Increasing attention has been given to the potential metabolic implications of inadequate sleep. Short sleep and, to a lesser extent, long sleep duration may increase the risk of developing obesity(Reference Gangwisch, Malaspina and Boden-Albala1–Reference Courno, Ruidavets and Marquie4) and metabolic diseases(Reference Ayas, White and Al-Delaimy5, Reference Knutson, Ryden and Mander6), possibly via alterations in metabolic regulation and appetite(Reference Tasali, Leproult and Ehrmann7, Reference Patel and Hu8).
Inadequate sleep duration may also modify eating patterns, thereby mediating or contributing to the observed relationship between sleep duration and obesity. Sleep duration in theory influences the time available for eating and the time of the day that eating occurs. Like most physiological actions and behaviours, eating practices are regulated in response to the sleep–wake cycle, which is the master output rhythm of the circadian clock(Reference Laposky, Bass and Kohsaka9). Moreover, several characteristics of eating practices such as skipping breakfast, late eating time and low meal frequency have been related to altered metabolic response, poor nutritional quality and obesity(Reference Bellisle, McDevitt and Prentice10–Reference Turek, Joshu and Kohsaka15). In a previous study, a higher prevalence of skipping breakfast was observed in persons with short sleep than in persons with normative sleep hours(Reference Qin, Li and Wang16). However, we are unaware of studies characterizing eating patterns in relation to sleep duration.
A major objective of the present study was to examine the relationship between sleep duration and eating patterns. It has been well documented that individual eating episodes are highly interrelated; i.e. the timing and satiety of the previous eating episode largely determine the time and size of the following eating episode(Reference Ma, Bertone and Stanek12). Therefore, we first used principal component analysis to identify major eating patterns among 27 983 women in the Sister Study and then evaluated their relationships with sleep duration. We also chose obesity and older age (age ≥55 years) a priori as potential effect modifiers based on previous literature suggesting altered meal and snack eating patterns in obese(Reference Bellisle, McDevitt and Prentice10, Reference Berteus, Lindroos and Sjostrom13) and older individuals(Reference Quandt, Vitolins and DeWalt17, Reference Slesinger, McDivitt and O’Donnell18).
Materials and methods
Sister Study
The National Institute of Environmental Health Sciences (NIEHS) Sister Study (www.SisterStudy.org) is a prospective cohort study of environmental and genetic risk factors for breast cancer and other endpoints in approximately 50 000 women aged 35–74 years who do not have breast cancer themselves but have a sister who was diagnosed with the disease. Participants are volunteers recruited through professional and volunteer organizations, breast cancer advocacy groups, health professionals, the media, outreach to specific populations, the Internet and word of mouth. Eligibility criteria include residence in the USA or Puerto Rico, age 35 to 74 years, ability to complete the study in English or Spanish, and having a full or half-sister who has had breast cancer. Recruitment began in four US cities in August 2003, opened nationally in October 2004 and ended in April 2009. Women who joined the study and provided written informed consent completed self-administered questionnaires (diet, family history, use of personal care products) and a two-part computer-assisted telephone interview to collect information on known and suspected breast cancer risk factors. Participating women also had a home visit for blood collection during which questionnaires and other biological and environmental samples were retrieved and height, weight, hip circumference, waist circumference and blood pressure were measured. The study was approved by the Institutional Review Boards of NIEHS, the National Institutes of Health (NIH) and the Copernicus Group.
Out of 29 316 women who completed the interview by June 2007, 28 735 provided information on both daily sleep duration (no. missing = 29) and eight meal and snack frequency questions (no. missing = 552). We further excluded thirty-two women who reported implausible values on sleep duration (predefined as <3 or >12 h of sleep daily), 471 women with implausible daily energy intake (defined as <2092 kJ/d (<500 kcal/d) or >14 644 kJ/d (>3500 kcal/d) derived from an FFQ) and 249 women with poor self-reported health status who might have had unusual sleep and eating behaviours, leaving 27 983 in the final analysis.
Main study variables
Sleep duration
Women were asked about average daily sleep duration using the following question: ‘About how many hours and/or minutes of sleep per (night/day) do you get on average?’ Study subjects were categorized into seven groups based on average daily sleep duration (3–4·9, 5–5·9, 6–6·9, 7–7·9, 8–8·9, 9–9·9 or 10–12 h).
Eating pattern
Habitual meal frequencies were asked using the following questions: ‘During the past year, on average, how many days per week did you eat … (breakfast, lunch or dinner/supper)?’ Snacking frequency was assessed using the following questions: ‘During the past year, on average, how many days per week did you have a snack … (before breakfast, between breakfast and lunch, between lunch and dinner/supper, between supper and bedtime, or after bedtime)?’ Snack was defined to exclude foods eaten at meals but to include all beverages except coffee, tea, diet drinks and water. Response categories were <1 time/week, 1–2 times/week, 3–4 times/week, 5–6 times/week or 1 time/d for all of the questions; in the analyses they were converted to 0·58, 1·5, 3·5, 5·5 and 7 times/week, respectively.
Dietary data
The information on dietary intake was obtained from a modified Block FFQ (Block Dietary Data Systems, Berkeley, CA, USA)(Reference Block, Hartman and Dresser19). The questionnaire was structured to collect average food intake during the previous year and a standard portion size was given for each food item. The Healthy Eating Index (HEI) was calculated as the sum of ten dietary components including the degree of conformity to the recommended number of servings for the five major food groups of the Food Guide Pyramid (meat, dairy, fruits, vegetables and grains), proportion of total energy from fat and saturated fat, total cholesterol and sodium intake, and diet variety(Reference Kennedy, Ohls and Carlson20). The glycaemic load was derived from the amount of carbohydrate intake and glycaemic index based on the white bread scale(Reference Wolever, Nuttall and Lee21).
Statistical analysis
Characteristics of the study subjects by daily sleep duration are described using the mean and standard deviation for continuous variables and the number and proportion for categorical variables. Principal component analysis was used to identify major eating patterns from weekly frequencies of three meals and five snacks at different times across the day. An individual’s score on each of the identified components was computed by summing across the product of the individual’s response to each eating frequency item and the corresponding weight (component loading). Nutritional characteristics were compared among four groups categorized on the basis of quartiles of the component scores using ANOVA.
Generalized linear regression models were used to estimate mean component scores for each of the two identified eating patterns by daily sleep duration category. The multivariable models included covariates that are related to either sleep duration or dietary practice as follows: age at enrolment (35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74 years); race (non-Hispanic white, black, Hispanic, others); annual household income (<20 000, 20 000–49 999, 50 000–99 999, 100 000–199 999, ≥200 000 US dollars); level of education (high school or less, some college or associate/technical degree, college graduate or higher); current employment status (currently working for ≥20 h/week or not); marital status (married/living as married, widowed/divorced/separated, single); having a child aged 18 years or younger; measured BMI (categorized into three groups using the cut-off points recommended by WHO(22), where BMI < 25 kg/m2 is defined as normal, BMI > 25 to <30 kg/m2 is defined as grade 1 overweight and BMI ≥ 30 kg/m2 is defined as grade 2 overweight or obese); menopause status; smoking status (never, past, current); use of alcohol in the past 6 months; physical activity in the past 12 months (grouped into quintiles based on the distribution of the sample in MET × h/week, where MET is metabolic equivalent)(Reference Ainsworth, Haskell and Leon23); self-rated health status (excellent, very good, good, fair); and perceived level of stress during the past 30 d, measured on the basis of the four-item version of the perceived stress scale developed by Cohen et al.(Reference Cohen, Kamarck and Mermelstein24) and categorized into three groups (low, 0–2; medium, 3–7; high, 8–16), based on total score from the four items.
Current obesity (BMI < 30 kg/m2v. BMI ≥ 30 kg/m2) and age group (<55 years v. ≥55 years) were evaluated as potential effect modifiers of the relationship between daily sleep duration and eating patterns using likelihood ratio tests, comparing models with and without multiplicative interaction terms with sleep duration. Analyses were performed using the STATA statistical software package version 10·0 (Stata Corporation, College Station, TX, USA) and all statistical tests were two-sided, with α level of 0·05.
Results
Characteristics of study subjects by sleep duration
Approximately 40 % of the women reported sleeping an average of 7–7·9 h daily, while those with 5–5·9 h or <5 h of sleep daily comprised 5·3 % (n 1485) and 1·3 % (n 376), respectively (Table 1). Similarly, very few women reported sleeping for 9–9·9 h daily (3·8 %) or ≥10 h daily (0·7 %). Compared with women sleeping 7–7·9 h daily, those with short sleep were more often non-white, current smokers and had a higher BMI. Women who reported sleeping for ≥10 h daily had similar characteristics to those with short sleep, and they also tended to be physically inactive. Total energy intake and macronutrient composition did not substantially vary by daily sleep duration.
Table 1 Characteristics of the study subjects according to daily sleep duration, Sister Study, 2003–2007 (N 27 983)
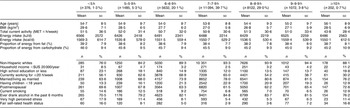
MET, metabolic equivalent.
Identification and characterization of eating patterns
Using a principal component analysis, we identified two major components characterizing the overall eating patterns in our study population (Table 2). The two components accounted for 44 % of total variance, had eigenvalues greater than 1 (1·9 and 1·6, respectively), and there was a long break between components 2 and 3 on the scree plot (figure not shown), supporting retention of just two components. The two components were also highly interpretable. For the first component, the loading was positively correlated with all three meal frequency items (breakfast, lunch and dinner) and with snacks only between breakfast and dinner times (snack between breakfast and lunch and snack between lunch and dinner); therefore, the first component might be interpreted as tendency for eating during conventional eating hours (i.e. from breakfast to dinner). The second component was positively correlated with all five snack frequency items, but inversely correlated with all three meal frequency items. Therefore, the second component may be interpreted as a degree of snack dominance over meals.
Table 2 Component loading for eating patterns from principal component analysis, Sister Study, 2003–2007 (N 27 983)

Women with increased tendency for eating during conventional eating hours had higher total energy intake and glycaemic load, but they consumed proportionally less fat and sweets and had higher HEI scores (Table 3). On the other hand, increasing snack dominance over meals was related to lower HEI scores and greater intakes of sweets and fat for energy (Table 4).
Table 3 Nutritional characteristics according to quartile of conventional eating scoreFootnote *, Sister Study, 2003–2007 (N 27 983)

* All nutritional characteristics compared were significantly different among the four groups (P < 0·01).
Table 4 Nutritional characteristics according to quartile of snack dominance scoreFootnote *, Sister Study, 2003–2007 (N 27 983)
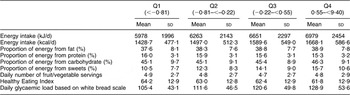
* All nutritional characteristics compared were significantly different among the four groups (P < 0·01) except for daily number of fruit/vegetable servings (P = 0·06).
Association between sleep duration and eating patterns
The tendency for eating during conventional hours (from breakfast to dinner) increased with increasing sleep duration up to 7–7·9 h: adjusted mean score of −0·54 (95 % CI −0·68, −0·41) in women sleeping <5 h daily v. 0·08 (95 % CI 0·06, 0·11) among those with 7–7·9 h of sleep daily (Fig. 1). The extent of snack dominance over meals decreased with increasing sleep duration, with mean score of 0·24 (95 % CI 0·11, 0·38) in those with <5 h of sleep daily v. −0·07 (95 % CI −0·10, −0·04) in women sleeping for 8–8·9 h daily. Women with long daily sleep duration (≥10 h) had scores for both eating patterns similar to those with less than normal sleep duration (<6 h).
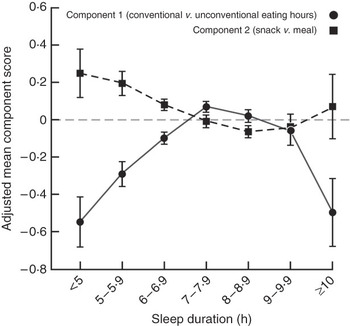
Fig. 1 Adjusted mean component scores for eating patterns by sleep duration among 27 983 women aged 35–74 years in the Sister Study, 2003–2007. Each circle represents adjusted mean score for eating during conventional eating hours and each square represents adjusted mean score for snacking by daily sleep duration category, estimated from multivariable models including age, race, household income, education, marital status, having children <18 years old, smoking status, current alcohol use, menopausal status, self-rated health, perceived level of stress, physical activity and measured BMI. 95 % confidence intervals represented by vertical bars
The relationship between daily sleep duration and eating patterns did not vary by obesity. Instead, we observed that obesity was associated with a lower tendency for eating during conventional eating hours and a greater degree of snack dominance over meals regardless of sleep duration (Fig. 2a). We also found a significant interaction between sleep duration and age for the degree of snack dominance over meals (likelihood ratio test P = 0·02; Fig. 2b).

Fig. 2 Adjusted mean component scores for eating patterns by sleep duration, according to obesity (○, □, BMI < 30 kg/m2; ●, ▪, BMI ≥ 30 kg/m2) and age (![]() ,
, ![]() , <55 years;
, <55 years; ![]() ,
, ![]() , ≥55 years) among 27 983 women aged 35–74 years in the Sister Study, 2003–2007. Each circle represents adjusted mean score for eating during conventional eating hours and each square represents adjusted mean score for snacking by daily sleep duration category, estimated from multivariable generalized linear models including age, race, household income, education, marital status, having children <18 years old, smoking status, current alcohol use, menopausal status, self-rated health, perceived level of stress, physical activity and measured BMI. 95 % confidence intervals represented by vertical bars. (a) The relationship between sleep duration and eating patterns did not vary by obesity (P = 0·13 for component 1 and P = 0·65 for component 2, likelihood ratio test); (b) the association between sleep duration and eating during conventional hours did not vary by age (P = 0·53, likelihood ratio test), but the association between sleep duration and snack dominance eating pattern was stronger in women aged <55 years than among women aged ≥55 years (P = 0·02, likelihood ratio test)
, ≥55 years) among 27 983 women aged 35–74 years in the Sister Study, 2003–2007. Each circle represents adjusted mean score for eating during conventional eating hours and each square represents adjusted mean score for snacking by daily sleep duration category, estimated from multivariable generalized linear models including age, race, household income, education, marital status, having children <18 years old, smoking status, current alcohol use, menopausal status, self-rated health, perceived level of stress, physical activity and measured BMI. 95 % confidence intervals represented by vertical bars. (a) The relationship between sleep duration and eating patterns did not vary by obesity (P = 0·13 for component 1 and P = 0·65 for component 2, likelihood ratio test); (b) the association between sleep duration and eating during conventional hours did not vary by age (P = 0·53, likelihood ratio test), but the association between sleep duration and snack dominance eating pattern was stronger in women aged <55 years than among women aged ≥55 years (P = 0·02, likelihood ratio test)
Discussion
In this analysis of 27 983 women, we identified distinctive eating patterns that were associated with sleep duration. Compared with women with normative sleep duration (7–7·9 h daily), short sleep duration and, to a lesser extent, long sleep duration were both associated with reduced tendency for eating during conventional eating hours and greater degree of snack dominance over meals.
Eating during unconventional hours (i.e. before breakfast and after bedtime) may reflect accommodations made to adjust to short sleep duration. In our exploratory analysis, short sleepers tended to go to bed later than those with normative sleep duration, thereby having more opportunities for eating at later hours. Furthermore, staying awake at night alone may also lead to physiological changes that promote hunger. In an experimentally controlled study, those who stayed awake later at night had lower concentrations of the anorexigenic hormone leptin without a notable peak at night compared with subjects who were asleep by 22.30 hours(Reference Qin, Li and Wang16). Likewise, women with short sleep duration also tend to get up early and are more likely to replace breakfast with an early morning snack, supported by the previous findings of a higher prevalence of skipping breakfast in persons with short sleep duration(Reference Qin, Li and Wang16). Given that the preceding meal largely determines the size and the time of the following meal(Reference Bernstein, Zimmerman and Czeisler25), substitution of breakfast with an early morning snack could subsequently affect eating patterns throughout the day. Thus, greater snack dominance over meals observed in habitual short sleepers may represent deviations in eating patterns arising from early rising or late bedtime.
The present study also showed that the eating patterns prevalent among short and long sleepers were reflective of a nutritionally poor diet; both eating during unconventional eating hours and preponderance of snacks over meals were related to lower intakes of fruits and vegetables and higher intakes of sweets and fat as a percentage of energy.
Snacks are generally composed of foods with higher carbohydrate and lower protein, and frequent snacking was found to be a good predictor of nutritionally empty and energy-laden diet in a previous study(Reference Berteus, Lindroos and Sjostrom13). On the other hand, breakfast often consists of foods with low fat and high carbohydrate and fibre contents(Reference Morgan, Zabik and Stampley26, Reference Ruxton and Kirk27), and skipping breakfast is considered to lead to intake of compensatory snacks with relatively poor nutritional quality or overeating in later meals(Reference Ruxton and Kirk27). Compared with individuals who regularly eat breakfast, those who skip breakfast had a lower micronutrient intake, a higher energy intake from fat and a higher level of LDL cholesterol(Reference Ruxton and Kirk27, Reference Wyatt, Grunwald and Mosca28).
Night-time eating also has been positively correlated with fat, carbohydrate and total energy intakes(Reference de Castro29), and has been more commonly reported in obese women than among non-obese women(Reference O’Reardon, Ringel and Dinges30). Moreover, food intake at a later time of day may induce different physiological responses to food: it has been suggested that food intake in the evening or night is more likely than food consumed at other times of the day to preserve glycogen levels in muscles, ultimately leading to higher adiposity(Reference Keim, Van Loan and Horn31).
Some limitations of the present study should be acknowledged. First, our participants were volunteers. As in other volunteer populations, compared with the general US population, they were more educated and had higher incomes on average, which might limit the external generalizability of our results. However, the associations between sociodemographic factors and sleep duration observed in our study were similar to those from the National Health Interview Survey(Reference Adams and Schoenborn32), suggesting that, at least in terms of sleep duration, our participants are comparable to a nationally representative US sample.
Second, there have been no established approaches to define what constitutes a ‘meal’ v. a ‘snack’(Reference Gatenby33). In the present study, ‘snack’ was loosely defined as an eating episode between meals except for non-caloric drinks and no clear definition of ‘meal’ was provided; thus, number, size and timing of ‘meals’ may be subject to individual variations in sociocultural norms and chronological trends(Reference Gatenby33). However, we were unable to evaluate the impact of such misclassification on the present findings.
Finally, although it was assumed that sleep duration was a prior event influencing eating patterns, we recognize that the relationship between sleep duration and eating patterns is likely to be bidirectional. Data suggest that sleep loss alters physiological regulation of metabolic hormones, which not only influences diet and eating patterns but also feeds back to affect the sleep–wake regulatory process itself(Reference Laposky, Bass and Kohsaka9). Conversely, it is also possible that short sleep duration and the associated eating patterns may be a manifestation of either genetically or non-genetically altered circadian rhythms(Reference Laposky, Bass and Kohsaka9). Mice with mutations in the clock gene that disrupt cellular rhythmicity showed a significant increase in energy intake and body weight, and higher levels of leptin and glucose, compared with wild-type mice(Reference Turek, Joshu and Kohsaka15). In man, polymorphisms in the clock gene have been associated with sleep deregulation(Reference Prasai, George and Scott34).
In conclusion, our data show that short and very long sleep duration are associated with eating during unconventional eating hours and snacking. Combined with growing evidence indicating circadian variation in physiological responses to foods(Reference Keim, Van Loan and Horn31), these results suggest that disrupted eating patterns may exacerbate the development of obesity and metabolic diseases in habitual short and very long sleepers via poor nutritional composition and altered physiological responses to nutrients.
Acknowledgements
Sources of funding: This research was funded by the Intramural Research Program of the NIH, NIEHS (Z01 ES04400509) with additional support from the National Center on Minority Health and Health Disparities. Conflict of interest: No actual or potential conflict of interest is declared. Authors’ contributions: S.K. conceptualized the study and analysed the data. S.K. drafted the manuscript; S.K., L.A.D. and D.P.S. contributed to the interpretation of the data, discussion of the findings and revision of the manuscript. D.P.S. obtained the funding. Acknowledgements: The authors thank Drs Honglei Chen and Matthew Longnecker for helpful comments on this manuscript.








