Colorectal cancer (CRC) is one of the most commonly diagnosed cancers worldwide( Reference Jemal, Bray and Center 1 ). The development of CRC in the normal colorectal epithelium results from genetic alterations, epigenetic modifications and environmental factors( Reference Potter 2 – Reference Hill 4 ). Diet may be an important aetiological factor in CRC( Reference Steinmetz and Potter 5 ) and several biological mechanisms may explain the relationship between diet and CRC risk( Reference Steinmetz and Potter 5 ).
Vitamin B12 is an essential coenzyme for methionine synthase, which maintains adequate intracellular methionine levels in one-carbon metabolism( Reference Vidal, Grant and Williams 6 , Reference Lim, Schenk and Kelemen 7 ) for several intracellular biological processes, including methylation reactions, nucleotide biosynthesis and DNA repair( Reference Kim 8 ). Recent studies have shown that one-carbon metabolism may be related to colorectal carcinogenesis( Reference Williams 9 , Reference Strohle, Wolters and Hahn 10 ). Therefore, vitamin B12 deficiency may increase CRC risk( Reference Kune and Watson 11 , Reference Williams, Satia and Adair 12 ). However, several studies have reported that vitamin B12 is not associated with the risk of CRC( Reference Harnack, Jacobs and Nicodemus 13 , Reference Murtaugh, Curtin and Sweeney 14 ), or that folic acid plus vitamin B12 is associated with increased cancer risk( Reference Ebbing, Bonaa and Nygard 15 ).
The results on the association between vitamin B12 intake and CRC risk are still controversial and no relevant pooled analyses have been performed. Therefore the aim of our meta-analysis study was to quantitatively and comprehensively summarize whether vitamin B12 intake or blood vitamin B12 level is related to CRC risk.
Methods
Literature search
A literature search for relevant studies was performed using the PubMed and EMBASE databases up to April 2014. The search terms used were: (vitamin B12 OR cyanocobalamin OR cobalamins OR cobalamin OR hydroxocobalamin OR 5′-deoxyadenosyl cobalamin) AND (colorectal cancer OR colon cancer OR rectal cancer). Moreover, we manually screened the references of the relevant studies and reviews to check for other potentially relevant studies.
Eligibility criteria
The studies which met the following eligible criteria were included: (i) cohort study or case–control study; (ii) the exposure of interest was vitamin B12 intake, or blood vitamin B12 level, for three or more quantitative categorized levels; (iii) the outcome of interest was related to CRC; and (iv) the risk estimates (OR, risk ratio (RR) or hazard ratio (HR)) and corresponding 95 % CI were reported or calculated from published data with a category-specific number of cases and a category-specific number of either person-years or non-cases. When several studies were based on the same population, only the most informative study was included.
Data extraction and quality assessment
Data were independently extracted by two reviewers. For each study, the following data were extracted: first author, publication year, publication country, study design, study name, population characteristics (sex and age), follow-up period, sample size, type of vitamin B12 intake (total intake=dietary intake plus dietary supplements; dietary intake; supplemental intake), measures and ranges of exposure, adjusted variables, and risk estimates with corresponding 95 % CI for each category. Risk estimates that reflected the greatest degree of adjustment for potential confounders were extracted.
The quality of the included studies was assessed according to the Newcastle–Ottawa Scale criteria( Reference Stang 16 ). A funnel plot was used to assess publication bias. Any disagreement on the data extraction and quality assessment of the studies was resolved through comprehensive discussion.
Statistical analysis
A dose–response analysis was first utilized to assess the relationship between vitamin B12 intake and blood vitamin B12 level and CRC risk using the generalized least squares method because this method can resolve the problem that the included studies used different FFQ( Reference Greenland and Longnecker 17 , Reference Orsini, Bellocco and Greenland 18 ). The analytical method required the distribution of case and person-years, median values of vitamin B12 intake or blood vitamin B12 levels, and corresponding risk estimates in each category for each study. If there were results on both dietary and total B12 intake in one study, we used the results on total vitamin B12 intake for the main analyses. For each study, the mean or median value of vitamin B12 intake and blood vitamin B12 level in each category was assigned to each corresponding risk estimate. The assigned value of the lowest category was designated as a reference level. If the study did not provide mean or median values of exposure, the midpoint of the upper and lower boundaries in each category was assigned the median value of exposure( Reference Berlin, Longnecker and Greenland 19 ). For the open-ended exposure categories, the length of the open-ended interval was assumed to be the same as that of the adjacent interval( Reference Keum, Aune and Greenwood 20 ).
We used random-effect restricted cubic splines with three knots at the 25 %, 50 % and 75 % percentiles of the distribution to examine a potential non-linear dose–response relationship between vitamin B12 intake and blood vitamin B12 level with CRC risk( Reference Orsini, Li and Wolk 21 , Reference Bagnardi, Zambon and Quatto 22 ). A P value for non-linearity was calculated by testing the null hypothesis that the regression coefficient of the second spline was equal to zero( Reference Orsini, Li and Wolk 21 ). To test and verify the non-linear model, a meta-analysis comparing the appropriate open categories (or highest category) of exposure with the lowest category was performed. The non-linear dose–response relationship was also verified by several representative point values and the risk estimates of a subgroup analysis based on the range of exposure.
RR was used as a measure of the association between exposure and CRC risk. The OR provided by a case–control study was used as an estimate of the RR because the incidence of CRC was sufficiently rare( Reference Greenland 23 ). When the category-specific risk estimates were reported separately for different polymorphisms in the 5,10-methylenetetrahydrofolate reductase gene, we combined the multiple risk estimates into a pooled estimate using a fixed-effects model for further meta-analysis( Reference Orsini 24 ). When the RR of different population databases or population sex were presented in one study, we considered each population database as one study.
The heterogeneity of the studies was evaluated using the Cochran Q test and the I 2 statistic( Reference Higgins, Thompson and Deeks 25 ). A P value <0·10 for the Q statistic and/or I 2>50 % were considered to indicate statistically significant heterogeneity. The random-effects model was then used if there was significant heterogeneity; otherwise, the fixed-effects model was used. A subgroup analysis and a Galbraith plot were used to explore the sources of heterogeneity. Publication bias was evaluated with Egger’s and Begg’s tests( Reference Egger, Davey Smith and Schneider 26 , Reference Begg and Mazumdar 27 ), and a trim-and-fill analysis was conducted if publication bias was detected( Reference Duval and Tweedie 28 ). To explore the association between sample size and the RR for CRC, we constructed a sampling-based scatter plot graphically summarizing the association by modelling sample size as a continuous variable. A subgroup analysis was performed based on sample size. We also conducted subgroup analyses stratified by geographic region, type of vitamin B12 taken, range of exposure and tumour site.
A two-sided P value <0·05 was considered statistical significance. All statistical analyses were conducted using the statistical software package Stata version 12·0.
Results
Selection of studies
Figure 1 shows a detailed flowchart of the literature search and the study inclusion procedure. A total of 1362 studies were initially identified with this literature search, but 1231 studies were excluded after screening the titles and abstracts. Then 114 studies were excluded after a full text review. Finally, fourteen studies of vitamin B12 intake and three studies of blood vitamin B12 level were identified as eligible for our meta-analysis.
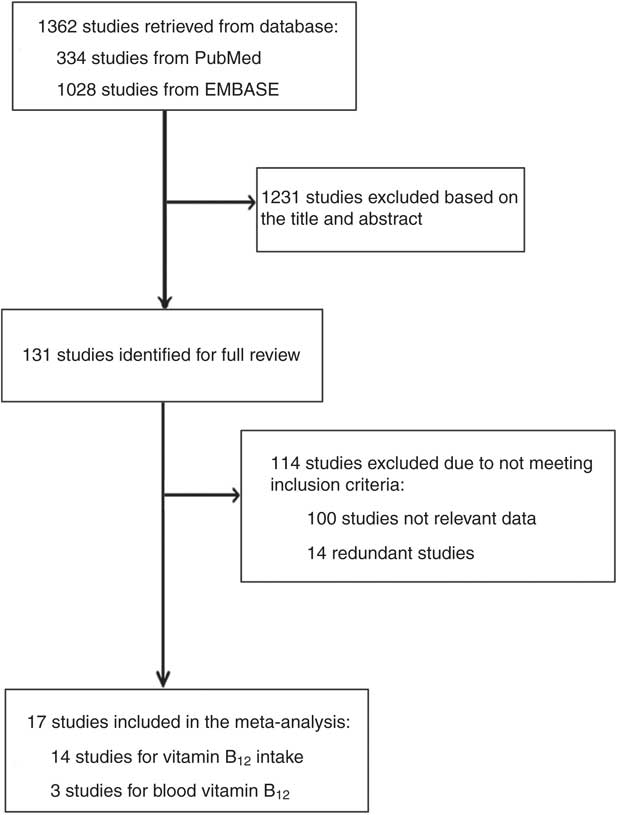
Fig. 1 Flowchart showing the literature search and study selection
Study characteristics
The fourteen studies of vitamin B12 intake included a total of 9693 cases (Table 1)( Reference Kune and Watson 11 – Reference Murtaugh, Curtin and Sweeney 14 , Reference Zschabitz, Cheng and Neuhouser 29 – Reference Otani, Iwasaki and Hanaoka 38 ). The studies were conducted in Asia, Europe and North America, and were published between 2008 and 2013. In terms of the study design, five studies were cohort studies( Reference Harnack, Jacobs and Nicodemus 13 , Reference Zschabitz, Cheng and Neuhouser 29 , Reference Bassett, Severi and Hodge 31 , Reference Shrubsole, Yang and Gao 34 , Reference Schernhammer, Giovannuccci and Fuchs 36 ) and nine studies were case–control studies. Of these fourteen studies, eight studies provided results only for dietary vitamin B12 intake from food( Reference Kune and Watson 11 , Reference Morita, Yin and Yoshimitsu 30 – Reference Shrubsole, Yang and Gao 34 , Reference Le Marchand, Wilkens and Kolonel 37 , Reference Otani, Iwasaki and Hanaoka 38 ), three studies provided results only for total vitamin B12 ( Reference Harnack, Jacobs and Nicodemus 13 , Reference Sharp, Little and Brockton 35 , Reference Schernhammer, Giovannuccci and Fuchs 36 ), and three studies provided both total and dietary vitamin B12 intakes( Reference Williams, Satia and Adair 12 , Reference Murtaugh, Curtin and Sweeney 14 , Reference Zschabitz, Cheng and Neuhouser 29 ). Besides the main end point of CRC, six studies provided sub-cohort results for colon cancer and/or rectal cancer separately( Reference Kune and Watson 11 , Reference Harnack, Jacobs and Nicodemus 13 , Reference Murtaugh, Curtin and Sweeney 14 , Reference Bassett, Severi and Hodge 31 , Reference Liu, Scherer and Poole 32 , Reference Schernhammer, Giovannuccci and Fuchs 36 ). Three case–control studies on blood vitamin B12 levels were conducted in the USA and Sweden, and were published between 2008 and 2010, comprising a total of 908 cases and 2387 controls (Table 2)( Reference Van Guelpen, Dahlin and Hultdin 39 – Reference Weinstein, Albanes and Selhub 41 ).
Table 1 The baseline characteristics of included studies on vitamin B12 intake and colorectal cancer
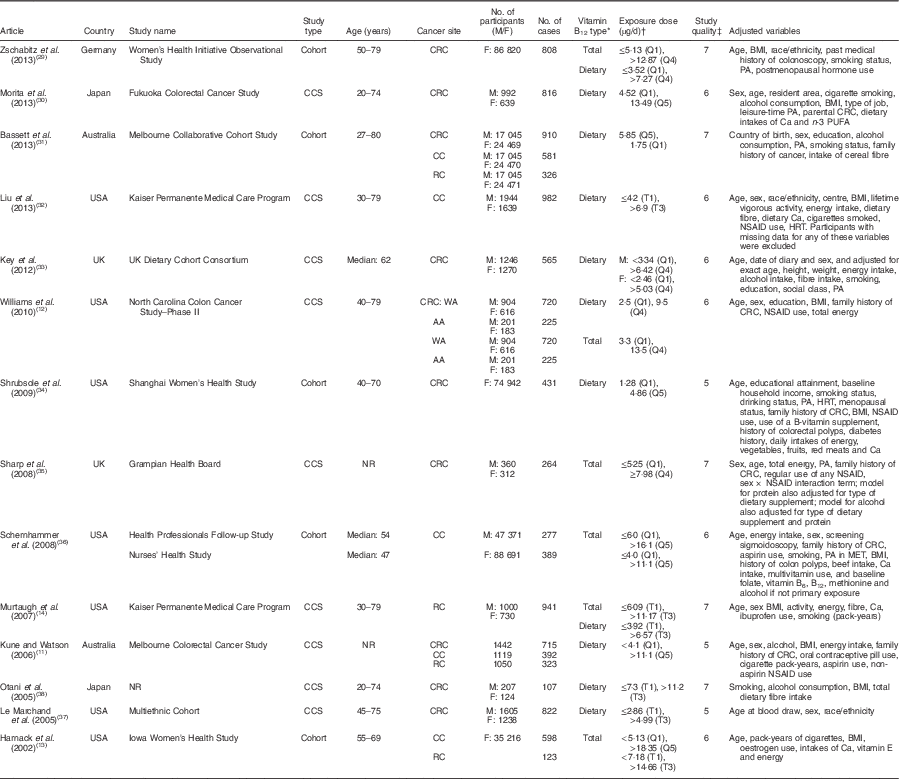
M, males; F, females; CCS, case–control study; NR, not reported; CRC, colorectal cancer; CC, colon cancer; RC, rectal cancer; WA, white Americans; AA, African Americans; Q, quartile/quintile; T, tertile; PA, physical activity; NSAID, non-steroidal anti-inflammatory drug; HRT, hormone replacement therapy; MET, metabolic equivalent of task.
* Dietary vitamin B12 intake included vitamin B12 intake from foods only, and total vitamin B12 intake included vitamin B12 intake from foods and supplements.
† Exposure dose means the cut-off points or distribution for the highest and lowest categories of vitamin B12 intake.
‡ The quality of the included studies was assessed with the nine-star Newcastle–Ottawa Scale criteria.
Table 2 The baseline characteristics of included studies on blood vitamin B12 level and colorectal cancer
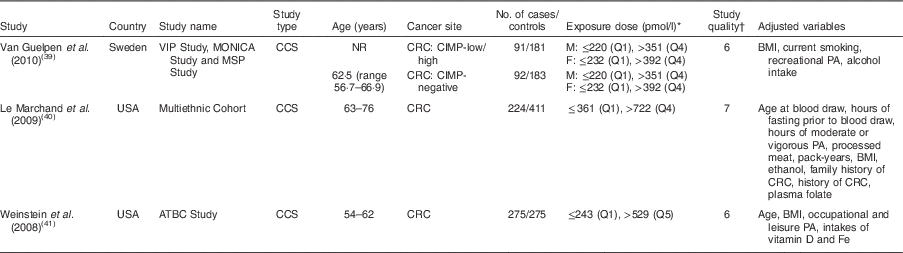
VIP, Västerbotten Intervention Programme; MONICA, MONitoring of trends and determinants in CArdiovascular disease; MSP, Mammography Screening Project; ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention; CCS, case–control study; NR, not reported; CRC, colorectal cancer; CIMP, CpG island methylator phenotype; M, males; F, females; Q, quartile/quintile; PA, physical activity.
* Exposure dose means the cut-off points or distribution for the highest and lowest categories of blood vitamin B12 level.
† The quality of the included studies was assessed with the nine-star Newcastle–Ottawa Scale criteria.
Dose–response association between vitamin B12 intake and colorectal cancer risk
We first evaluated the non-linear dose–response relationship between vitamin B12 intake and CRC risk. Heterogeneity existed (P heterogeneity=0·017) in the overall analysis of vitamin B12 intake, with a significant non-linear dose–response relationship (P non-linearity=0·026; Fig. 2). In the non-linear dose–response relationship, there was a slight trend towards a reduction in CRC risk when vitamin B12 intake was less than 12·85 μg/d, although the reduction was not statistically significant, and a significantly reduced risk was observed when vitamin B12 intake was more than 12·85 μg/d. A slight reduction in CRC risk was also observed with every 4·5 μg/d increment in vitamin B12 intake (RR=0·961; 95 % CI 0·930, 0·994) when we evaluated the relationship in a separate analysis with a linear model, suggesting that similar trends were observed with linear and non-linear models.
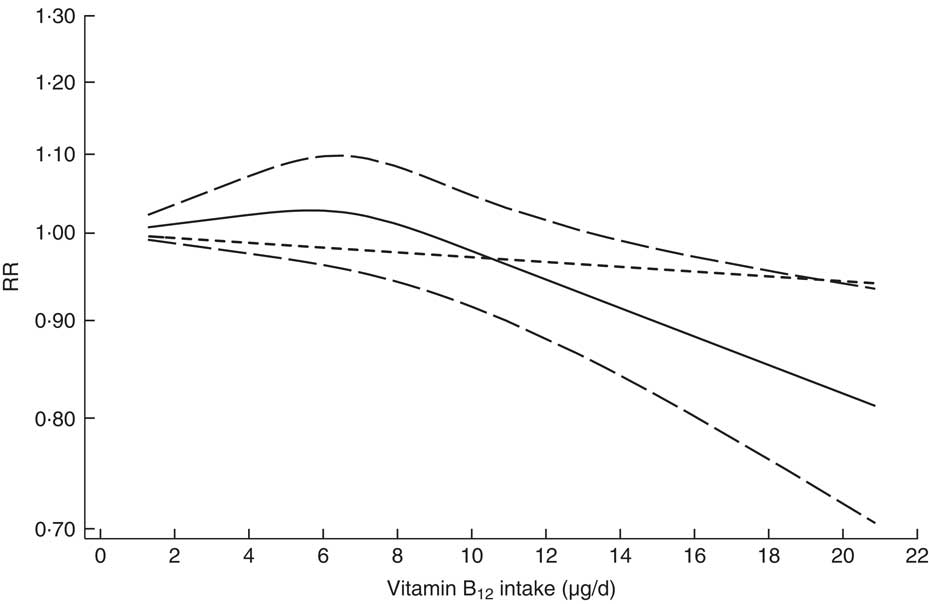
Fig. 2 Dose–response relationship between vitamin B12 intake and risk of colorectal cancer. Relative risks (RR; ———) and the corresponding 95 % CI (— — —) were summarized for the dose–response relationship between vitamin B12 intake (μg/d) and risk of colorectal cancer. Data were modelled with random-effects restricted cubic spline models, where ---- represents the linear trend
In addition, dose–response analyses for the association between vitamin B12 intake and CRC risk were stratified based on the type of vitamin B12 intake (total intake and dietary intake). There was no evidence of a non-linear association between total vitamin B12 intake and CRC risk (P non-linearity=0·690), and every 4·5 μg/d increment in total vitamin B12 intake was inversely associated with CRC risk (RR=0·963; 95 % CI 0·928, 0·999) without significant heterogeneity (P heterogeneity=0·138). For dietary vitamin B12 intake there was significant evidence of non-linearity, and every 4·5 μg/d increment in dietary vitamin B12 intake was inversely associated with CRC risk (RR=0·914; 95 % CI 0·856, 0·977) under the linear model.
The non-linear dose–response relationship (Fig. 2) graphically showed that vitamin B12 intake above a certain threshold (high dose, i.e. >12 μg/d) was inversely associated with CRC risk. We used several representative point values to test and verify the non-linear dose–response relationship by pooling the CRC risks for appropriate open categories of vitamin B12 intake when the lowest category in each study was regarded as a reference level. Our results indicated a significantly decreased risk of CRC when the vitamin B12 intake was above a certain threshold (8·5 μg/d: RR=0·898; 95 % CI 0·824, 0·979; 11 μg/d: RR=0·843; 95 % CI 0·731, 0·971; or 13 μg/d: RR=0·881; 95 % CI 0·802, 0·968), thereby enhancing the non-linear dose–response relationship.
Dose–response association between blood vitamin B12 level and colorectal cancer risk
There was no heterogeneity (P heterogeneity=0·327) in the overall analysis of blood vitamin B12 levels, with an insignificant non-linear dose–response relationship between blood vitamin B12 level and CRC risk (P non-linearity=0·219). The results of the linear model indicated that every 150 pmol/l increment in blood vitamin B12 level was not associated with CRC risk (RR=1·023; 95 % CI 0·881, 1·187) without significant heterogeneity (P heterogeneity=0·303).
High v. low vitamin B12 intake or blood vitamin B12 level
In the crude analyses, the highest vitamin B12 intake or blood vitamin B12 level v. the lowest intake or blood level was insignificantly associated with CRC risk (vitamin B12 intake: RR=0·942; 95 % CI 0·829, 1·070; blood vitamin B12 level: RR=0·927; 95 % CI 0·560, 1·534). We also performed in-depth subgroup analysis of the vitamin B12 intake. The results indicated that the association between vitamin B12 intake and CRC risk was stronger in studies with a wider range of vitamin B12 intake (>8 μg/d difference in assigned value compared with the reference level: RR=0·831; 95 % CI 0·711, 0·971; Fig. 3) compared with studies with a narrower range of vitamin B12 intake (≤8 μg/d: RR=1·016; 95 % CI 0·878, 1·175), thereby enhancing the non-linear dose–response relationship. The highest v. lowest total vitamin B12 intake was significantly associated with CRC risk (RR=0·870; 95 % CI 0·782, 0·967).
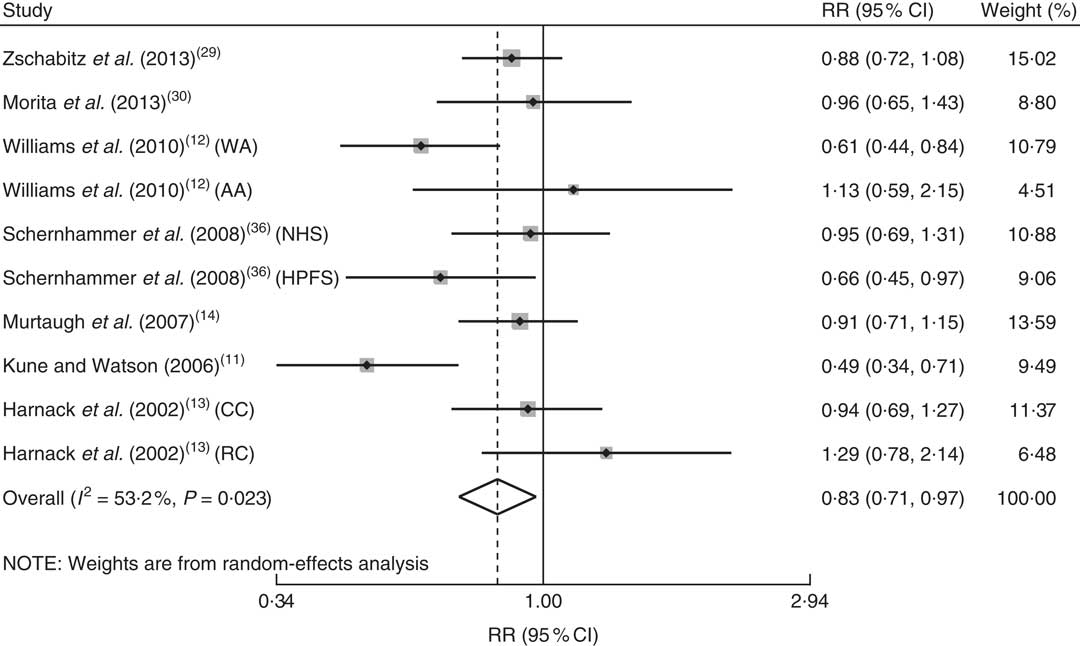
Fig. 3 Adjusted relative risk (RR) of colorectal cancer for a wider range of vitamin B12 intake (range >8 μg/d); the adjusted RR was summarized for the association between a wider range of vitamin B12 intake and risk of colorectal cancer. The study-specific RR and 95 % CI are represented by the black dot and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond presents the pooled RR risk and its width represents the pooled 95 % CI (NHS, Nurses’ Health Study; HPFS, Health Professionals Follow-up Study; CC, colon cancer; RC, rectal cancer)
The association between sample size and the RR of CRC is summarized in Fig. 4 with a sampling-based scatter plot. The subgroup analysis based on sample size showed that the sample sizes of the studies did not obviously influence our results. Detailed subgroup analyses based on CRC sites, sex of participants, regions of participants and the adjusted covariates were also conducted, and the results are summarized in Table 3.
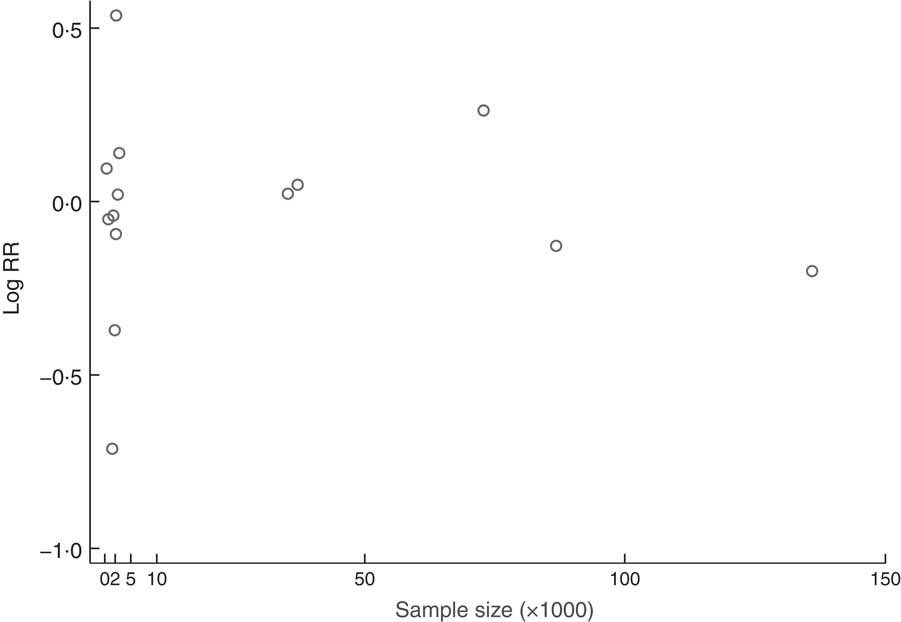
Fig. 4 The association between sample size and the relative risk (RR) of colorectal cancer; a sampling-based scatter plot summarized the association between sample size and the RR of colorectal cancer
Table 3 The results of subgroup analyses for the relationship between vitamin B12 intake and colorectal cancer risk
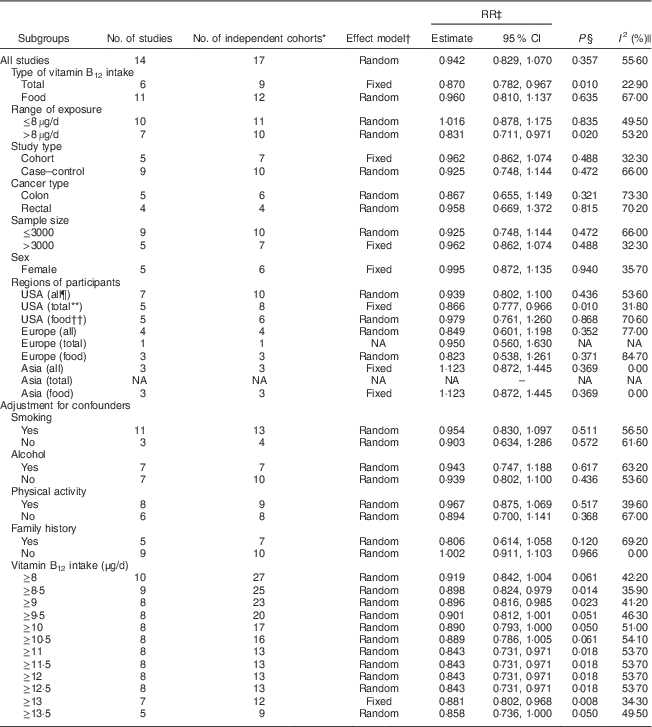
RR, relative risk; NA, not applicable.
* The actual number of ‘independent study cohorts’ that can be included in the corresponding analysis because we considered each population database as one study cohort for statistical analyses if the RR of different populations or population sex were available (one study); thus the number of independent study cohorts can be larger than the number of studies.
† Random-effects model was used if there was significant heterogeneity; otherwise, fixed-effects model was used.
‡ RR for colorectal cancer risk of the highest v. lowest categories of vitamin B12 intake.
§ P for the RR.
|| I 2 shows the degree of heterogeneity among studies.
¶ ‘All’ means that the analysis included either total vitamin B12 intake or dietary vitamin B12 intake.
** ‘Total’ means that the analysis included only total vitamin B12 intake.
†† ‘Food’ means that the analysis included only dietary vitamin B12 intake.
Publication bias
Begg’s test and Egger’s test showed no evidence of publication bias for the overall analysis of vitamin B12 intake (P Begg=0·192, P Egger=0·266) and blood vitamin B12 level (P Begg=1·000, P Egger=0·447), and the subgroup analysis of total vitamin B12 intake (P Begg=0·348, P Egger=0·616).
Discussion
The one-carbon metabolism pathway requires adequate vitamin B12 and this raises the possibility that vitamin B12 may have an important role in CRC risk. However, the association between vitamin B12 and CRC risk is still under debate due to a lack of sufficient evidence. To the best of our knowledge, our present dose–response meta-analysis is the first study to systematically evaluate the association between vitamin B12 and CRC risk.
In crude overall analyses, vitamin B12 intake or blood vitamin B12 level was insignificantly associated with CRC risk. Interestingly, current results found a non-linear dose–response relationship between vitamin B12 intake and CRC risk. The association was insignificant if vitamin B12 intake was under a certain threshold (low dosage, i.e. <7 μg/d), whereas vitamin B12 intake above a certain threshold (high dosage, i.e. >12 μg/d) was inversely associated with CRC risk. Moreover, this non-linear dose–response relationship was enhanced by several representative point values and the risk estimates of subgroup analysis based on range of exposure (Fig. 3).
The biological mechanisms responsible for the protective effect of high-dosage vitamin B12 are unclear. One possible explanation is that vitamin B12 is an essential coenzyme in one-carbon metabolism for methylation reactions, nucleotide biosynthesis and DNA repair through transforming homocysteine into methionine. Indeed, Choi et al. reported that the colonic DNA of vitamin B12-deficient rats has a 35 % decrease in genomic methylation and a 105 % increase in uracil incorporation, which might increase susceptibility to carcinogenesis( Reference Choi, Friso and Ghandour 42 ). Furthermore, B-group vitamin supplementation, including vitamin B12, may have antioxidant and anti-inflammatory effects( Reference Ullegaddi, Powers and Gariballa 43 , Reference Ullegaddi, Powers and Gariballa 44 ). In addition, animal experiments have shown that vitamin B12 can inhibit the proliferation of cancer cells( Reference Nishizawa, Yamamoto and Terada 45 ).
We did not find statistically significant evidence for a non-linear relationship between blood vitamin B12 level and CRC risk (P non-linearity=0·219). Every 150 pmol/l increment in blood vitamin B12 level was not associated with CRC risk, enhanced by the analysis of the highest blood vitamin B12 levels v. lowest blood levels. One explanation may be that all included studies roughly assessed blood vitamin B12 level in relation to CRC risk while ignoring the fact that only methylcobalamin and 5′-deoxyadenosylcobalamin are the principal active coenzyme forms of vitamin B12 compared with other forms of vitamin B12 in blood (e.g. cyanocobalamin and hydroxocobalamin)( Reference Donaldson 46 – Reference Jones, Russell and Greetham 48 ). Thus, these inactive forms of blood vitamin B12 may underestimate the effect of active forms of vitamin B12 on CRC risk. Similar to studies regarding pyridoxal 5′-phosphate (PLP, the active form of vitamin B6), the association between the active forms of vitamin B12 and CRC risk should be deeply investigated by further multicentre clinical studies.
Our meta-analysis indicated no association between blood vitamin B12 level and CRC risk, different from the association found between vitamin B12 intake and CRC risk. One potential reason may be that plasma vitamin B12 level has been found to be only slightly correlated with dietary vitamin B12 intake (multivariable Pearson correlation coefficient, r=0·08) and total vitamin B12 intake (r=0·25)( Reference Zhang, Willett and Selhub 49 ).
The strength of the current meta-analysis study was that our results and conclusions were enhanced by an in-depth subgroup analysis. A subgroup analysis based on the type of vitamin B12 intake indicated that the association between total vitamin B12 and CRC risk was stronger than the association between dietary vitamin B12 intake alone and CRC risk (Table 3). As expected, heterogeneity was reduced to low levels in the assessment of total vitamin B12 intake. Supplemental vitamin B12 intake may play an important role in CRC risk, and Zschabitz et al. showed that supplemental vitamin B12 intake was marginally associated with CRC risk( Reference Zschabitz, Cheng and Neuhouser 29 ). However, the interaction between supplemental vitamin B12 intake and dietary vitamin B12 intake is unclear. Thus, the type of vitamin B12 intake may be important for the assessment of vitamin B12 intake and CRC risk, and future clinical studies should not ignore the role of supplemental vitamin B12 intake. The association between vitamin B12 intake and CRC risk may be various among different races of population (Table 3), in agreement with the study by Williams et al. in which the associations between vitamin B12 and distal CRC differed for whites compared with African Americans( Reference Williams, Satia and Adair 12 ). This may be due to polymorphisms in genes related to one-carbon metabolism, supported by a previous study in which gene–nutrient interactions in folate-mediated one-carbon metabolism played an important role in modifying the risk of CRC( Reference Liu, Scherer and Poole 32 ). No evidence of publication bias was detected, indicating that the entire pooled results may be unbiased.
Several limitations of our meta-analysis must be acknowledged. First, the controls were not always of uniform ascertainment in case–control studies, although most studies matched controls to cases based on age and sex. Some controls might therefore have had benign diseases or other risk factors that contribute to CRC risk and misclassifications of controls or vitamin B12 intake possibly exist among several included studies. Second, some important confounding factors were not measured in several studies and some inherently confounding factors in included studies could not be solved perfectly in our study. Thus, an inadequate control of confounding factors may lead to an underestimation of risk estimates of vitamin B12 on CRC. Third, the limited number of included studies may impact the statistical power and performance of some in-depth subgroup analysis. The assigned value of vitamin B12 in each category (midpoint, mean and median) was not available in all included studies for dose–response analysis. Therefore, various assigned values might affect the accuracy of the dose–response relationship. In addition, a considerable degree of heterogeneity was observed among studies and could not be explained completely. Our subgroup analysis indicated that study design may have been one source of heterogeneity. After the case–control studies were excluded, the heterogeneity among the studies decreased (I 2=32·3 %) and the fixed-effects model was used. Similarly, our results also showed that the types of vitamin B12 intake and population sex contributed to the heterogeneity (total vitamin B12 intake group: I 2=22·9 %; female group: I 2=35·7 %). For individual studies, the result of the Galbraith plot (Fig. 5) indicated that the studies by Liu et al.( Reference Liu, Scherer and Poole 32 ) and Kune and Watson( Reference Kune and Watson 11 ) contributed substantial heterogeneity. Indeed, the heterogeneity was clearly reduced after the removal of one or both of these studies (without Liu et al.: I 2=49·5 %; without Kune and Watson: I 2=32·7 %; without Liu et al. and Kune and Watson: I 2=9·4 %). The remaining unexplained heterogeneity may have been caused by differences in the population characteristics and methodological differences (e.g. differences in the estimates of vitamin B12 intake in FFQ and the control of confounding factors). The heterogeneity did not influence or dominate the quality or stability of the results. Despite these limitations, the present study is the first dose–response meta-analysis to quantitatively assess the association between vitamin B12 and CRC risk.
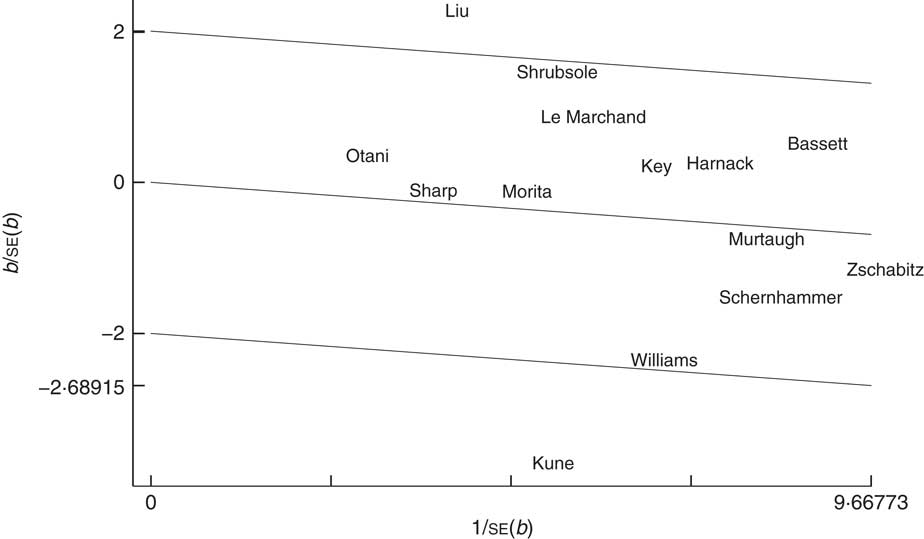
Fig. 5 Galbraith plot for exploring the sources of heterogeneity in the fourteen studies examining the relationship of vitamin B12 intake and risk of colorectal cancer. ——— represent fitted lines; those at ±2 from the fitted (regression-through-the-origin) line represent the approximate 95 % confidence region; the studies are denoted by the first author’s surname
Conclusion
In conclusion, results from the present meta-analysis indicate that vitamin B12 intake is inversely associated with CRC risk when the dosage of vitamin B12 intake is above a certain threshold, and that the association between total vitamin B12 and CRC risk is stronger than the association between dietary vitamin B12 intake alone and CRC risk. In addition, there was an insignificant association between blood vitamin B12 level and CRC risk. Further studies are required to investigate the associations between the active forms of blood vitamin B12 and CRC risk and to evaluate the influence of vitamin B12 supplementation.
Acknowledgements
Financial support: This work was supported by the Natural Science Foundation of Liaoning Province (grant number 2014029201), Program of Education Department of Liaoning Province (grant number L2014307). The sponsors had no role in study design, data collection, data analysis, data interpretation or writing of the report. Conflict of interest: None. Authorship: N.-H.S., X.-Z.H., S.-B.W. and Z.-N.W. were responsible for the conception and design of the study. N.-H.S. and X.-Z.H. conducted the statistical analyses and wrote the article. S.-B.W., Y.L., L.-Y.W. and H.-C.W. contributed to the literature search, acquisition of data, tables and figures. Z.-N.W. provided clinical expertise and interpretation of the data. C.-W.Z., C.Z. and H.-P.L. provided the draft of the article and statistical expertise. All the investigators have read and approved the final manuscript. Ethics of human subject participation: Ethical approval was not required.











