5 Quality appraisal of cohort studies using Newcastle–Ottawa scale(Reference Wells, Shea and O’Connell21)

Effect of vitamin D on biomarkers of immune function
Inflammation
Of the nine studies reporting on biomarkers of inflammation (three RCT; five cross-sectional studies; and one cohort study), most were performed in adults(Reference Chandler, Scott and Drake23,Reference Sinha-Hikim, Duran and Shen24,Reference Antwi, Huffman and Sullivan30,Reference Bobbitt, Peters and Li31,Reference Legeai, Vigouroux and Souberbielle34,Reference Xiao, Wood and Swist35,Reference Szeto, Zucker and LaSota37) , including one study in pregnant women(Reference Bobbitt, Peters and Li31). Six studies focused on subjects with inflammatory conditions(Reference Sinha-Hikim, Duran and Shen24,Reference Varshney, Khadgawat and Gahlot25,Reference Antwi, Huffman and Sullivan30,Reference Gopal, Thevarajah and Ng33) or acute/chronic infections(Reference Legeai, Vigouroux and Souberbielle34,Reference Szeto, Zucker and LaSota37) . Average baseline 25(OH) status in RCT ranged from 20·9 to 54·9 nmol/l. Among these trials, two did not report the prevalence of vitamin D deficiency (<25 nmol/l) at baseline(Reference Chandler, Scott and Drake23,Reference Sinha-Hikim, Duran and Shen24) , while one reported a prevalence of 70·6 %(Reference Varshney, Khadgawat and Gahlot25). The vitamin D3 dosing regimens varied, ranging from 25 to 100 µg daily for three months(Reference Chandler, Scott and Drake23), average weekly doses (mean ± sd) of 2133 µg ± 400 for 1 year(Reference Sinha-Hikim, Duran and Shen24) or monthly doses of 3000 µg or 300 µg for 12 months(Reference Varshney, Khadgawat and Gahlot25). The attained 25(OH)D concentrations following these interventions are described in Table 1. The main biomarkers measured were CRP (n 8)(Reference Chandler, Scott and Drake23–Reference Varshney, Khadgawat and Gahlot25,Reference Bobbitt, Peters and Li31,Reference Gopal, Thevarajah and Ng33–Reference Xiao, Wood and Swist35,Reference Szeto, Zucker and LaSota37) and IL-6 (n 8)(Reference Chandler, Scott and Drake23–Reference Varshney, Khadgawat and Gahlot25,Reference Antwi, Huffman and Sullivan30,Reference Bobbitt, Peters and Li31,Reference Gopal, Thevarajah and Ng33,Reference Legeai, Vigouroux and Souberbielle34,Reference Szeto, Zucker and LaSota37) . Three studies found a significant association between vitamin D status and at least one inflammatory biomarker in black populations(Reference Chandler, Scott and Drake23,Reference Bobbitt, Peters and Li31,Reference Legeai, Vigouroux and Souberbielle34) . No significant change was found in IL-6, IL-10, TNF alpha (TNF-α), CRP or soluble TNF receptor 2 (sTNF-R2) monitored within an RCT following vitamin D supplementation compared to placebo or control in African American, Latino or Asian Indian ethnic groups(Reference Chandler, Scott and Drake23–Reference Varshney, Khadgawat and Gahlot25).
Chandler et al. found a significant inverse association between baseline 25(OH)D status and CRP concentrations in African American adults, but no significant changes were found following vitamin D3 supplementation of 25 µg, 50 µg or 100 µg daily(Reference Chandler, Scott and Drake23). In the remaining two RCT measuring CRP, no significant difference between treatment groups was found following average weekly doses (mean ± sd) of 2133 µg ± 400 in Latino and African American adults with pre-diabetes and hypovitaminosis D(Reference Sinha-Hikim, Duran and Shen24) or monthly doses of 3000 µg or 300 µg in Asian Indian obese children and adolescents(Reference Varshney, Khadgawat and Gahlot25). There were no significant findings after adjusting for covariates in four observational studies measuring CRP concentrations in American pregnant women(Reference Bobbitt, Peters and Li31), Asian participants with rheumatoid arthritis(Reference Gopal, Thevarajah and Ng33), black adults with HIV(Reference Legeai, Vigouroux and Souberbielle34) and black adults hospitalised with COVID-19(Reference Szeto, Zucker and LaSota37).
Bobbitt et al. found a significant inverse association between 25(OH)D and IL-1β concentrations in African American pregnant women after adjusting for covariates(Reference Bobbitt, Peters and Li31). No significant difference between treatment groups was found in three RCT measuring IL concentrations (IL-6 or IL-10) following 25 µg, 50 µg or 100 µg daily in African American adults(Reference Chandler, Scott and Drake23), average weekly doses (mean ± sd) of 2133 µg ± 400 in Latino and African American adults with pre-diabetes and hypovitaminosis D(Reference Sinha-Hikim, Duran and Shen24) or monthly doses of 3000 µg or 300 µg in Asian Indian obese children and adolescents(Reference Varshney, Khadgawat and Gahlot25). There were no significant findings after adjusting for covariates in four observational studies measuring IL concentrations (IL-6 or IL-10) in non-Hispanic black type 2 diabetic and non-diabetic adults(Reference Antwi, Huffman and Sullivan30), Asian participants with rheumatoid arthritis(Reference Gopal, Thevarajah and Ng33), black adults with HIV(Reference Legeai, Vigouroux and Souberbielle34) and black adults hospitalised with COVID-19(Reference Szeto, Zucker and LaSota37).
Five studies investigated TNF-α (n 2), sTNF-R2 (n 2) or soluble TNF receptor 1 (sTNF-R1) (n 1))(Reference Chandler, Scott and Drake23–Reference Varshney, Khadgawat and Gahlot25,Reference Bobbitt, Peters and Li31,Reference Legeai, Vigouroux and Souberbielle34) . Legeai et al. found black adults with HIV and vitamin D deficiency (<25 nmol/l) had significantly higher sTNF-R2 concentrations compared to those who were not deficient(Reference Legeai, Vigouroux and Souberbielle34). No significant difference between treatment groups was found for these biomarkers in three RCT measuring IL concentrations (IL-6 or IL-10) following 25 µg, 50 µg or 100 µg daily in African American adults(Reference Chandler, Scott and Drake23), average weekly doses (mean ± sd) of 2133 µg ± 400 in Latino and African American adults with pre-diabetes and hypovitaminosis D(Reference Sinha-Hikim, Duran and Shen24) or monthly doses of 3000 µg or 300 µg in Asian Indian obese children and adolescents(Reference Varshney, Khadgawat and Gahlot25). Bobbit et al. found no significant association between 25(OH)D concentrations and TNF-α in African American pregnant women(Reference Bobbitt, Peters and Li31).
Antibody concentrations
Two cross-sectional studies investigated antibody concentrations(Reference Chen, Wang and Kao32,Reference Yao, Tu and Chang36) , yet no significant association with 25(OH)D was found for IgE concentrations in Asian children and adolescents(Reference Yao, Tu and Chang36) or measles antibody titres in Mexican American, non-Hispanic black or Other Hispanic participants after adjusting for covariates(Reference Chen, Wang and Kao32).
Effect of vitamin D on acute respiratory tract infection incidence
Seven studies reported on incidence of ARTI (four RCT; three cohort studies)(Reference Aloia, Islam and Mikhail26–Reference Hibbs, Ross and Ann Kerns29,Reference Binks, Smith-Vaughan and Marsh38–Reference Meltzer, Best and Zhang40) . Among the RCT, most were performed in adults (n 3)(Reference Aloia, Islam and Mikhail26–Reference Denlinger, King and Cardet28), while one study was performed in infants(Reference Hibbs, Ross and Ann Kerns29). Average baseline 25(OH)D status of RCT ranged from 46·9 to 63·0 nmol/l. The prevalence of vitamin D deficiency (<25 nmol/l) at baseline was reported in three out of four RCT, ranging from 0 to 13 %(Reference Aloia, Islam and Mikhail26–Reference Hibbs, Ross and Ann Kerns29). The vitamin D3 dosing regimens included bolus doses of 2500 µg then 100 µg daily for 28 weeks(Reference Denlinger, King and Cardet28) or 5000 µg then 2500 µg monthly for 3 years(Reference Camargo, Sluyter and Stewart27), an adjusted daily dose (mean ± sd) of 87·3 µg ± 36·6 for 3 years(Reference Aloia, Islam and Mikhail26) or 10 µg daily regardless of dietary intake(Reference Hibbs, Ross and Ann Kerns29). The attained 25(OH)D concentrations following these interventions are described in Table 1. RCT investigated ARTI incidence, and cohort studies measured COVID-19 incidence or hospitalisation due to lower respiratory tract infections.
Most RCT(Reference Aloia, Islam and Mikhail26,Reference Camargo, Sluyter and Stewart27,Reference Hibbs, Ross and Ann Kerns29) did not find any significant difference between treatment groups. However, Denlinger et al. found a significantly higher incidence of ARTI in the placebo group compared to the intervention group in African American adults with asthma, but no significant difference between treatment groups was found in Hispanic, Asian/Pacific Islander or Other ethnic groups(Reference Denlinger, King and Cardet28). Of the two cohort studies investigating COVID-19 incidence in black and white individuals, only one found a significant inverse association between serum 25(OH)D and SARS-CoV-2 positivity in black individuals(Reference Meltzer, Best and Zhang40). Binks et al. in Indigenous mother-infant pairs found that lower mean cord blood 25(OH)D concentrations were associated with lower respiratory tract infection hospitalisation in infants compared to those that were not hospitalised(Reference Binks, Smith-Vaughan and Marsh38).
Meta-analysis for the effect of vitamin D3 supplementation on acute respiratory tract infection incidence
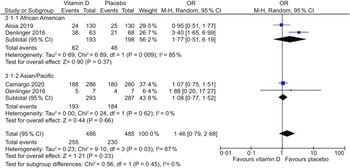
Three placebo-controlled RCT in adults provided sufficient data to be included in the meta-analysis(Reference Aloia, Islam and Mikhail26–Reference Denlinger, King and Cardet28). There was no statistically significant difference in ARTI incidence between vitamin D supplementation and placebo groups overall (OR, 1·14; 95 % CI: 0·84, 1·56; P = 0·41) and in the subgroup analysis for ethnic minority groups (South Asian, Pacific, Asian/Pacific Islander, Māori, American Indian/Alaska Native, African American, Hispanic, Other) (OR, 1·40; 95 % CI: 0·70, 2·79; P = 0·34) (Fig. 2). In the subgroup analysis, there was high heterogeneity in the ethnic minority group (I 2 = 83 %). A second subgroup analysis was performed in African American and Asian/Pacific (South Asian, Pacific, Asian/Pacific Islander) groups (n 2). No statistically significant differences were found overall (OR, 1·46; 95 % CI: 0·79, 2·68; P = 0·23) and in African American (OR, 1·77; 95 % CI: 0·51, 6·19; P = 0·37) or Asian/Pacific (OR, 1·08; 95 % CI: 0·77, 2·68; P = 0·66) groups (Fig. 3). In this subgroup analysis, heterogeneity was higher in the African American group (I 2 = 85 %).

Fig. 2 Forest plot of placebo-controlled trials investigating the effect of vitamin D supplementation on acute respiratory tract infection (ARTI) incidence in adults, sub-grouped by white and ethnic minority groups. Individual trial effect estimates (boxes) and pooled effect estimate (diamond) for ARTI incidence are shown. Values are OR with error bars representing the 95 % CI determined with the use of M-H random-effects models. Heterogeneity was quantified by I 2 at a significance of P < 0·10

Fig. 3 Forest plot of placebo-controlled trials investigating the effect of vitamin D supplementation on acute respiratory tract infection (ARTI) incidence in adults, sub-grouped by African American and Asian/Pacific ethnic groups. Individual trial effect estimates (boxes) and pooled effect estimate (diamond) for ARTI incidence are shown. Values in the plot are OR with 95 % CI determined with the use of M-H random-effects models. Heterogeneity was quantified by I 2 at a significance of P < 0·10
Discussion
This systematic review investigated whether vitamin D status or vitamin D supplementation is associated with biomarkers of immune function and prevention of ARTI in dark-skinned ethnic groups with diverse subject characteristics and geographical settings. While some cohort and cross-sectional studies found vitamin D status to be inversely associated with inflammation and ARTI, the results were conflicting. No evidence was identified to support the use of vitamin D supplementation to reduce inflammation or ARTI. However, the existing literature addressing this issue is limited.
Previous human-derived immune cell studies have shown that vitamin D has many effects on cells within the immune system, including the production of antimicrobial peptides and cytokines, regulating of nuclear factor-kB and reducing the activation of genes encoding inflammatory factors(Reference Bishop, Ismailova and Dimeloe41). In this review, significant inverse associations with vitamin D status were identified for CRP(Reference Chandler, Scott and Drake23), sTNF-R2(Reference Legeai, Vigouroux and Souberbielle34) and IL-1β(Reference Bobbitt, Peters and Li31) in black/African American individuals. However, no significant association was found in studies investigating the relationship between vitamin D status and IL-6(Reference Chandler, Scott and Drake23,Reference Antwi, Huffman and Sullivan30,Reference Bobbitt, Peters and Li31,Reference Gopal, Thevarajah and Ng33,Reference Legeai, Vigouroux and Souberbielle34,Reference Szeto, Zucker and LaSota37) , IL-10(Reference Chandler, Scott and Drake23,Reference Bobbitt, Peters and Li31) , sTNF-R1(Reference Legeai, Vigouroux and Souberbielle34), TNF-α(Reference Bobbitt, Peters and Li31,Reference Legeai, Vigouroux and Souberbielle34) , IgE(Reference Yao, Tu and Chang36) or measles antibody tires(Reference Chen, Wang and Kao32) in dark-skinned ethnic groups. The subject populations that showed significant results had average serum 25(OH)D concentrations <40 nmol/l(Reference Chandler, Scott and Drake23,Reference Bobbitt, Peters and Li31,Reference Legeai, Vigouroux and Souberbielle34) , indicating that conflicting results may be due to threshold effects. Nonetheless, there were no significant findings for the effect of vitamin D supplementation on concentrations of IL-6, TNF-α, CRP(Reference Chandler, Scott and Drake23–Reference Varshney, Khadgawat and Gahlot25), IL-10 or sTNF-R2(Reference Chandler, Scott and Drake23) in dark-skinned ethnic groups. These results are consistent with a systematic review that found no significant effect on CRP and TNF-α in overweight and obese adults(Reference Zuk, Fitzpatrick and Rosella42). However, while previous meta-analyses of RCT investigating vitamin D supplementation on inflammatory biomarkers have reported inconsistent findings(Reference Rodriguez, Mousa and Ebeling43–Reference Mousa, Naderpoor and Teede45), some have shown significant effects for at least one biomarker of inflammation, including IL-6, TNF-α or CRP, in patients with heart failure(Reference Rodriguez, Mousa and Ebeling43), type 2 diabetes(Reference Mousa, Naderpoor and Teede45) and abnormal glucose homeostasis(Reference Dashti, Mousavi and Larijani44).
The interaction between adiposity and immunomodulatory or inflammatory mediators may influence the differential response to vitamin D3. For instance, in the vitamin D and Omega-3 Trial, vitamin D supplementation was linked to lower advanced cancer rates in participants, with the strongest reduction found in individuals with normal BMI and no reduction in individuals who were overweight or obese(Reference Chandler, Chen and Ajala46). Additionally, it has been found that the 25(OH)D increase in the intervention arm and population heterogeneity might impact the power of vitamin D RCT investigating immunomodulatory mediators(Reference Zgaga, Shraim and Bolger47) or respiratory infections(Reference Wyse, Mangan and Zgaga48). Moreover, large increases in 25(OH)D concentrations might not achieve sufficient power if the population is already 25(OH)D sufficient(Reference Wyse, Mangan and Zgaga48). In this review, only one RCT investigated a predominantly vitamin D deficient population (70·6 %) at baseline(Reference Varshney, Khadgawat and Gahlot25). Following high-dose vitamin D supplementation, only 41 % of subjects in the intervention group achieved adequate vitamin D concentrations (>75 nmol/l) and 32 % remained vitamin D insufficient (<50 nmol/l). Therefore, sufficient concentrations of 25(OH)D may not have been achieved to identify significant effects on inflammatory biomarkers, highlighting a need for further well-designed RCT.
The preventative role of vitamin D against both bacterial and viral ARTI has been proposed due to its antibacterial and antiviral properties(Reference Bishop, Ismailova and Dimeloe41). In this review, two cohort studies identified an inverse association between vitamin D status and ARTI incidence in black(Reference Meltzer, Best and Zhang40) and Indigenous groups(Reference Binks, Smith-Vaughan and Marsh38). However, conflicting results were found in studies investigating the association between vitamin D status and COVID-19 positivity in black and white individuals(Reference Crandell, Rockwell and Whitehead39,Reference Meltzer, Best and Zhang40) . These studies used 25(OH)D measurements at 90 d and 1 year before the COVID-19 test, which could account for the conflicting findings as vitamin D status can change over time(Reference Crandell, Rockwell and Whitehead39).
The evidence presented in the meta-analysis of RCT did not show a statistically significant difference when comparing vitamin D supplementation and a placebo among ethnic minorities, or African American or Asian/Pacific subgroups. The null results in the current study should be interpreted with caution due to the low number of studies included in the meta-analysis. In contrast, several meta-analyses in adults, children or adolescents reported a significant reduction in ARTI with vitamin D supplementation compared to the control(Reference Pham, Rahman and Majidi12,Reference Martineau, Jolliffe and Hooper16–Reference Abioye, Bromage and Fawzi18,Reference Charan, Goyal and Saxena49) . Contradictory reports to this review may be attributed to differences in subject populations, as previous studies did not investigate ethnic groups. Additionally, there may be possible differences in ARTI pathogens, as lower respiratory tract infections and upper respiratory tract infections are associated with different aetiologies(Reference Assane, Makhtar and Abdoulaye50).
There did not appear to be any clear trends in the quality scores and the significance of the findings. However, while scoring criteria were developed for the Jadad and Newcastle–Ottawa Scales to minimise bias from the subjective interpretation of scoring, these assessment methods were not appropriately designed for all studies and the quality scores were therefore used as a general guide to assess quality.
Strengths and limitations
This review has several strengths, such as the broad range of outcomes examined to provide a comprehensive overview of the relationship between vitamin D and both immune function and ARTI. As this subject area is an emerging area of research, the search terms and inclusion criteria were extensive to capture all relevant clinical studies.
Limitations to this review include the low number of studies together with considerable heterogeneity between studies for ethnic groups studied, study design, quality and method for analysing 25(OH)D concentrations. The accuracy of vitamin D status may be reduced as only two studies reported using LC-MS/MS to measure 25(OH)D, which is considered the gold standard(Reference Altieri, Cavalier and Bhattoa51). The health status varied between studies, with several populations having pre-existing medical conditions that may impact immune function or ARTI.
The method for classifying ethnicity was not mentioned in 50 % of included studies, potentially leading to inaccuracies in reporting. However, of the eight studies that did report the collection method, most used self-reported ethnic identification which is generally considered the ideal method of collection(Reference Iqbal, Johnson and Szczepura52). Moreover, it is recognised that combining ethnic minorities into one group in a meta-analysis is a limitation as ethnic group differences may be overlooked. While a second analysis was conducted, not all individual ethnic groups were able to be combined due to country-specific differences in ethnic classification and/or author reporting, limiting the comparability of certain ethnic groups.
The definition of vitamin D deficiency used in the present study is <25 nmol/l, as recommended by the Scientific Advisory Committee on Nutrition(Reference Bresson, Burlingame and Dean2), whereas the Institute of Medicine and European Food Safety Authority use < 30 nmol/l to indicate an increased risk of vitamin D deficiency(1,53) . The lack of consensus on these thresholds may be a reason for the low number of studies that provided data on vitamin D deficiency. Of the studies that did provide data, there was a lack of studies investigating populations with low baseline 25(OH)D status. Another possible limitation may be the heterogeneity in dosing regimens adopted by the studies. Daily dosing regimens using standard doses (10–25 µg) taken for up to 12 months have been found to provide the most benefit against ARTI(Reference Jolliffe, Martineau and Griffiths17), yet most RCT investigating ARTI in this review used large daily(Reference Aloia, Islam and Mikhail26) or monthly doses(Reference Camargo, Sluyter and Stewart27,Reference Denlinger, King and Cardet28) .
Future research
Drawing accurate conclusions is challenging due to the small number of studies found, with only seven RCT included in this review. The limited number of studies compared to prior reviews on vitamin D supplementation and ARTI that did not consider ethnic groups(Reference Jolliffe, Martineau and Griffiths17,Reference Abioye, Bromage and Fawzi18) highlights the importance of improved reporting of ethnicity, and the need for further research in this area, particularly RCT. Despite many studies included in this review having large sample sizes, 65 % of all subjects were of white ethnicity. This highlights the underrepresentation of dark-skinned populations, despite their higher risk of vitamin D deficiency(Reference Schleicher, Sternberg and Looker3,Reference Vearing, Hart and Charlton4) and ARTI-related adverse outcomes(Reference Thompson, Jungk and Hancock6,Reference Mathur, Rentsch and Morton54) . To increase the participation rate when targeting ethnic groups, it has been suggested that recruitment strategies should be culturally sensitive, including community outreach and researchers who are members of the same ethnic group(Reference Burlew, Peteet and McCuistian55). Therefore, improved guidelines on collecting and reporting ethnicity in future research are needed.
None of the studies included in this review investigated the association between vitamin D and differential cell counts, leucocyte types, lymphocyte subsets or antimicrobial proteins in dark-skinned populations, highlighting a gap in the literature. Previous research conducted in vitro and in vivo has demonstrated that vitamin D can alter many cells in the immune system(Reference Bishop, Ismailova and Dimeloe41). A study by Liu et al. (Reference Liu, Stenger and Li56) reported that African American subjects had significantly lower vitamin D3 compared to Caucasians, and induction of cathelicidin mRNA was significantly reduced in the presence of serum from African American subjects. This evidence suggests that adequate vitamin D concentrations are required for cathelicidin production. Hence, future trials investigating a range of immune biomarkers in dark-skinned populations are warranted.
Conclusions
This review found a lack of conclusive evidence supporting an association between vitamin D status and immune function or ARTI incidence in dark-skinned ethnic groups. No evidence was found to support the use of vitamin D supplementation in reducing inflammation or ARTI within these populations. The findings are limited by the small number of RCT with considerable heterogeneity between studies for baseline 25(OH)D status and vitamin D dosing regimens and a lack of studies investigating low vitamin D status. Hence, further RCT investigating dark-skinned populations with low vitamin D status and using appropriate vitamin D dosing regimens are needed to elucidate the link between vitamin D health, immune function and ARTI.
Acknowledgements
Not applicable.
Financial support
The work of ARB was funded by UK Research and Innovation and Biotechnology and Biological Sciences Research Council FoodBioSystems Doctoral Training Partnership grant no: BB/T008776/1.
Conflict of interest
A.R.B., A.L.D., K.H.H. and I.D.G. declare they have no competing interests. J.A.L. is Deputy Chair of the UK Government’s Scientific Advisory Committee on Nutrition (SACN). SLN is a member of SACN and also the European Food Safety Authority Committee on the Tolerable Upper Limit for vitamin D. She is Research Director of D3Tex Ltd which holds the UK and Gulf Corporation Council Patents for the use of UVB material for combatting vitamin D deficiency in women who dress for cultural style.
Authorship
All authors contributed to the study’s conception and design. A.R.B., S.A.L.-N. and K.H.H. were involved in screening the articles and gave input on the eligibility criteria. Data acquisition, statistical analysis and interpretation of the data were performed by A.R.B., with revision support from A.L.D. The first draft of the manuscript was written by A.R.B., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Ethics of human subject participation
Not applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980024001861