The combination of poverty-related infectious diseases and lifestyle-related non-communicable diseases, both driven by malnutrition, causes considerable problems for South Africa. The 2005 National Food Consumption Survey Fortification Baseline (NFCS-FB-I) revealed that stunting and underweight were the most prominent nutritional disorders in children aged 1–9 years( Reference Labadarios, Swart and Maunder 1 ). This was confirmed by the South African National Health and Nutrition Examination Survey (SANHANES-1) executed in 2011–2012. In children younger than 5 years the prevalence of stunting has increased slightly and the prevalence of underweight has decreased since 2005( Reference Shisana, Labadarios and Rehle 2 ). Moreover, the NFCS-FB-I showed that one out of seven children was Fe-deficient, 45·3 % of children had inadequate Zn status and two out of three children had poor vitamin A status( Reference Labadarios, Swart and Maunder 1 ). The National Food Consumption Survey (NFCS) of 1999 is the only national survey that reports nutrient intake data for children. It reported that the essential micronutrient intake of one out of two 1–9-year-old children was less than half of what is recommended, especially in rural areas. The micronutrients that were found to be consumed at less than 67 % of the RDA were Fe, vitamin A, Zn and Ca( Reference Labadarios, Steyn and Maunder 3 ).
Healthy and nutritious diets for populations depend on the availability and accessibility of a variety of plant and animal foods, in a context that promotes and supports healthy behaviour. Traditional biodiversity use, instead of Westernized diets, in the socio-cultural context can be a powerful tool for maintaining and enhancing health and nutritional status( Reference Johns 4 ). Uusiku et al.( Reference Uusiku, Oelofse and Duodu 5 ), Faber et al. ( Reference Faber, Venter and Benadé 6 ) and Nesamvuni et al. ( Reference Nesamvuni, Steyn and Potgieter 7 ) in South Africa underscored the important contribution of African leafy vegetables (ALV) as a potential source of micronutrients, in particular Fe, Zn and vitamin A (β-carotene). The Fe, Zn and β-carotene content of ALV is known to range from 0·2 to 12·8 mg, from 0·02 to 18·5 mg and from 99 to 1970 µg retinol equivalents (RE) per 100 g edible portion, respectively( Reference Uusiku, Oelofse and Duodu 5 ). Moreover, apart from being good plant sources of Fe, Zn and β-carotene, dark-green ALV supply other nutrients such as folate and ascorbic acid, as well as phytochemicals( Reference Uusiku, Oelofse and Duodu 5 , Reference Tontisirin, Nantel and Bhattacharjee 8 ).
Consumption of ALV can potentially contribute to dietary diversity and the reduction of micronutrient malnutrition, and further improve nutritional status and human health. However, to our knowledge, no studies have been carried out on how these vegetables can make a contribution to the nutritional status of school-aged children in farm communities in Africa, including South Africa. The objectives of the present study therefore included establishing the micronutrient content and consumer acceptance of dishes made with selected ALV and investigating whether inclusion of these ALV dishes in school children’s diets would improve their nutritional status. Hence, the effect of consumption of selected ALV on blood Hb, serum ferritin (SF), serum transferrin receptor (sTfR), Zn protoporphyrin (ZnPP), serum Zn and serum retinol concentrations of school children was investigated.
Materials and methods
Setting
The study was conducted in two farm schools in a malaria-free rural area approximately 50 km from Potchefstroom in the North West Province, South Africa. Both these primary schools were located in similar farm surroundings (where the main farming activities include maize, sunflower and chicken) and were fully funded by the South African Department of Education and by the farm owners. Children attending these schools receive one meal each school day (at about 10.30 hours) as part of the National School Nutrition Programme in South Africa. These farm schools were selected because farm communities in South Africa are regarded as the least privileged and the most vulnerable population in terms of income, health status, education and household nutrition security( Reference Chopra, Drimie and Whitten 9 , Reference Kruger, Lemke and Phometsi 10 ).
Participants
The sample size requirement was calculated by using data from De Pee et al. ( Reference De Pee, West and Permaesih 11 ). A sample size of sixty-three learners per group would be adequate to detect a 20 % increase in SF and a 20 % increase in serum retinol at a 5 % significance level with 80 % statistical power. The study was conducted from March to June 2012. All children from grade R to grade 4 (6–12 years old) were invited to participate in the study. The parents/guardians of these 239 children were invited to the school to discuss the study. Parents of 171 children gave consent to participate, resulting in a response rate of 71·5 %. Participating children were dewormed at baseline with an oral dose of 400 mg albendazole (Cipla-Medpro (Pty) Ltd, Cape Town, South Africa). Participants were apparently healthy and had no signs and symptoms of acute illness at the time of baseline blood collection. Children with Hb concentration <8 g/dl were excluded from the study and referred for medical treatment. Children who were taking micronutrient supplements were also excluded from the study.
Study design
The study used a parallel-group randomized controlled design (see Fig. 1); participants were stratified by school and by grade, and were randomly individually allocated to either the intervention (n 86) or control group (n 81) by means of a random number generator (http://stattrek.com/Tables/Random.aspx). All measurements were done blind. Two weeks before the start of the intervention, which took place from 1 March 2012 until 8 June 2012, baseline data were collected at both schools. Each child in the intervention group received 300 g of a cooked ALV dish with the starch of the school meal for sixty-two school days. This included thirty times the amaranth-and-cowpea dish, twenty-five times the amaranth-and-pumpkin dish, three times the amaranth-and-spiderplant dish and three times the amaranth dish. The ALV used in the intervention were not commercially available on the scale needed at the time of the study and were therefore cultivated especially for the intervention. The yields of the various ALV used were different and this resulted in a non-equal distribution. The children in the control group received the normal school meal. Meals were eaten in the classrooms under the supervision of a fieldworker who recorded adherence and absence, including the reason. Adherence was measured by how much of the meal was consumed in four categories (plate empty, plate 75 % empty, plate 50 % empty or plate full). If the child ate very little (less than 25 %) of the meal then this was recorded as non-compliance. This fieldworker also monitored potential food sharing and contamination or spill-over effects.
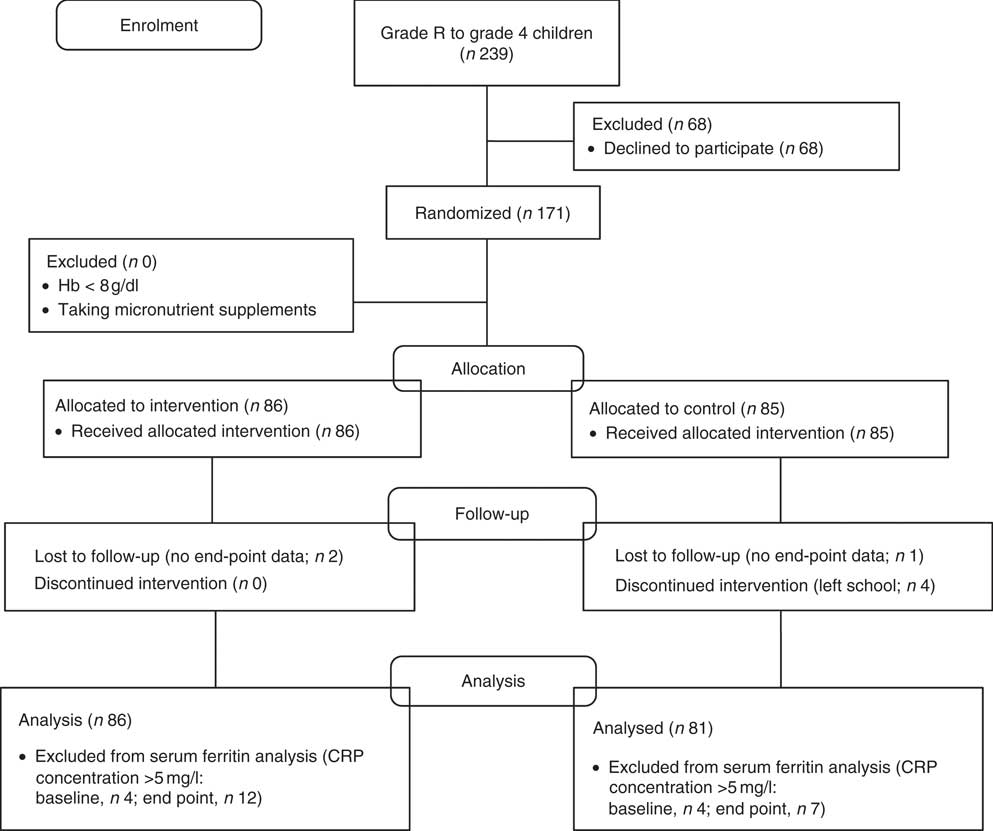
Fig. 1 Flow diagram of the intervention study (CRP, C-reactive protein)
Intervention product
The following ALV were used to prepare the intervention dish: Amaranthus cruentus (amaranth), Cleome gynandra (spiderplant), Cucurbita maxima (pumpkin) and Vigna unguiculata (cowpea). These ALV were found to be those mostly consumed and used in urban and rural areas in the North West Province( Reference Matenge, Van der Merwe and Kruger 12 ). The vegetables were cultivated on farmland approximately 50 km from Potchefstroom in the North West Province, South Africa from October 2011 until February 2012. Harvesting took place from 9 January 2012 until 2 February 2012. After harvesting, the vegetables were brought to the research kitchen on the Potchefstroom Campus of the North-West University and stored at +5°C. Either on the day of harvesting or the next day, the vegetables were processed into the intervention dishes using an oil-jacketed pot (Vulcan BP-225; Vulcan Catering Equipment (Pty) Ltd, Johannesburg, South Africa). The ALV in the first dish consisted of 100 % amaranth; the ALV in the other three dishes consisted of 80 % amaranth plus 20 % of spiderplant, pumpkin or cowpea. The ALV content of each dish (raw ingredients) was 49 %. The remaining raw ingredients included chopped tomatoes and onion mix (22 %), vegetable oil (3 %), a commercially available instant gravy powder (5 %) and water (21 %). Three different flavours of the same gravy were used to avoid product fatigue during consumption and thereby assuring better adherence. The packaging of the intervention dishes consisted of four portions of 300 g each, labelled with the type of dish and the harvest and processing date and stored at –20°C. The recipe that was used to prepare the dishes was based on the study results by Matenge et al. ( Reference Matenge, Van der Merwe and De Beer 13 ) in the North West Province, which showed that this recipe was found to be the most acceptable and preferred of the traditional dishes used in the area. During the intervention study, the dish (300 g) was served together with the starch (125 g) that was provided by the school meal. The control meal consisted of the same starch portion accompanied by a serving spoon of relish, including vegetables (often cabbage) or legumes and sometimes meat or soya mince (not more than a teaspoon).
Acceptance of the dishes containing African leafy vegetables
Prior to the intervention, the ALV dishes were evaluated by children (n 80) from grade 2–4 attending the selected schools on sensory attributes in order to ensure adherence to the intervention during the entire intervention period. Swiss chard (Beta vulgaris) was included as a reference dish and was prepared in the same way as the ALV dishes. The methods used for this sensory evaluation have been described in Van der Hoeven et al. ( Reference Van der Hoeven, Osei and Greeff 14 ).
Nutrient composition of the dishes containing African leafy vegetables
The ALV dishes were analysed for Fe and Zn content. At least three composite samples (each consisting of three randomly selected individual dishes) of each ALV dish were prepared at the beginning, mid-way and end of the harvest and processing time. The amaranth-and-spiderplant dish was prepared only at the beginning of the harvest and processing time, because the yield of the cultivated spiderplant was low. The composite samples were sent to Microchem Laboratories in Cape Town, which is accredited by the South African National Accreditation System. Inductively coupled plasma–optical emission spectrometry (Optima 5300 DV; PerkinElmer, Johannesburg, South Africa) was used for the analysis of Fe and Zn. The nutrient content of the ALV dishes was used to calculate their contribution to the children’s Fe and Zn intake requirements.
Dietary intake assessment
Dietary intake was assessed by means of a 24 h dietary recall method and a quantitative FFQ (QFFQ) among a sub-sample of the study population (n 100) after completion of the intervention study. The 24 h recall was used to collect information on the nutrient intakes of the intervention and control groups and included dietary intake on both weekdays and weekend days. The QFFQ collected data on the food items consumed in the previous month. Food portion sizes were estimated using fresh foods (stiff and soft maize-meal porridge and rice), plastic food models, household utensils and food packaging materials. The adult responsible for the food preparation for the child was present during the 24 h recall and QFFQ interview with the child. These interviews were conducted in the mother tongue of the interviewees by two trained and experienced interviewers. All 24 h recalls and QFFQ were checked for completion (e.g. preparation methods included and portion sizes) and possible errors. Data were coded and quantified manually by a registered dietitian using the MRC Food Quantities Manual ( Reference Langenhoven, Conradie and Wolmarans 15 ). Data were computerized and analysed using the MRC FoodFinder3 software, which is based on the South African food composition (SAFOODS) database( Reference Wolmarans, Danster and Dalton 16 ). The vitamin A values for plant foods, given as µg RE in the SAFOODS database, were divided by 2 to obtain µg retinol activity equivalents (RAE) values; for animal foods and fortified staple foods, µg RE is equal to µg RAE.
Biochemical indicators
At baseline and intervention end, registered nurses drew venous blood samples (10 ml) into a 7 ml serum tube (only 6 ml blood) and a 4 ml EDTA-coated tube from non-fasting participants, using a sterile Venofix® infusion set (23G, 0·65 mm×20 mm; Braun, Melsungen, Germany) and syringes. Whole blood collected in the serum tube was first allowed to clot in the tube at room temperature. All blood samples were kept on ice and transported in a cooler box within 1–2 h after collection to the laboratory at the Centre of Excellence for Nutrition, North-West University. Hb and erythrocyte ZnPP were measured immediately after arriving at the laboratory, whereas for all other measurements, baseline and end samples were analysed paired-wise immediately after the completion of the study. Hb concentration was measured in whole blood in the EDTA tubes using a haematology analyser (Coulter® Ac·T™ 5diff CP; Beckman Coulter Inc., Pasadena, CA, USA). After the Hb was measured, the tubes were centrifuged for 10 min at 3500 rpm. The plasma was collected and stored at −80°C. The remaining erythrocytes were washed three times with 5 ml saline solution, inverted after each addition and spun off. ZnPP was measured on these washed erythrocytes using a ZPP haematofluorometer (model 206D; Aviv Biomedical Inc., Lakewood, NJ, USA). Care was taken throughout to ensure that the samples were protected from direct light. The micro-tubes used for the storage of serum for Zn analysis were trace element-free (micro-tubes washed with 10−20 % v/v HNO3) to avoid contamination. For serum preparation, whole blood collected in the 7 ml serum tube was centrifuged at 3500 rpm for 10 min at room temperature (Universal 16RTM Centrifuge; Hettich, Tuttlingen, Germany), then transferred into micro-tubes and stored at −80°C. SF was measured using a ferritin ELISA kit (Ramco Laboratories Inc., Stafford, TX, USA). sTfR was determined by using an in vitro enzyme immunoassay (Ramco Laboratories Inc.). Serum retinol, Zn and C-reactive protein (CRP) were measured at the laboratory of the South African Medical Research Council in Cape Town. Serum retinol was determined by a reversed-phase HPLC method described by Catignani and Bieri( Reference Catignani and Bieri 17 ) and using a Spectra SERIES chromatographic system (Thermo Separation Products, Fremont, CA, USA). Serum Zn was analysed with a flame atomic absorption spectrophotometer (Philips Pye Unicam SP9, Cambridge, UK) using a commercial control serum (Seronorm Trace Elements Serum; SERO AS, Billingstad, Norway) as a quality control. CRP was measured by an immunoturbidimetric method (Technicon method no. SM4-0183G89, Technicon RA-1000 auto analyser; Technicon Instruments, Tarrytown, NY, USA), using Bayer TESTpoint Serum Protein Controls (Bayer Diagnostics, Fernwald, Germany).
Anaemia was defined as Hb concentration <11·5 g/dl( 18 ). Fe deficiency was defined as SF concentration <15 µg/l( Reference Zimmermann 19 ), ZnPP concentration >70 µmol/mol haem( Reference Metzegeroth, Adelberger and Dorn-Beineke 20 ) or sTfR concentration >8·3 mg/l (test-kit reference value). Fe-deficiency anaemia was defined as having both Hb<11·5 g/dl and SF<15 µg/l. Inflammation was defined as CRP concentration >5 mg/l( Reference Thurnham, McCabe and Haldar 21 ); SF and serum retinol values of participants with inflammation were excluded from the analyses because of the confounding effects of inflammation on SF and serum retinol. Zn deficiency was defined as serum Zn concentration <65 μg/dl( Reference Hotz, Peerson and Brown 22 ). Subclinical vitamin A deficiency was defined as serum retinol<20 μg/dl( 23 ).
Anthropometric measurements
Body weight was measured without shoes and wearing minimal clothing to the nearest 0·1 kg using a digital Tanita Ironman Innerscan body composition monitor scale (BC 554–Elite Series; Tanita Corporation of America Inc., Arlington Heights, IL, USA), which was calibrated by using fixed weights. Height was measured with the Seca Leicester height measure mounted on a board (Seca Ltd, Birmingham, UK). During height measurement, the child stood upright without shoes on a flat surface against a wall with the head in the Frankfort plane. Height-for-age Z-score (HAZ), weight-for-age Z-score (WAZ) and BMI-for-age Z-score (BAZ) were calculated using the 2007 WHO reference for children aged 5−19 years( Reference De Onis, Onyango and Borghi 24 ). Stunting was defined as HAZ<−2 and mild stunting as −2<HAZ<–1. Underweight was defined as WAZ<−2 and mild underweight as –2<WAZ<–1. WAZ was available only for children under 11 years. WAZ cannot be used as a monitoring tool for growth beyond childhood, since it is not able to distinguish between relative height and body mass( Reference De Onis, Onyango and Borghi 24 ). Overweight was defined as BAZ>1 and obesity as BAZ>2.
Ethical considerations
The study was conducted according to the guidelines laid down by the Declaration of Helsinki. All procedures involving human subjects were approved by the Ethics Committee of the North-West University (NWU-00033-09-A1). Permission was also granted by the Department of Education of the North West Province (Dr Kenneth Kaunda district) and the governing bodies of the two schools. Meetings with the parents/guardians were held in their preferred language to explain the purpose and the procedures of the study and they were given the opportunity to ask questions or make remarks. Afterwards guardians were asked to give their written consent for their children to participate in the study. Only children who obtained parental consent and gave verbal assent for the study were included. Participation was voluntary and participants could withdraw any time without any consequences. The intervention study was registered at clinicaltrails.gov as NCT01920646 with the title ‘Effect of African Leafy Vegetables on nutritional status of South African school children (ALV)’. The full trial protocol can be requested from the authors.
Statistical methods
Statistical analyses were performed using the statistical software package IBM SPSS 20·0 for Windows. Data were checked for normal distribution and the presence of outliers (±3 sd from the mean). Skewed variables were transformed before analysis. Five imputations were produced for all outcome variables for missing data for the intervention study (<3 %) under the assumption that values were missing at random. These multiple imputations were produced using an iterative Markov chain Monte Carlo method and a linear regression model, which included the intervention (ALV consumption), age, sex, school and adherence as independent predictors. Independent t tests were conducted to investigate potential differences in baseline characteristics between the intervention and the control group. Estimated intervention effects were analysed by using ANCOVA on the end-point measurement with baseline value, sex, age, school and adherence as individual-level covariates. Baseline differences in nutritional status outcomes between the intervention and control groups, between boys and girls, between children who were stunted and those who were not, and between deficient and non-deficient children were analysed by independent t tests. A two-factor ANCOVA including sex (boys compared with girls) or HAZ (stunted compared with not stunted) or deficiencies (deficient compared with not deficient) and ALV consumption as fixed factors was performed to investigate potential interactions of sex, stunting or deficiencies with ALV consumption on end-point nutritional status outcomes. For the consumer acceptance, each verbal anchor used in the sensory evaluation was allocated a value ranging from ‘super good’ (value=5) to ‘super bad’ (value=1). Mean values for the different attributes were calculated. Data were found not to be normally distributed; therefore the Kruskal–Wallis test was used to compare the ALV dishes. A P value of <0·05 was regarded as statistically significant.
Results
Acceptance of the intervention dish
Each child (n 80) evaluated four different samples and each dish was evaluated at least sixty-two times. Table 1 shows the mean rating scores of the four ALV dishes with regard to colour, smell, taste and overall acceptability. Although all dishes were rated as acceptable, Swiss chard had higher rating scores. The ALV dishes were rated ‘good’ or ‘super good’ by 62·3 % of the participants for colour, by 57·2 % for smell, by 52·2 % for taste and by 56·8 % for overall acceptance (data not shown).
Table 1 Sensory evaluation scoresFootnote * (mean and standard deviation) for different dishes made with African leafy vegetables (ALV) among grade 2 to grade 4 children (n 80) of two farm schools, North West Province, South Africa, 2012

a,b,c,dMean values within a column with unlike superscript letters were significantly different (Kruskal–Wallis test; P<0·05).
* Score: five-point ordinal scale ranging from 5 (‘super good’) to 1 (‘super bad’).
† Each dish (raw ingredients) consisted of 49 % ALV, tomato and onion mix (22 %), vegetable oil (3 %), a commercially available instant gravy powder (5 %) and water (21 %).
Nutrient composition of the dishes containing African leafy vegetables
Table 2 shows the Fe and Zn content of the ALV dishes and their contribution to the RDA for Fe and Zn. One single serving (300 g) of an ALV dish provided 116–158 % of the RDA for Fe and 28–74 % of the RDA for Zn (based on RDA for children aged 4–8 years( Reference Otten, Hellwig and Meyers 25 )). The dish made with 80 % amaranth and 20 % pumpkin contributed most to the RDA for Fe (15·8 mg per 300 g portion) and the dish made with 100 % amaranth contributed most to the RDA for Zn (3·7 mg per 300 g portion).
Table 2 Percentage contribution of iron and zinc content of different African leafy vegetable (ALV) dishes (per 300 g serving) to the RDA

* Each dish (raw ingredients) consisted of 49 % ALV, tomatoes and onion mix (22 %), vegetable oil (3 %), a commercially available instant gravy powder (5 %) and water (21 %).
† The RDA for Fe and Zn are 10 mg/d and 5 mg/d, respectively, for children aged 4–8 years. For children aged 9–13 years, the RDA for both Fe and Zn is 8 mg/d( Reference Otten, Hellwig and Meyers 25 ).
Intervention
In total 167 children completed the study, four children left the school and for three children only baseline data were collected (Fig. 1). Baseline characteristics are shown in Table 3. There were no relevant differences in any baseline characteristics between the intervention and the control group. Morbidity patterns were similar for the intervention and control group. Adherence was high in both the intervention (79 %) and the control group (89 %), taking into account absenteeism and leftovers. The meat included in the control meals was a small portion (less than a teaspoon). If meat was included, it was only once or twice every two weeks. Children from the intervention group were allowed to also eat the control meal, but without the starch, after they finished their intervention meals. During the study only two children from the intervention group ate the control meal (not the starch) as well. The full portion of the intervention meal was eaten on 71 % of the days and the full portion of the control meal was eaten on 89 % of the days. For the intervention group the mean daily ALV dish consumption was 236 (sd 1·6) g.
Table 3 Baseline characteristics of study population: grade R to grade 4 children (6–12 years old) of two farm schools, North West Province, South Africa, 2012
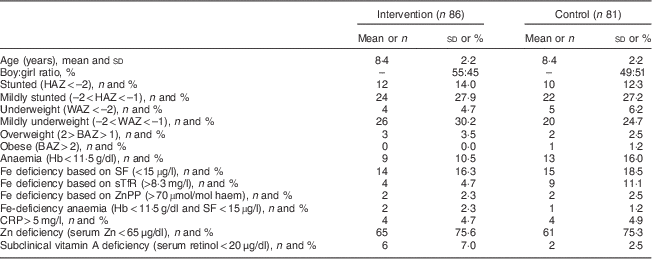
HAZ, height-for-age Z-score; WAZ, weight-for-age Z-score; BAZ, BMI-for-age Z-score; SF, serum ferritin; sTfR, serum transferrin receptor; ZnPP, zinc protoporphyrin; CRP, C-reactive protein.
Values are reported as mean and standard deviation, or as number and percentage.
SF values of participants with a CRP concentration >5 mg/l were excluded.
WAZ available only for participants aged <11 years (n 141).
Dietary intake
Analysis of the macronutrient intake showed no differences between the intervention and the control group (data not shown). The median (interquartile range) energy intake (based on QFFQ) was 7291 (5768–9960) kJ for children in the intervention group. The control group had a median (interquartile range) energy intake of 6493 (5258–8457) kJ. The median macronutrient intake (as a percentage of energy) was within the acceptable macronutrient distribution range for both groups as determined by the Institute of Medicine( 26 ). Table 4 shows that children in both the intervention and the control group (based on QFFQ) had a median intake above the estimated average requirement (EAR) for Fe, vitamin A and Zn, and most children’s intake was above the EAR for Fe and Zn. However, the percentage of children with an intake below the EAR for vitamin A ranged from 8·1 % to 27·5 % depending on the dietary assessment method (Table 4). The food items providing these micronutrients were mainly cereals and cereal products and meat. Stiff maize-meal porridge and bread, both mandatorily fortified, were among the food items contributing to more than one micronutrient and provided the largest amounts of Fe, vitamin A and Zn (Table 5). Table 5 shows that almost every child consumed both fortified stiff maize-meal porridge and bread. Taking into account the median portion size of both the 24 h recall and the QFFQ, the porridge provided 27–32 %, 20–23 % and 18–22 % of the RDA for children 4–8 years old for Fe, vitamin A and Zn, respectively. The bread was a richer source of Zn than the porridge; it provided 14–16 %, 7–8 % and 30–36 % of the RDA for children 4–8 years old for Fe, vitamin A and Zn, respectively.
Table 4 Daily micronutrient intake (median and interquartile range) and percentage below the EAR for grade R to grade 4 children (6–12 years old) of two farm schools, North West Province, South Africa, 2012

EAR, estimated average requirement; 24 h recall, 24 h dietary recall method, QFFQ, quantitative FFQ; Q, quartile; RAE, retinol activity equivalent.
Dietary intake was assessed among a sub-sample of the study population (n 100) after completion of the intervention study and included mandatorily fortified products.
* EAR for children aged 4–8 years( Reference Otten, Hellwig and Meyers 25 ).
Table 5 Consumption of fortified food items rich in iron, vitamin A and zinc, based on the QFFQ, among grade R to grade 4 children (6–12 years old) of two farm schools, North West Province, South Africa, 2012
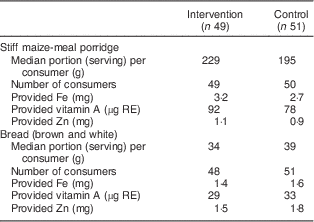
QFFQ, quantitative FFQ; RE, retinol equivalent.
Dietary intake was assessed among a sub-sample of the study population (n 100) after completion of the intervention study.
Intervention effects
Concentrations of biochemical indicators of Fe, Zn and vitamin A status at baseline and end point for both the intervention and control group are shown in Table 6. There were no significant estimated intervention effects. There were no significant sex × ALV consumption interaction effects on the biochemical outcomes either. Other deficiencies (anaemia, Zn deficiency and Fe deficiency based on SF) did not have a significant interaction with ALV consumption. In both groups Hb and serum Zn concentrations improved significantly from baseline: in the intervention group, P=0·001 and P=0·022, respectively; and in the control group, P<0·001 and P=0·006, respectively. There was no significant difference between baseline and end point for serum retinol and SF in either of the two groups. The prevalence of subclinical vitamin A deficiency decreased significantly in the intervention group from 7·0 % at baseline to 1·3 % at end point (P=0·015), but there was no change in the control group. The anaemia prevalence decreased from 10·5 % to 1·9 % in the intervention group (P=0·015) and from 16·0 % to 2·5 % in the control group (P=0·001). The prevalence of Zn deficiency decreased as well; however, non-significantly in the intervention group (from 75·6 % to 68·8 %) and significantly (from 75·3 % to 53·8 %) in the control group (P<0·001). The prevalence of Fe deficiency based on SF decreased non-significantly from 16·3 % to 15·0 % in the intervention group and increased non-significantly from 18·5 % to 21·3 % in the control group.
Table 6 Effects of African leafy vegetable consumption on biochemical indicators of iron, zinc and vitamin A statusFootnote * among grade R to grade 4 children (6–12 years old) of two farm schools, North West Province, South Africa, 2012
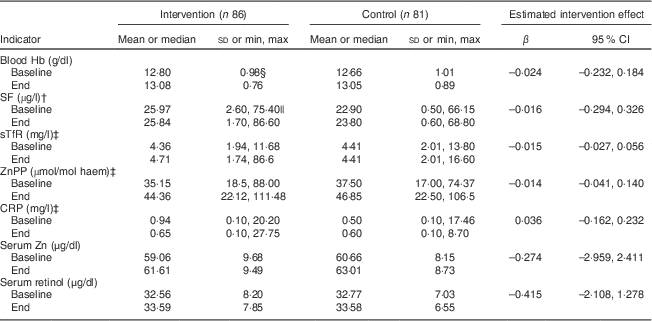
SF, serum ferritin; sTfR, serum transferrin receptor; ZnPP, zinc protoporphyrin; CRP, C-reactive protein.
* Intervention effects were estimated by using ANCOVA adjusted for respectively baseline value, sex, age, school and adherence.
† Data were square-root transformed to perform ANCOVA. SF values of participants with CRP concentration >5 mg/l were excluded (at end point: intervention, n 12; control, n 7).
‡ Data were log transformed to perform ANCOVA.
§ Values are presented as mean and standard deviation (all such values).
|| Values are presented as median and minimum, maximum (all such values).
Discussion
The present randomized controlled trial contributes to the limited existing evidence on the effect of dark-green leafy vegetables, including ALV, on the micronutrient status of children( Reference De Pee, West and Permaesih 11 , Reference Nawiri, Nyambaka and Murungi 27 – Reference Tang, Gu and Hu 29 ). The ALV used in the study were selected for their potential contribution towards dietary Fe, vitamin A and Zn. The ALV dishes were found to be acceptable with regard to colour, taste and smell for school children, confirming our previous findings( Reference Van der Hoeven, Osei and Greeff 14 ). The study population was selected for its expected vulnerable nutritional status and assumed high prevalence of anaemia and Fe, vitamin A and Zn deficiencies. Although this was true for Zn deficiency, the study population only had mild Fe and vitamin A deficiencies. When adjusted for baseline value, sex, age, school and adherence, no intervention effect of ALV consumption on the Fe, vitamin A and Zn status of children was found. This is in contrast to Agte et al. ( Reference Agte, Jahagirdar and Chiplonkar 30 ), who found that daily intake of 100 g green leafy vegetables with 10 g oil improved plasma β-carotene, Hb, plasma vitamin C and plasma Zn in forty young healthy adults.
In terms of previous studies that assessed the effect of green leafy vegetables on anaemia in children, Nawiri et al. ( Reference Nawiri, Nyambaka and Murungi 27 ) found that cooked dishes made of fresh cowpea and amaranth leaves improved Hb concentrations in pre-school children, of whom about 70 % were anaemic (Hb<12·0 g/dl), with an improvement of 5·9 % although not significant. Takyi( Reference Takyi 28 ) found that consumption of cooked dark-green leafy vegetables by pre-school children, of whom 92 % had Fe-deficiency anaemia, did not reduce the prevalence of anaemia.
Previous studies have shown that consumption of dark-green leafy vegetables improved the serum retinol concentrations in children with vitamin A deficiency( Reference Nawiri, Nyambaka and Murungi 27 , Reference Takyi 28 ) or a marginal vitamin A status( Reference De Pee, West and Permaesih 11 ). A combination of dark-green leafy and yellow vegetables (including spinach, Chinese chives, broccoli, carrots and red yam) improved the serum retinol concentration in children with a sufficient vitamin A status( Reference Tang, Gu and Hu 29 ). There was no change in mean serum retinol concentration observed in our study, probably because ALV seem to be more effective at improving low levels of serum retinol( Reference Nawiri, Nyambaka and Murungi 27 ); the mean serum retinol of the children included in our study was relatively high. The decrease in subclinical vitamin A deficiency in the intervention group in our study appeared to confirm this. According to Takyi( Reference Takyi 28 ), homogenization releases β-carotene molecules better from their food matrix in order to be incorporated into mixed micelles before intestinal absorption. However, homogenization was not used in meal preparation in studies found in the literature observing an improvement in serum retinol and in our study( Reference De Pee, West and Permaesih 11 , Reference Nawiri, Nyambaka and Murungi 27 , Reference Tang, Gu and Hu 29 ). Although we did not analyse the β-carotene levels in our vegetables due to technical reasons, van Jaarsveld et al. ( Reference Van Jaarsveld, Faber and van Heerden 31 ) reported that amaranth, cowpea, spiderplant and pumpkin leaves are rich in β-carotene (ranging from 4·2 to 7·1 mg per 100 g edible fresh weight( Reference Van Jaarsveld, Faber and van Heerden 31 )).
Our intervention showed no effect on Zn status, despite the fact that serum Zn was low in many children. Taking into account the Zn content of the dish as well as the low bioavailability( Reference Hassan, Umar and Dangoggo 32 ), a possible explanation for the significant increase in mean serum Zn in both groups is regression to the mean, as the baseline and end samples were analysed paired-wise after completion of the intervention. Furthermore, a generally accepted sensitive and specific biomarker for Zn status is currently lacking. In the meanwhile, serum (or plasma) Zn remains the most widely used biomarker( Reference Moran, Stammers and Median 33 ).
The nutritional status of the children at baseline was better than expected. We assumed a high prevalence of micronutrient deficiencies in the selected study population based on the results of the 2005 NFCS-FB-I( Reference Labadarios, Swart and Maunder 1 ). Results of smaller more localized studies do, however, suggest that the prevalence of anaemia and vitamin A deficiency in children may have improved in certain regions of the country since the NFCS-FB-I( Reference Taljaard, Covic and Van Graan 34 , Reference Faber, van Jaarsveld and Kunneke 35 ). The 2012 SANHANES-1 also suggested that the micronutrient status of children in South Africa has improved in the last years( Reference Shisana, Labadarios and Rehle 2 ). Screening the study population for the intended micronutrient deficiencies before the implementation of a randomized controlled trial is therefore imperative in future studies. In South Africa, a mandatory national food fortification programme was introduced in 2003 whereby the two most commonly eaten staple foods, i.e. wheat flour (bread) and maize meal, are fortified by addition of a fortification mix containing vitamin A, Fe, Zn, as well as other micronutrients( 36 ). The national food fortification programme probably contributed towards the better than expected nutritional status of the children at baseline.
The ALV dish was served with the starch of the school meal. Traditionally, ALV are consumed mainly as relish together with other ingredients to accompany a starch dish (mostly maize-meal porridge), as was the case in the present study. The high phytic acid concentration in the maize meal is regarded as the principal dietary factor that influences the absorption of naturally occurring and added Fe and Zn, especially in a plant-based diet( Reference Hurrell and Egli 37 – Reference Miller, Krebs and Hambidge 39 ). Processing and preparation methods of ALV, including traditional recipes, should be optimized, focusing on both the reduction of anti-nutrients and the increase of micronutrient bioavailability.
The children included in the intervention group were not reluctant to eat a substantial portion of ALV five days per week for a period of three months. Vegetables and fruit are often neglected, contributing to a monotonous diet in many South African households( Reference Samuel, Egal and Oldewage-Theron 38 , Reference Labadarios, Steyn and Nel 40 ). It is well known that the consumption of sufficient amounts of fruit and vegetables reduces the risk of disease( Reference Naude 41 , Reference Lock, Pomerleau and Causer 42 ). It has been argued that ALV can play an essential role in the WHO’s global initiative on the consumption of fruit and vegetables( Reference Smith and Eyzaguirre 43 ). ALV, when in season, can also play an important role in achieving the guidelines to include vegetables and fruit in the daily school meal, as stipulated by the National School Nutrition Programme. This is not always implemented at school level due to limited access to these perishables and lack of storage space( Reference Faber, Laurie and Maduna 44 ).
Conclusion
In conclusion, acceptance of the ALV dish and adherence during the intervention were good. Yet the study showed no intervention effect on micronutrient status. Daily consumption of mandatory fortified maize-meal porridge and bread, mild deficiencies of Fe and vitamin A in the children and the relatively short duration of the intervention could have contributed towards the lack of intervention effect. Also, the bioavailability of Fe and Zn in plant sources is known to be low and can be further compromised by the presence of anti-nutrients. Bioavailability of Fe and Zn in ALV, and the effect of including ALV dishes in maize-based meals, should be further investigated. Novel, but culturally acceptable processing and preparation methods to improve the bioavailability of micronutrients from plant sources should be investigated.
Acknowledgements
Acknowledgements: The authors would like to thank the participating children and their teachers for their contribution; the Agricultural Research Council for providing the ALV seed; the farmer and his team of Muiskraal, Rysmierbult, South Africa for the cultivation of the ALV; and their colleagues at the Centre of Excellence for Nutrition of the North-West University for their assistance during the field and laboratory work. Financial support: This research was supported by a grant from Sight and Life and Program to Support Pro-poor Policy Development (PSPPD), a partnership between the Presidency of South Africa and the European Union. The funders only supported the study financially and had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: C.M.S., M.F., A.K. and M.v.d.H. designed the research; M.v.d.H., J.O. and C.M.S. conducted the research; M.v.d.H. analysed the data; M.v.d.H., M.F. and C.M.S. wrote the first draft of the manuscript and all authors had access to data and were involved in the interpretation of results. M.v.d.H. had the primary responsibility for final content. All authors read and approved the final manuscript. Ethics of human subject participation: The study was conducted according to the guidelines laid down by the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committee of the North-West University (NWU00033-09-A1). Permission was also granted by the Department of Education of the North West Province (Dr Kenneth Kaunda district) and the governing bodies of the two schools. Parents/guardians gave written consent for their children to participate in the study; only children who obtained parental consent and gave verbal assent for the study were included.










