The growing prevalence of cardiometabolic disease, which includes CVD, stroke, and type 2 diabetes mellitus (T2DM)(Reference Micha, Penalvo and Cudhea1,2) , is a leading concern for policymakers in the United States and globally(Reference Ronto, Wu and Singh3). While the US Burden of Disease Collaboration has reported that a decrease has been observed in some aspects of CVD over the past two decades, other components have increased(Reference Roth and Johnson4). Specifically, ischemic heart disease (IHD) and ischemic stroke were the first and tenth leading causes of mortality in 2016, accounting for 544 800 and 113 300 deaths, respectively(Reference Roth and Johnson4). Moreover, diabetes incidence increased from the twelfth to the eighth cause of death between 1990 and 2016, and was the third highest cause for disability in 2016, behind low-back pain and major depressive disorders(Reference Roth and Johnson4). Individuals with T2DM are also at higher risk for CVD(Reference Garber, Abrahamson and Barzilay5). While the pathogenesis of cardiometabolic disease is multifactorial, diet has been identified as a major risk factor, and was implicated in 529 299 US deaths in 2016, with >85 % due to CVD and diabetes(Reference Roth and Johnson4).
It is now clear that diet plays an important role in risks of CVD and diabetes, but elucidating the impact of specific dietary factors is challenging, primarily due to the slow manifestation of these diseases and the complex interactions among nutrients. Therefore, much past work has focused on diet and biomarkers, such as total cholesterol (tChol), low-density lipoprotein cholesterol (LDL-C), ratio of LDL-C to high-density lipoprotein cholesterol (HDL-C), and blood glucose. For example, a strong relationship has been established between LDL-C and risk of CVD, with the data supporting that a 1 mmol/l reduction in LDL-C is associated with a 20 % reduction in coronary artery disease (CAD)(Reference Baigent and Blackwell6). This has led to past US Dietary Guidelines emphasizing a reduction of dietary cholesterol(Reference Sacks, Lichtenstein and Wu7).
Egg yolks are among the cholesterol-rich foods, delivering around 141 and 234 mg of cholesterol per one chicken egg, depending on the size, and, therefore, past dietary guidelines have included limiting egg consumption. However, more recent data have suggested that dietary cholesterol is not a major contributor to tChol and LDL-C increases, with other factors in foods, primarily saturated and trans fats, playing a larger role(Reference Sacks, Lichtenstein and Wu7,Reference Soliman8) . Furthermore, blood cholesterol is under tight homeostasis, mainly regulated by the endogenous cholesterol synthesis in the liver(Reference Kuang, Yang and Zhang9). Thus, in line with this, the 2015–2020 US Dietary Guidelines(10) removed the limit on dietary cholesterol although the policy continued to emphasize that cholesterol intake should be kept as low as possible. Further, the policy stated that, although egg yolk is high in cholesterol, eggs being a nutrient dense food can be consumed as part of a healthy diet ‘along with a variety of other choices and within and across the subgroup recommendations of the protein food groups’.
Early reviews of intervention studies on eggs and blood lipids (e.g. LDL-C) often combined data from low-cholesterol diets, such as decreasing saturated fat-containing meats along with eggs(Reference Weggemans, Zock and Katan11). Given that eggs are low in saturated fat (1·56 g per large chicken egg) and contain components that may decrease risk of certain cardiometabolic diseases, combining data on eggs with saturated fat-rich foods may not reflect the actual effect of eggs alone on health outcomes(Reference Clayton, Fusco and Kern12). Indeed, in contrast to the intervention studies that measured biomarkers, prospective cohort studies that assessed cardiometabolic disease outcomes reported associations of eggs with either decreased or null risks in the general population(Reference Fuller, Sainsbury and Caterson13). Data from sub-groups of prospective studies, however, have been inconsistent, particularly in diabetics(Reference Clayton, Fusco and Kern12,Reference Fuller, Sainsbury and Caterson13) . Although numerous reviews on eggs and health have been published, most are narrative and, until recently, only a few systematic reviews and meta-analyses on the effect of eggs on cardiometabolic-related health outcomes have become available(Reference Li, Zhou and Zhou14,Reference Shin, Xun and Nakamura15) .
Given the role of eggs in delivering important nutrients, as well as the recent change in the policy on dietary cholesterol, renewed discussions on eggs and health have occurred in the literature(Reference Kuang, Yang and Zhang9,Reference David Spence16) . In addition, within the last two years, numerous evidence-based reviews have also been published. The objective of this umbrella review was to assess the totality of the evidence-based literature on the dietary consumption of eggs and cardiometabolic health in order to understand the areas of consensus, as well as the gaps in the existing evidence.
Materials and methods
Literature search and selection criteria
The literature search and reporting followed the standards of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), and was conducted independently by two authors (DL, OC). The initial search was conducted in the PubMed (MEDLINE) database for reviews published from inception through 3 October 2018 with terms that included egg and eggs with dietary, consumption, intake or food (see Supplemental Table S1a). An updated search in PubMed (MEDLINE) was conducted on 29 April 2019, along with searches in Web of Science (WOS), Cochrane Library, the Nutrition Evidence Systematic Review (NESR), formerly the Nutrition Evidence Library (NEL), and Agency for Healthcare Research and Quality (AHRQ) EPC Evidence-Based Reports. Only studies that incorporated a systematic literature search, or were designated as systematic reviews or meta-analyses, and that were published in English and included assessment of studies conducted in humans were included. In addition, only studies that provided specific data on egg consumption were included. Studies on cancer, immune and allergy-related outcomes, and reproduction, as well as reviews not including studies conducted in humans, were excluded. Additional hand-searching and review of reference lists from the recent narrative reviews were also conducted, as well as ad hoc searches on Google.
Data extraction and analysis
The following were extracted from each review by two investigators (EM and DL) and reviewed by a third (OC): (i) first author name and year of publication; (ii) objective of the review; (iii) databases used for the search and years of publications covered in the review; (iv) general search terms and selection criteria; (v) number and type of studies included in the assessment; (vi) specific references/studies used by the authors for their assessments. For each systematic review, additional extracted information included: (vii) the authors’ conclusions; and (viii) limitations noted by the authors. For each meta-analysis, additional data extracted included (ix) the statistical analysis approach; and (x) the most adjusted and study-specific estimates for relative risk (RR) or hazard ratio (HR) with 95 % CI. Given that this review covers disparate endpoints of cardiometabolic health, no formal data analysis was conducted. However, outcomes of each meta-analysis were compared qualitatively, and the data used for each study compared to assess the amount of independent data that was included and level of independent analyses.
Quality of the reviews
Adherence to reporting quality of each review was assessed using the PRISMA checklist (http://www.prisma-statement.org/). Results for each review were expressed as a percentage of the required items, which included twenty-four for meta-analyses and nineteen for systematic reviews. Methodological quality was assessed using the Risk of Bias in Systematic Reviews (ROBIS; https://www.bristol.ac.uk/population-health-sciences/projects/robis/robis-tool/) assessment tool and the AMSTAR2 checklist (https://amstar.ca/Amstar-2.php). For quality assessments of systematic review using the ROBIS tool, the checklist items were adjusted to remove the elements not applicable to non-meta-analyses (i.e. item 13, identification of principal summary measures; item 14, description of methods of handling data and combining results of studies; item 15, description of assessment of risk of bias that may affect the cumulative evidence; item 21, reporting of results of each meta-analysis; and item 22, reporting of results of any assessment of risk of bias across studies). The score was adjusted accordingly with low, unclear, and high bias results indicated per each review, and a conclusion of low bias indicating higher quality. The AMSTAR2 checklist included sixteen items, with three items not applicable to systematic reviews (i.e. item 11, use of appropriate methods for meta-analysis; item 12, assessment of risk of bias in individual studies for meta-analysis; and item 15, inclusion of small study publication bias and the impact on the results). The original AMSTAR2 does not provide an overall score; however, subsequent validation papers(Reference Shea, Bouter and Peterson17,Reference Shea, Hamel and Wells18) awarded each item scoring ‘yes’ one point and summed these to calculate an overall score. We modified this approach by adjusting the sum to a percentage, based on the maximum attainable points of 13 and 16 for systematic reviews and meta-analyses, respectively. Adherence to reporting quality and methodological quality scoring was conducted independently by two investigators (EM and OC) and scoring details for individual reviews are provided in supplemental materials (Supplemental Table S2).
Selection and characteristic of reviews
Search and selection results
The search returned 1098 references and 1009 of these were excluded based on abstract screening (Fig. 1). A further seventy-one were excluded based on full-text screening (Supplemental Table S1b). A total of twenty-three reviews were included, of which seven were reviews utilizing systematic approaches, but without meta-analysis, and sixteen were meta-analyses of at least one cardiometabolic-relevant outcome.
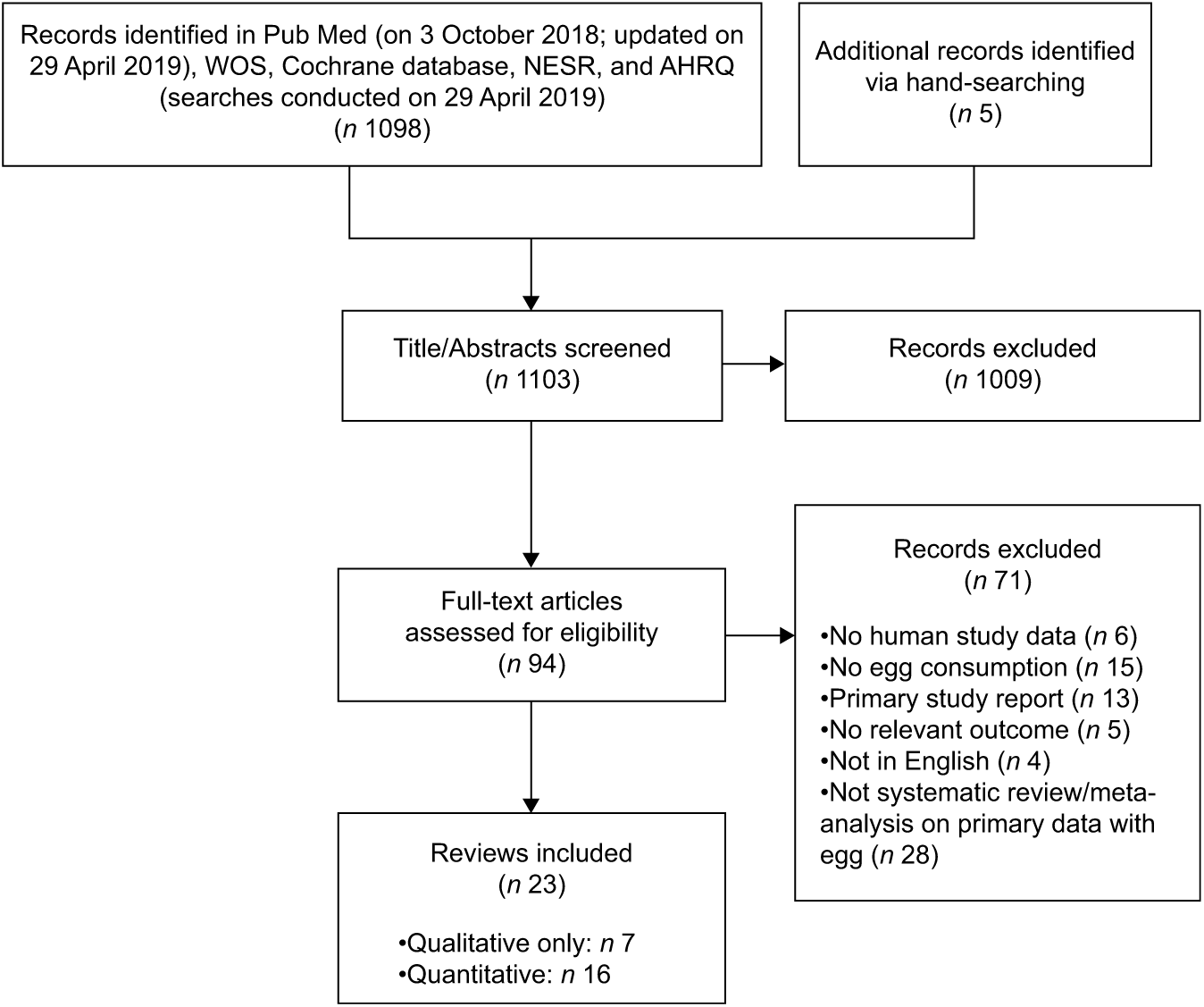
Fig. 1 PRISMA diagram
Of the seven reviews utilizing systematic approaches, three were on eggs and a relevant outcome(Reference Mente, de Koning and Shannon19-Reference Tran, Barraj and Heilman21), two represented comprehensive assessments but not full systematic reviews(Reference Geiker, Larsen and Dyerberg22,Reference Park, Jung and Choi23) , and two were separate reports of the same search strategy and outcome relationship by the same author(Reference Kerley24,Reference Kerley25) and are, thus, considered together. Therefore, overall, six independent reviews were identified (Table 1). Three of the reviews included both prospective cohort and intervention studies(Reference Tran, Barraj and Heilman21-Reference Park, Jung and Choi23), whereas one reported on intervention studies only(Reference Richard, Cristall and Fleming20) and three included only prospective cohort studies(Reference Mente, de Koning and Shannon19,Reference Kerley24,Reference Kerley25) .
Table 1 Characteristics of systematic and comprehensive narrative reviews on egg intake and CVD and/or diabetes
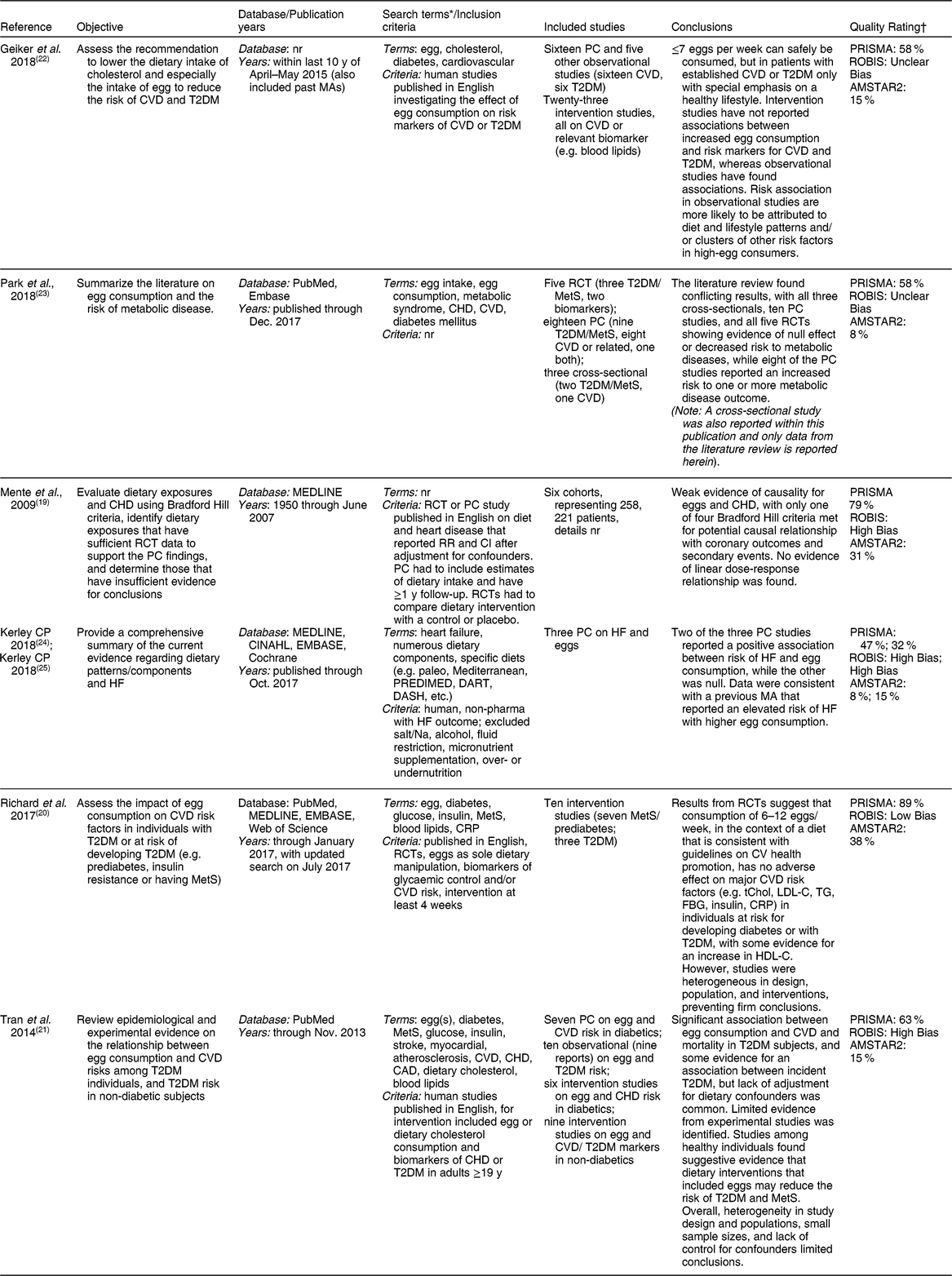
CAD, coronary artery disease; CRP, C-reactive protein; CV, cardiovascular; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; HF, heart failure; LDL-C, low-density lipoprotein cholesterol; MA(s), meta-analysis(es); MetS, metabolic syndrome; nr, not reported; PC, prospective cohort; PRISMA, preferred reporting items for systematic reviews and meta-analyses; RCT, randomized controlled trial; ROBIS, risk of bias in systematic reviews; RR, relative risk; T2DM, type-2 diabetes mellitus; tChol, total-cholesterol; TG, triglycerides.
* Terms summarized for comparison of scope or search. See specific reports for full list of search terms and combinations.
† Details on PRISMA, ROBIS, and AMSTAR2 assessments are provided in the methods section. Briefly, AMSTAR2 checklist items were scored at 1-point if present and zero if missing, and final assessment scores were presented as a percentage of adjusted the maximum attainable scores (19 and 13 points, respectively), with a higher score indicating a higher quality. ROBIS assessments for systematic reviews did not include Domain 4, as it was not applicable to non-meta-analyses, and the overall assessment was adjusted accordingly, with low risk of bias indicating higher quality. Finally, adherence to the PRISMA checklist was presented as a percentage of the maximum attainable scores (19 for systematic reviews).
The fifteen meta-analyses (Table 2) included nine reports of studies on risk of a relevant CVD outcome (e.g. heart disease, hypertension (HTN), heart failure (HF), and stroke) in the general population from prospective cohort data(Reference Li, Zhou and Zhou14,Reference Shin, Xun and Nakamura15,Reference Alexander, Miller and Vargas26-Reference Zhang and Zhang31) , and two meta-analyses included data from intervention studies on CVD risk factors (i.e. lipid biomarkers and obesity)(Reference Rouhani, Rashidi-Pourfard and Salehi-Abargouei32,Reference Schlesinger, Neuenschwander and Schwedhelm33) . Three of these meta-analyses also included data on CVD risk in T2DM populations(Reference Li, Zhou and Zhou14,Reference Shin, Xun and Nakamura15,Reference Rong, Chen and Zhu29) . Finally, two of these meta-analyses, along with five other meta-analyses, included data on risk of T2DM in the general population as well(Reference Li, Zhou and Zhou14,Reference Shin, Xun and Nakamura15,Reference Djousse, Khawaja and Gaziano34-Reference Wallin, Forouhi and Wolk38) .
Table 2 Characteristics of meta-analyses on egg intake and CVD and/or type 2 diabetes
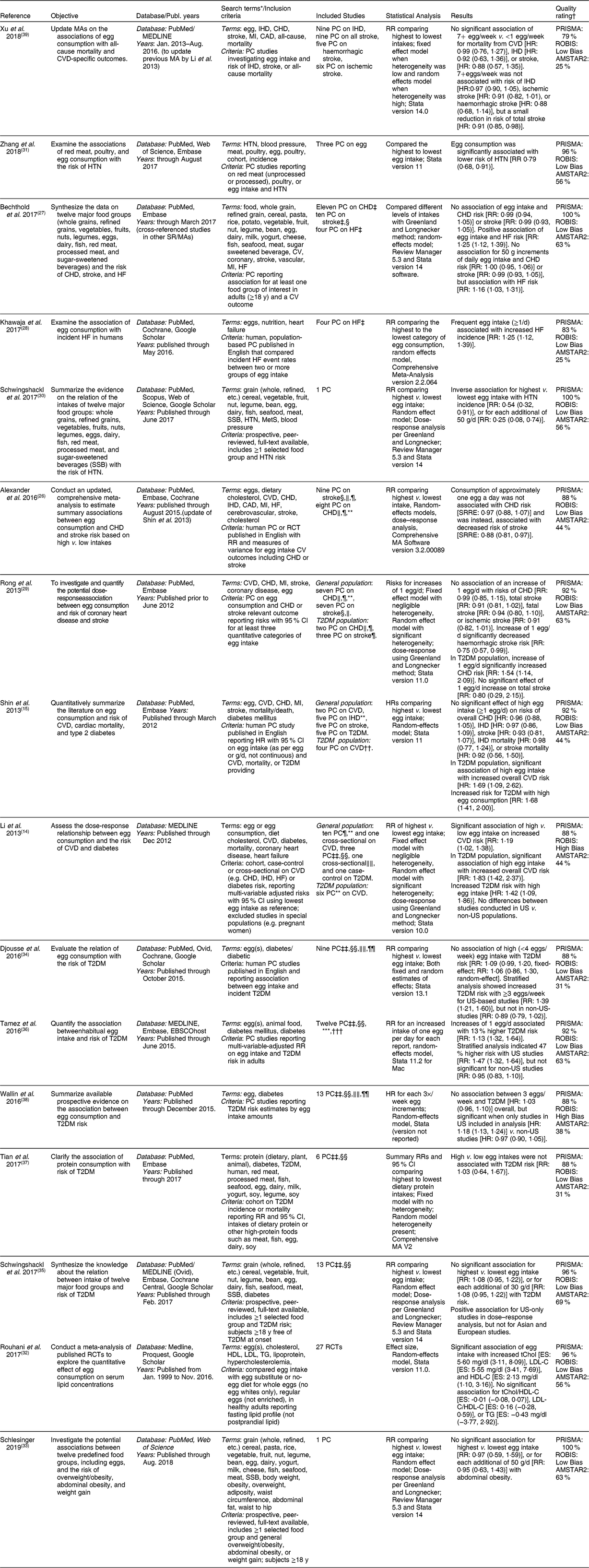
CAD, coronary artery disease; CV, cardiovascular; d, day; dL, deciliter; ES, effect size; g, gram; HDL-C, high-density lipoprotein cholesterol; HF, heart failure; HR, hazard ratio; HTN, hypertension; LDL-C, low-density lipoprotein cholesterol; MA(s), meta-analysis(es); mg, milligram; PC, prospective cohort; PRISMA, preferred reporting items for systematic reviews and meta-analyses; RCT, randomized controlled trial; ROBIS, risk of bias in systematic reviews; RR, relative risk; SSB, sugar-sweetened beverage; SR, systematic review; SRRE, summary relative risk estimates; T2DM, type-2 diabetes mellitus; tChol, total-cholesterol; TG, triglycerides.
* Terms summarized for comparison of scope or search. See specific reports for full list of search terms and combinations.
† Each PRISMA and AMSTAR2 required checklist items were assigned 1 point if reported in the manuscript and 0 if not, with maximum achievable scores of 24 and 16 points, respectively. The scores were adjusted as a percentage of the maximum available points.
‡ Two cohorts (Swedish Mammography Cohort and Cohort of Swedish Men) were analysed separately and included in one report (Larsson et al., 2013).
§ Two cohorts (Health Professionals Follow-Up Study and Nurses’ Health Study) were analysed separately and included in one report (Bernstein et al. 2012).
|| Males and females from the same cohort were analysed separately in one report (Nakamura et al. 2004).
¶ Males and females from the same cohort were analysed separately in one report (Scrafford et al. 2010).
** Two cohorts (Health Professionals Follow-Up Study and Nurses’ Health Study) were analysed separately and included in one report (Hu et al. 1994).
†† Males and females from the same cohort were analysed separately in one report (Qureshi et al. 2007).
‡‡ Two cohorts (Physicians’ Health Study I and Women’s Health Study) were analysed separately and included in one report (Djousse et al. 2009).
§§ Males and females from the same cohort were analysed separately in one report (Djousse et al. 2010).
|||| Males and females from the same sample were analysed separately in one report (Shi et al. 2011).
¶¶ Males and females from the same cohort were analysed separately in one report (Djousse et al. 2015).
*** Males and females from the same cohort were analysed separately in one report (Kurotani et al. 2014).
††† Two cohorts were analysed together and included in one report (Vang et al. 2008).
Systematic reviews
The systematic reviews on egg consumption and cardiometabolic health are described in Table 1. Three of these reviews were generally broad, and included evidence on eggs and risk of cardiovascular-related outcomes and/or T2DM in the general population(Reference Mente, de Koning and Shannon19,Reference Geiker, Larsen and Dyerberg22,Reference Park, Jung and Choi23) , with one of these combining data from a primary cross-sectional study in Korean adults with the literature review(Reference Park, Jung and Choi23). Two of the reviews focused specifically on CVD risk in individuals with T2DM subjects or at risk of developing diabetes(Reference Richard, Cristall and Fleming20,Reference Tran, Barraj and Heilman21) , while two focused on HF risk only(Reference Kerley24,Reference Kerley25) .
Although most of the systematic reviews had similar study objectives, the number and type of studies included varied widely among reviews. This is likely due to the difference in search dates and eligibility criteria. For example, Tran et al.(Reference Tran, Barraj and Heilman21) included studies that not only examined the effect of egg intake, but also dietary cholesterol in general. Meanwhile, Richard et al.(Reference Richard, Cristall and Fleming20) selected only studies whereby eggs were the sole aspect of the diet that was manipulated with interventions that were at least four weeks long. In contrast, Geiker et al.(Reference Geiker, Larsen and Dyerberg22), which included the most number of studies, had few restrictions on the studies that were included. The systematic reviews that included the fewest studies were those of Mente et al. (six cohorts)(Reference Mente, de Koning and Shannon19) and Kerley (three cohorts)(Reference Kerley24,Reference Kerley25) , and the Kerley reviews(Reference Kerley24,Reference Kerley25) were also the most limited in scope focusing exclusively on HF.
As shown in Table 1, adherence to the PRISMA reporting checklist by the systematic reviews were mostly poor to fair, with Kerley et al.(Reference Kerley24,Reference Kerley25) having the poorest adherence (<50 %), and Richard et al.(Reference Richard, Cristall and Fleming20) and Mente et al.(Reference Mente, de Koning and Shannon19) with the best adherence (89 and 79 %, respectively). Methodological quality scores using AMSTAR2 generally followed the PRISMA reporting quality scores as well, with Kerley et al.(Reference Kerley24) having one of the lowest AMSTAR2 scores along with Park et al.(Reference Park, Jung and Choi23) and Richard et al.(Reference Richard, Cristall and Fleming20), and Mente et al.(Reference Mente, de Koning and Shannon19) having the highest scores. Risk of bias using ROBIS scoring was low for only one study(Reference Richard, Cristall and Fleming20), with unclear bias for another two(Reference Geiker, Larsen and Dyerberg22,Reference Park, Jung and Choi23) . The Kerley reviews(Reference Kerley24,Reference Kerley25) were rated as having high risk of bias owing to poor ratings for ROBIS Domain 2 (identification and selection of studies) and Domain 3 (data collection and study appraisal), Mente et al.(Reference Mente, de Koning and Shannon19) was rated as having high risk of bias owing to poor ratings for ROBIS Domain 1 (study eligibility criteria) and Domain 2, and Tran et al.(Reference Tran, Barraj and Heilman21) was rated having high risk of bias owing to poor ratings in ROBIS Domains 1, 2 and 3.
Meta-analyses
The characteristics of the fifteen meta-analyses are provided in Table 2. These reviews covered different aspects of risk of cardiometabolic outcomes, with the vast majority (n 13) of the reviews including only evidence from prospective cohort studies(Reference Shin, Xun and Nakamura15,Reference Alexander, Miller and Vargas26-Reference Zhang and Zhang31,Reference Djousse, Khawaja and Gaziano34-Reference Xu, Lam and Jiang39) , while one study included searches for prospective cohorts, case-controls, and cross-sectionals(Reference Li, Zhou and Zhou14) and another included only intervention studies(Reference Rouhani, Rashidi-Pourfard and Salehi-Abargouei32). Most meta-analyses estimated RR or HR by comparing the highest category of egg consumption with the lowest for each study.
Seven meta-analyses assessed the effect of egg consumption on CVD outcomes in the general population(Reference Li, Zhou and Zhou14,Reference Shin, Xun and Nakamura15,Reference Alexander, Miller and Vargas26-Reference Rong, Chen and Zhu29,Reference Xu, Lam and Jiang39) with three also focusing on the T2DM population(Reference Li, Zhou and Zhou14,Reference Shin, Xun and Nakamura15,Reference Rong, Chen and Zhu29) . Most of these included point estimates for an aspect of cardiovascular health, i.e. coronary heart disease (CHD), HF, myocardial infarction, or IHD (Table 2) while one review combined data on three outcomes (CHD, IHD, and HF)(Reference Li, Zhou and Zhou14). Three meta-analyses only assessed risk factors for cardiometabolic disease, specifically HTN(Reference Schwingshackl, Schwedhelm and Hoffmann30,Reference Zhang and Zhang31) and blood lipids(Reference Rouhani, Rashidi-Pourfard and Salehi-Abargouei32). Seven assessed the effects of egg consumption on T2DM risk in the general population(Reference Li, Zhou and Zhou14,Reference Shin, Xun and Nakamura15,Reference Djousse, Khawaja and Gaziano34-Reference Wallin, Forouhi and Wolk38) and calculated point estimates for incidence of T2DM. Additionally, a few meta-analyses(Reference Li, Zhou and Zhou14,Reference Alexander, Miller and Vargas26,Reference Bechthold, Boeing and Schwedhelm27,Reference Rong, Chen and Zhu29,Reference Schwingshackl, Schwedhelm and Hoffmann30,Reference Rouhani, Rashidi-Pourfard and Salehi-Abargouei32,Reference Djousse, Khawaja and Gaziano34-Reference Tamez, Virtanen and Lajous36,Reference Wallin, Forouhi and Wolk38) also investigated the dose–response relationship of egg consumption with the respective outcomes.
As shown in Table 2, the majority of the meta-analyses had good adherence to the PRISMA reporting checklist, with three reviews including all 24 reporting requirements(Reference Bechthold, Boeing and Schwedhelm27,Reference Schwingshackl, Schwedhelm and Hoffmann30,Reference Schlesinger, Neuenschwander and Schwedhelm33) , with six additional reviews including at least 90 % of the required reporting items(Reference Shin, Xun and Nakamura15,Reference Rong, Chen and Zhu29,Reference Zhang and Zhang31,Reference Rouhani, Rashidi-Pourfard and Salehi-Abargouei32,Reference Schwingshackl, Hoffmann and Lampousi35,Reference Tamez, Virtanen and Lajous36) , and seven reviews including ~80 to 90 % of the required items(Reference Li, Zhou and Zhou14,Reference Alexander, Miller and Vargas26,Reference Khawaja, Singh and Luni28,Reference Djousse, Khawaja and Gaziano34,Reference Tian, Xu and Jiang37-Reference Xu, Lam and Jiang39) . Methodological quality assessed by AMSTAR2 generally followed that of the PRISMA rating, although scores were lower overall, with the highest quality (addressing 50–70 % of checklist items) found for eight studies(Reference Bechthold, Boeing and Schwedhelm27,Reference Rong, Chen and Zhu29-Reference Schlesinger, Neuenschwander and Schwedhelm33,Reference Schwingshackl, Hoffmann and Lampousi35,Reference Tamez, Virtanen and Lajous36) , while six reviews addressed between 30 and 50 % of items(Reference Li, Zhou and Zhou14,Reference Shin, Xun and Nakamura15,Reference Alexander, Miller and Vargas26,Reference Djousse, Khawaja and Gaziano34,Reference Tian, Xu and Jiang37,Reference Wallin, Forouhi and Wolk38) , and two reported low quality with less than 30 % of checklist items addressed(Reference Khawaja, Singh and Luni28,Reference Xu, Lam and Jiang39) . The ROBIS score indicated low bias, representing higher quality, for 13 reviews(Reference Shin, Xun and Nakamura15,Reference Alexander, Miller and Vargas26-Reference Rouhani, Rashidi-Pourfard and Salehi-Abargouei32,Reference Djousse, Khawaja and Gaziano34-Reference Tian, Xu and Jiang37,Reference Xu, Lam and Jiang39) . Two studies(Reference Li, Zhou and Zhou14,Reference Wallin, Forouhi and Wolk38) were rated as being at high risk for bias due to poor ratings for Domains 2 (identification and selection of studies) and 3 (data collection and study appraisal). Most studies explored potential sources of heterogeneity such as location, sex, length of follow-up, study quality, study design, number of participants, method of assessing dietary intake, age, whether the study controlled for diet or cholesterol level, and type of stroke or CVD. Only one analysis did not report assessing potential sources of heterogeneity(Reference Shin, Xun and Nakamura15).
Results
Three of the systematic reviews assessed intervention and observational data across different aspects of cardiometabolic outcomes and presented conclusions based on data in the general population as well as those at risk(Reference Tran, Barraj and Heilman21-Reference Park, Jung and Choi23). For example, the review of Park et al.(Reference Park, Jung and Choi23) addressed coronary heart health broadly as a component of cardiometabolic diseases and noted conflicting results across studies. In general, the only studies that found a positive association between risk of metabolic disease and egg consumption by these authors were prospective cohorts. Meanwhile, RCTs indicated that egg consumption did not affect lipid profile (n 2), inflammatory markers (n 2) or MetS risk (n 1). The Park et al. study(Reference Park, Jung and Choi23) also included primary cross-sectional data on the association of egg consumption with risk of MetS, as well as several biomarkers (e.g. HTN, blood lipids), but did not provide details on how the data were combined in support of their conclusions.
Geiker et al.(Reference Geiker, Larsen and Dyerberg22) concluded that up to seven eggs can be consumed per week without increasing risk of metabolic conditions in the generally healthy population, but did not conduct a separate meta-analysis. Instead, these authors based their conclusions primarily on five meta-analyses included in discussions below(Reference Li, Zhou and Zhou14,Reference Shin, Xun and Nakamura15,Reference Alexander, Miller and Vargas26,Reference Rong, Chen and Zhu29,Reference Djousse, Khawaja and Gaziano34) as well as a meta-analysis on dietary cholesterol and CVD(Reference Berger, Raman and Vishwanathan40), with the addition of six observational studies in healthy people or subjects at high CVD risk that were not included in the previously published analyses. Geiker et al.(Reference Geiker, Larsen and Dyerberg22) cautioned that the conclusion of up to seven eggs per day may not be applicable to people with established CVD or T2DM. Other systematic reviews focused more specifically on a particular cardiometabolic outcome and are, thus, discussed in the appropriate sections below.
Heart disease
Five meta-analyses(Reference Shin, Xun and Nakamura15,Reference Alexander, Miller and Vargas26,Reference Bechthold, Boeing and Schwedhelm27,Reference Rong, Chen and Zhu29,Reference Xu, Lam and Jiang39) and one systematic review(Reference Mente, de Koning and Shannon19) reported on an aspect of coronary heart health separately, while one meta-analysis(Reference Li, Zhou and Zhou14) combined data from all-cause mortality, CHD, IHD, stroke, and HF into one risk analysis (Table 3). Consistency was seen in the conclusions from these reviews for a null effect of eggs on total and fatal CHD, as all as IHD, but not for HF (see below). The datasets assessed in these reports varied but included from seven to eleven cohorts for each assessment (Table 4). For example, with respect to CHD and IHD, the recent reviews by Bechthold et al.(Reference Bechthold, Boeing and Schwedhelm27) and Xu et al.(Reference Xu, Lam and Jiang39) included the most datasets, with eleven and nine cohorts, respectively, and only four of these were in common across the reviews. Mente et al.(Reference Mente, de Koning and Shannon19) conducted an analysis of the strength of evidence of the link between dietary factors and CHD (Table 1). Based on nine prospective cohort studies, these authors reported that the overall evidence for eggs and CHD is weak using the Bradford-Hill criteria for strength, consistency, or biological gradient. Additionally, Bechthold et al. rated the level of evidence as having moderate confidence for the effect estimate such that further research may change the effect estimate(Reference Bechthold, Boeing and Schwedhelm27).
Table 3 Summary of results from meta-analyses
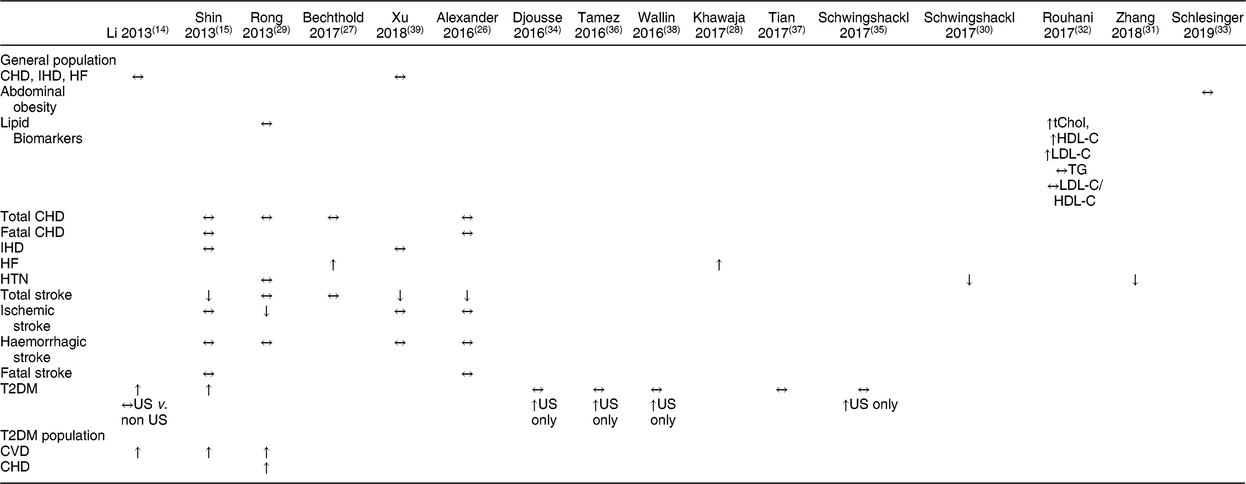
HF, heart failure; HTN, hypertension; T2DM, type 2 diabetes mellitus; US, United States; tChol, total cholesterol; HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol; TG, triglyceride.
↔ no change, ↑ increased risk, ↓ decreased risk.
Table 4 Study selection for reviews on egg intake and heart health outcomes
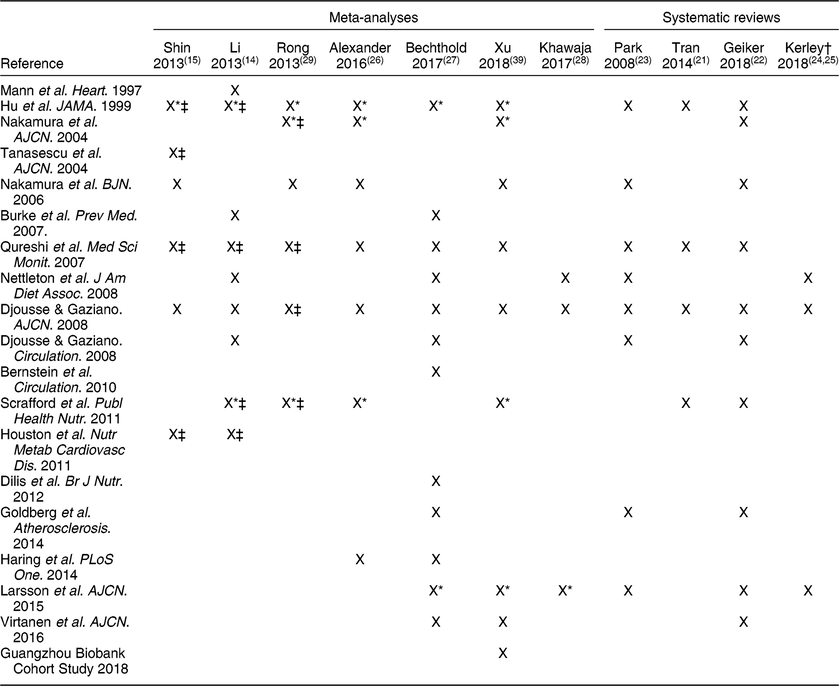
* Analysis was performed separately on the male and female cohorts.
† Kerley review included discussion of, and reference to, the Khawaja et al., 2017 meta-analysis.
‡ Studies that included data for CVD risk in T2DM population.
Heart failure
Two meta-analyses assessed egg intake and HF(Reference Bechthold, Boeing and Schwedhelm27,Reference Khawaja, Singh and Luni28) (Table 3), and both included the same data from three publications that represented four cohorts (Table 4). For the analyses, 105 999 subjects and 5059 cases of new onset HF were included in both analyses, which compared the highest (≥1 egg/d or 140 g/d) to the lowest intake, and both reported a pooled RR of 1·25 (95 % CI, 1·12, 1·39) with 0 % heterogeneity in the data. The meta-analysis conducted by Li et al.(Reference Li, Zhou and Zhou14) included two of these three publications in their assessment of eggs and CVD risk, but did not report data on HF alone. Kerley(Reference Kerley24,Reference Kerley25) based his reviews of dietary patterns on the same three publications used in these meta-analyses as well, and noted that three of the prospective cohorts(Reference Larsson, Akesson and Wolk41,Reference Nettleton, Steffen and Loehr42) reported a positive association, whereas a more recent cohort study only found an association in men who consumed >6 eggs weekly, but not in women or diabetics(Reference Djousse and Gaziano43). One meta-analysis rated the effect estimate as having a moderate confidence such that further research may change the effect estimate(Reference Bechthold, Boeing and Schwedhelm27).
Stroke
Five meta-analyses addressed eggs and stroke(Reference Shin, Xun and Nakamura15,Reference Alexander, Miller and Vargas26,Reference Bechthold, Boeing and Schwedhelm27,Reference Rong, Chen and Zhu29,Reference Xu, Lam and Jiang39) with mixed conclusions (Table 3). Of these, Rong et al.(Reference Rong, Chen and Zhu29) and Alexander et al.(Reference Alexander, Miller and Vargas26) included nearly identical data, with six studies in common and the latter review including an additional study that was published in 2012 (Table 5). The Bechthold et al.(Reference Bechthold, Boeing and Schwedhelm27) review included the most recent publically available data. In contrast, the review of Xu et al.(Reference Xu, Lam and Jiang39) included most of the earlier publications, along with a new prospective cohort that was presented within the review from the Guangzhou Biobank Cohort Study.
Table 5 Study selection for reviews on egg intake and cerebrovascular outcomes
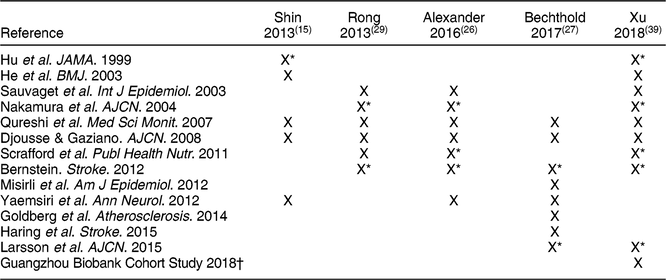
* Analysis was performed separately on the male and female cohorts.
† Primary prospective cohort data included as one dataset in the analysis.
Taken together, the Bechthold et al.(Reference Bechthold, Boeing and Schwedhelm27) and Xu et al.(Reference Xu, Lam and Jiang39) reviews include the totality of evidence used in the other three reviews. The Xu et al.(Reference Xu, Lam and Jiang39) study included nine studies that represented fourteen cohorts along with data from the Guangzhou Biobank Cohort Study, and Bechthold et al.(Reference Bechthold, Boeing and Schwedhelm27) included eight publications representing ten cohorts. Xu et al.(Reference Xu, Lam and Jiang39) compared higher (≥7 eggs/week) to low (<1 egg/week) consumers and found a small but significant reduction in stroke (HR: 0·91; 95 % CI, 0·85, 0·98), but no significant effect on mortality from stroke (HR: 0·88; 95 % CI, 0·57, 1·35). Bechthold et al.(Reference Bechthold, Boeing and Schwedhelm27) reported no association of egg with risk of stroke (RR: 0·99; 95 % CI, 0·94, 1·05) for highest (~75 g/d) to lowest (~0 g) intakes. Further, no evidence of a dose-response was found. The other reviews included less evidence, and either reported null effects or similarly small inverse effects on risk of stroke. Furthermore, Bechthold et al. rated the level of confidence for the effect estimate as moderate such that further research may change the effect estimate(Reference Bechthold, Boeing and Schwedhelm27). Therefore, the overall effect of eggs on risk of stroke was either null or slightly favourable, when comparing higher to lower intakes.
Diabetes risk
The meta-analyses on risk of T2DM with egg consumption included only prospective cohort studies, with the exception of Li et al.(Reference Li, Zhou and Zhou14) who included one cross-sectional and one case-control study that were not in the other reviews. Only two primary studies were in common across these meta-analyses (Table 6). Despite being the most recent report, Tian et al.(Reference Tian, Xu and Jiang37) only included five studies, in contrast with the other reviews published in the same year or the year before that included 8–12 studies(Reference Djousse, Khawaja and Gaziano34-Reference Tamez, Virtanen and Lajous36,Reference Wallin, Forouhi and Wolk38) .
Table 6 Study selection for meta-analyses on egg intake and type 2 diabetes mellitus risk
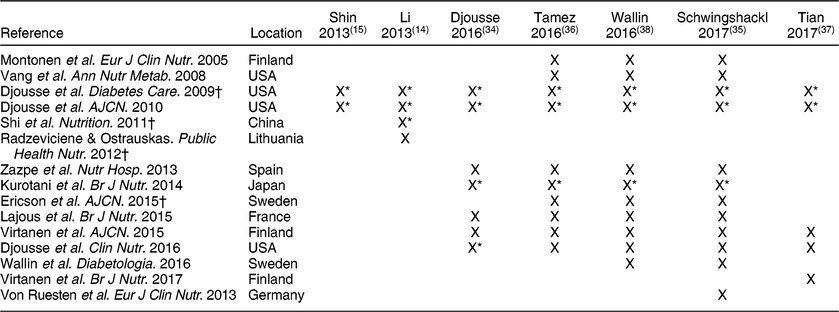
* Analysis was performed separately on the male and female cohorts.
† Studies that reported increased risk of T2DM with egg consumption.
For egg intake and T2DM risk in the general population, two older reports(Reference Li, Zhou and Zhou14,Reference Shin, Xun and Nakamura15) showed an increased risk for T2DM with high egg consumption. Li et al.(Reference Li, Zhou and Zhou14) compared results between US and non-US studies and did not observe any differences. However, the more recent meta-analyses suggest that egg intake is associated with increased T2DM risk only among studies conducted in the US. Djousse et al.(Reference Djousse, Khawaja and Gaziano34) reported that high egg consumption (<4 eggs/week) was not associated with the risk of T2DM in the total population, but a stratified analysis showed an elevated risk of T2DM with consumption of ≥3 eggs/week among US-based studies only. Similarly, Wallin et al.(Reference Wallin, Forouhi and Wolk38) reported that there was no overall association between egg intake of three servings/week and T2DM, but that consumption of three servings/week was positively associated with T2DM risk in studies conducted in the US only. Schwingshackl et al.(Reference Schwingshackl, Hoffmann and Lampousi35) also reported no significant association with T2DM when the lowest and highest egg intakes were compared and for each additional 30 g/d, but the consumption of 30 g/d was positively associated with T2DM risk in studies conducted in the US, but not in Asia or Europe. Finally, Tamez et al.(Reference Tamez, Virtanen and Lajous36) reported that an increase of one egg/d is associated with 13 % higher risk of T2DM (RR: 1·13 [95 % CI 1·32, 1·64]). When stratified based on location, analysis of studies conducted in the US showed that an egg per day was associated with a 47 % higher risk of T2DM, whereas the association for studies conducted elsewhere was not statistically significant(Reference Tamez, Virtanen and Lajous36). Tian et al.(Reference Tian, Xu and Jiang37) also did not observe any significant effect of egg intake on T2DM risk, but did not perform analysis comparing study locations. One meta-analysis rated the level of confidence for the effect estimate as moderate such that further research may change the effect estimate(Reference Schwingshackl, Hoffmann and Lampousi35).
CVD risk in people with T2DM
For CVD outcomes in the T2DM population, only one study was included in all three meta-analyses (Table 3). In addition, Shin et al.(Reference Shin, Xun and Nakamura15) and Li et al.(Reference Li, Zhou and Zhou14) had two studies in common, while Li et al.(Reference Li, Zhou and Zhou14) and Rong et al.(Reference Rong, Chen and Zhu29) had one study in common. In total, Li et al.(Reference Li, Zhou and Zhou14) included five studies, Shin et al.(Reference Shin, Xun and Nakamura15) included four studies, and Rong et al.(Reference Rong, Chen and Zhu29) included four studies. These three meta-analyses all found an increased risk for cardiovascular-related diseases with high egg consumption. Shin et al.(Reference Shin, Xun and Nakamura15) and Li et al.(Reference Li, Zhou and Zhou14) both reported that high egg intake was significantly associated with increased overall CVD risk among T2DM population. Rong et al.(Reference Rong, Chen and Zhu29) observed a RR:1·54 (95 % CI, 1·14, 2·09) for CHD with diabetes.
Based on their review of intervention studies, Richard et al.(Reference Richard, Cristall and Fleming20) reported that consumption of six to 12 eggs/week, in the context of a diet that is consistent with guidelines for heart health promotion, has no adverse effect on major CVD risk factors in individuals at risk of developing diabetes or with established T2DM. This is in agreement with the systematic review of prospective cohort studies by Tran et al.(Reference Tran, Barraj and Heilman21) for the general population; however, these authors noted that significant associations have been observed between increased egg consumption and CVD and mortality in people with T2DM. Both authors commented that the evidence was heterogeneous in study design, populations, and definitions of outcomes. Most notably, observational studies did not adjust analyses for confounders that could have an impact on study outcomes.
Risk factors for CVD
With respect to risk factors, only HTN and blood lipids appear to be well covered in the reviews on egg consumption. Geiker et al.(Reference Geiker, Larsen and Dyerberg22) included risk factors in their systematic review, and noted that only two of the twenty-three intervention studies they selected for inclusion reported an increase in LDL-C with increased egg consumption, leading to their conclusion that high-quality RCTs have not found significant effects of increasing egg consumption on risk factors for CVD in healthy subjects or subjects with T2DM. However, these authors did not conduct a quantitative analysis. The only meta-analysis that reported on intervention studies specifically with egg consumption on blood lipids was that of Rouhani et al.(Reference Rouhani, Rashidi-Pourfard and Salehi-Abargouei32), who reported that egg consumption was significantly associated with increases in tChol, LDL-C and HDL-C, but not triglyceride (TG) and the ratios of tChol/HDL-C or LDL-C/HDL-C. Of the twenty-seven intervention studies included by Rouhani et al.(Reference Rouhani, Rashidi-Pourfard and Salehi-Abargouei32) and the twenty-three reviewed by Geiker et al.(Reference Geiker, Larsen and Dyerberg22) only twelve were in common between the two reviews (Table 7). Additionally, both reviews reported quality assessments of all included studies and these ratings were not consistent between the two reviews.
Table 7 Selection of intervention studies that assessed blood lipids
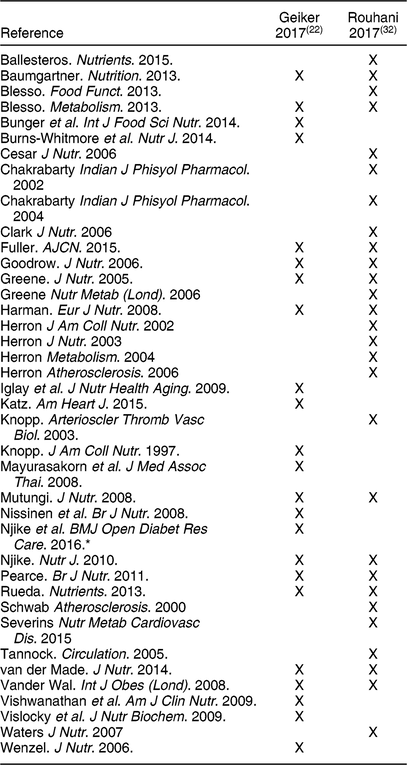
* Assessed glycated haemoglobin, anthropometrics, blood pressure, and diet quality, but not blood lipids.
The effect of eggs on the risk of HTN was assessed in two meta-analyses(Reference Schwingshackl, Schwedhelm and Hoffmann30,Reference Zhang and Zhang31) , and both reported that egg consumption was inversely associated with risk for HTN (Table 3). Both reviews were conducted on the effect of different food groups on HTN, with eggs as a sub-group analysis. The Schwingshackl et al.(Reference Schwingshackl, Schwedhelm and Hoffmann30) review included one prospective study with red meat, poultry, and eggs in an Iranian population (n 1152, 144 cases), which, comparing the highest tertile (~23 g/d) to the lowest tertile consumption, resulted in RR: 0·54 (95 % CI, 0·32, 0·91), with each 50 g/d intake associated with RR: 0·25 (95 % CI, 0·08, 0·74). Schwingshackl et al.(Reference Schwingshackl, Schwedhelm and Hoffmann30) also rated the level of confidence for the effect estimate as very low due to the very limited and uncertain meta-evidence available. The Zhang et al.(Reference Zhang and Zhang31) analysis included two additional studies (n 8942, 1987 cases) and reported the overall multi-adjusted RR: 0·79 (95 % CI, 0·68, 0·91, P = 0·001) for the highest compared with the lowest egg consumption group, with no significant heterogeneity or observed publication bias. Finally, Schlesinger et al.(Reference Schlesinger, Neuenschwander and Schwedhelm33) compared abdominal obesity in the highest and lowest egg consumers from two observational studies, one of which was a cross-sectional study in Korean adults (n 1663), reporting a RR: 0·97 (95 % CI, 0·59, 1·59), with each 50 g/d intake associated with RR: 0·95 (95 % CI, 0·63, 1·43)(Reference Schlesinger, Neuenschwander and Schwedhelm33). These authors indicated the confidence for the effect estimate was very low such that the meta-evidence is very limited and uncertain.
Discussion
Eggs are a nutrient dense food, but their high cholesterol content makes them a food of concern due to the association of dietary cholesterol intake and increased risk for cardiometabolic diseases. Past recommendations from US Dietary Guidelines for Americans, American Heart Association, and American College of Cardiology have indicated limited consumption of dietary cholesterol, including eggs. However, the 2015–2020 DGA modified this recommendation to state ‘cholesterol is not a nutrient of concern for overconsumption’ because ‘adequate evidence is not available for a quantitative limit for dietary cholesterol specific to the dietary guideline’. In this umbrella review, we provided a comprehensive overview of reported evidence from systematic reviews and meta-analyses regarding the impact of egg consumption on CVD and T2DM risks.
Existing reviews consisting of both prospective cohort studies and intervention studies on egg consumption and cardiometabolic outcomes suggest that (i) egg intake is not associated with increased CVD risk in healthy individuals; (ii) conclusions on the effect of egg consumption on CVD risk in subjects with T2DM are different between prospective cohort studies and intervention studies; (iii) the positive association between egg consumption and T2DM risk are specific to US-based studies; (iv) egg consumptions are associated with increased risk for heart failure; and (v) there is a negative association between egg intake and risk of HTN.
In general populations, there were no significant effects of egg intake on overall CVD risk(Reference Li, Zhou and Zhou14,Reference Xu, Lam and Jiang39) , ischemic stroke(Reference Alexander, Miller and Vargas26,Reference Rong, Chen and Zhu29,Reference Xu, Lam and Jiang39) , fatal stroke(Reference Alexander, Miller and Vargas26,Reference Rong, Chen and Zhu29) , total CHD(Reference Alexander, Miller and Vargas26,Reference Bechthold, Boeing and Schwedhelm27,Reference Rong, Chen and Zhu29) , fatal CHD(Reference Alexander, Miller and Vargas26,Reference Rong, Chen and Zhu29) , and ischemic heart disease(Reference Shin, Xun and Nakamura15,Reference Xu, Lam and Jiang39) . Two meta-analyses reported no effect of egg intake on total stroke(Reference Bechthold, Boeing and Schwedhelm27,Reference Rong, Chen and Zhu29) , whereas three meta-analyses reported that egg intake is significantly associated with lower risk of total stroke(Reference Shin, Xun and Nakamura15,Reference Alexander, Miller and Vargas26,Reference Xu, Lam and Jiang39) . For haemorrhagic stroke, three meta-analyses reported no effect of egg intake(Reference Shin, Xun and Nakamura15,Reference Alexander, Miller and Vargas26,Reference Xu, Lam and Jiang39) , while one(Reference Rong, Chen and Zhu29) reported that egg intake is significantly associated with lower risk of haemorrhagic stroke. Finally, the two meta-analyses that assessed egg intake and HF reported increased HF risk with egg intake(Reference Bechthold, Boeing and Schwedhelm27,Reference Khawaja, Singh and Luni28) . Overall, meta-analyses of prospective cohort studies suggest that egg consumption is not associated with increased risk of CVD in the general population, with the exception of HF.
The four meta-analyses on atherosclerosis cardiovascular disease (ASCVD; e.g. stroke, CHD) concluded that egg intake is not associated with ASCVD incidence in the overall population (diabetic and non-diabetic population)(Reference Li, Zhou and Zhou14,Reference Shin, Xun and Nakamura15,Reference Alexander, Miller and Vargas26,Reference Rong, Chen and Zhu29) . However, HF incidence was reported to be associated with egg intake in the overall population(Reference Khawaja, Singh and Luni28). The reason behind the disparate conclusions may be related to the pathogenesis of these different diseases/disorders. Unlike ASCVD which have their origins in atherosclerosis, HF is a clinical syndrome that can result from any structural or functional cardiac disorders that impair the ability of the ventricle to fill with or eject blood(Reference Hunt, Abraham and Chin44). While CAD, a type of ASCVD, is a major cause of HF, other major causes include HTN, diabetes, and dilated cardiomyopathy, whereby 30 % of cases have been reported to relate to genetics(Reference Hunt, Abraham and Chin44). While the link between atherosclerosis, circulating cholesterol, and dietary cholesterol is still being debated, the observations that egg consumption is associated with HF, but not ASCVD in general populations, suggest that eggs, a source of dietary cholesterol, do not contribute to atherosclerosis but may contribute to HF via a mechanism(s) that remains to be elucidated. Furthermore, a causal relationship must be established between them, with consideration of additional factors such as lifestyle, dietary habits, mental stress, and genetic dispositions. Thus, additional investigations into the role of eggs on different types of CVD, with a focus on atherosclerotic v. non-atherosclerotic diseases, are warranted. Furthermore, the opposite effect of egg consumption on risk for HF and HTN requires further investigations because HTN is a contributing cause of HF.
Meta-analyses on the association between egg consumption and risk of T2DM are inconclusive. Of the five meta-analyses that compared data from studies conducted within the US and those conducted outside the US, four reported a positive relationship between egg intake and T2DM risk only in US populations but not in European and Japanese populations(Reference Djousse, Khawaja and Gaziano34-Reference Tamez, Virtanen and Lajous36,Reference Wallin, Forouhi and Wolk38) . The other meta-analysis(Reference Li, Zhou and Zhou14) only included a total of four studies whereby two of the non-US-based studies were cross-sectional(Reference Shi, Yuan and Zhang45) and case-control(Reference Radzeviciene and Ostrauskas46) studies that were not included in the other meta-analyses. It should be noted that of the four US-based studies included among the five meta-analyses, only one prospective study reported significant positive association between egg consumption and T2DM risk and this study included two randomized trials, the Physicians’ Health Study I and the Women’s Health Study(Reference Djousse, Gaziano and Buring47). Subsequent studies conducted by the same group did not show a significant relationship between egg intake and T2DM risk(Reference Djousse, Kamineni and Nelson48,Reference Djousse, Petrone and Hickson49) and the different results between the newer and older studies merit some considerations. The difference in relationship between egg and T2DM risk between US-based and non-US based studies suggest that the impact of egg consumption may be confounded by lifestyle or dietary habits associated with the risk of T2DM. For example, the authors of these meta-analyses hypothesized that high consumers of eggs in the US may also indulge in other habits that contribute to T2DM risk, such as smoking, low physical activity, and excess saturated fat and caloric intakes. Additionally, food preparation methods (e.g. boiled or fried eggs, whole eggs or only egg whites) or concurrent consumption of other foods associated with increased diabetes risk (e.g. home fries, bacon) that are popular in the US may also partly account for the differences. Unfortunately, the authors of meta-analyses cited the lack of data on the overall quality of the diet and food preparation details within individual studies as a reason for not being able to pursue this line of investigation.
The positive association between egg consumption and risk for CVD and CHD among those with T2DM(Reference Li, Zhou and Zhou14,Reference Shin, Xun and Nakamura15,Reference Rong, Chen and Zhu29) raises the question of whether separate recommendations on egg consumption should be provided for individuals with diabetes. Currently, recommendations on egg consumption not only vary across countries, but also rarely account for diabetes status. However, these findings were based on a limited number (n ≤8) of older studies (2011 and older)(Reference Li, Zhou and Zhou14,Reference Shin, Xun and Nakamura15,Reference Rong, Chen and Zhu29) . Therefore, further research is needed to better define the role of eggs on CVD risk in the T2DM population.
A striking gap in knowledge in the area of egg consumption and CVD risk is the lack of meta-analyses on intervention studies. The authors of one narrative review(Reference Geiker, Larsen and Dyerberg22) attributed their decision to not conduct a meta-analysis on the effects of egg intake on CVD risk factors in individuals with T2DM or at risk for developing T2DM due to the heterogeneity of the studies, with the intervention trials differing in population, primary outcome, background diet, and amount of eggs used. Interestingly, the one meta-analysis on intervention studies reported that higher egg consumption increases tChol and LDL-C, surrogate markers for CVD in the general population(Reference Rouhani, Rashidi-Pourfard and Salehi-Abargouei32). This is in contrast to meta-analyses on prospective cohort studies, which reported no association between egg intake and ASCVD risk. Because it is not time and cost efficient for intervention studies to measure actual CVD incidence, intervention studies have resorted to measuring surrogate markers of ASCVD risk and not CVD risk itself.
It is possible that the contradicting observations of the meta-analysis on intervention studies and those on prospective cohort studies are due to limitations in extrapolating changes of surrogate markers to changes in CVD risk. For example, although LDL-C is a key target for CVD risk reduction, the residual risk (i.e. the ongoing appreciable risk of major CV events in statin-treated patients who have achieved evidence-based lipid goals(Reference Kones50)), suggests LDL-C should not be used as the sole basis for CVD risk prediction. Indeed, predictive equations such as the new Pooled Cohort ASCVD Risk equation, the Framingham CHD 10-year risk, and the Framingham Risk Score for CVD 10-year risk have paired LDL-C with other risk factors such as age, tChol, HDL-C, systolic BP, diabetes status, and current smoking status to increase the predictability of CVD risk. Although several intervention studies collect all the different elements of the aforementioned equations, none have calculated CVD risk and included that as a study outcome. Thus, future studies should consider combinations of various risk markers to take advantage of the better predictive potential than that of respective measures in isolation.
The reviews included may not represent all reviews on egg consumption and cardiometabolic health because reviews not published in English or not using a systematic search strategy were excluded, and those not published in PubMed, WOS, Cochrane Library, NESR, and AHRQ may have been missed. However, effort was made during this review to hand search all references in selected narrative reviews in addition to ad hoc searches on Google. In addition, because this is an umbrella review, the primary studies included in all the selected reviews were not assessed in detail.
Conclusions
Recent reviews addressing the specific effect of eggs on cardiometabolic health risks consistently report that higher egg consumption is not associated with CVD risk, but a positive association was found with risk of HF. A decreased risk of HTN with higher egg consumption has also been reported, which is inconsistent with the HF findings since HTN is a major risk factor for HF. However, these findings are based on limited data and further research is needed to better understand the role of eggs in HTN and HF. In addition, higher egg consumption is not associated with risk of T2DM in the general population, but is positively associated with T2DM in US-based studies only, which also requires additional research, specifically focused on differences in US-based and European populations. These varying results may reflect the complexity of cardiometabolic health, and differential individual responses to eggs, which can be affected by health status (e.g. presence or absence of diabetes) and response to dietary cholesterol, and other physiological and lifestyle factors.
Acknowledgements
Acknowledgements: The authors would like to thank Dr Chad Cook and Deena Wang for assistance with an initial scoping review that led to the development of this project. Financial support: Partial support for this work was provided by the Egg Nutrition Council to Biofortis, Mérieux NutriSciences. Conflict of interest: D.L. received support for conducting an initial review from the Egg Nutrition Council. The development of the manuscript was supported by Biofortis, Mérieux NutriSciences. The Egg Nutrition Council had no influence on the formulation of review aims, search and screening of reviews, data extraction, interpretation, and writing of the article. Authorship: Conceptualization (D.L.); search and screening (D.L., O.C.); data extraction (D.L., O.C., E.M.); writing (D.L., O.C., E.M.). All authors read and approved the final version. Ethics of human subject participation: Not applicable.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980019002441










