Children's food choices and preferences develop from a young age and may be influenced by genetic predisposition towards certain tastes, by food availability and by cultural and parental influences(Reference Birch and Fisher1–Reference Vereecken, Keukelier and Maes3). There is evidence that behaviours and attitudes inculcated at a young age track through childhood and into adulthood(Reference Mannino, Lee and Mitchell4–Reference Nicklaus, Boggio and Chabanet6). Therefore, the importance of establishing healthy eating habits in early life is crucial to reducing the risk for longer-term diet-related diseases in later years.
Experimental studies have revealed that infants who are breast-fed experience flavours in their mother's milk before they are exposed to the same flavours in solid foods(Reference Mennella, Forestell and Morgan7, Reference Mennella, Jagnow and Beauchamp8) and that breast-fed infants have a greater acceptability of new foods and flavours at 2–8 months of age compared with formula-fed infants(Reference Hausner, Nicklaus and Issanchou9–Reference Sullivan and Birch12). For example, breast-fed infants have been reported to have a greater diversity in foods and to consume less cordial and juice at 9 months of age(Reference Conn, Davies and Walker13). Furthermore, there is convincing evidence that, compared with formula-fed infants, breast-fed infants have a reduced risk of being overweight or obese and of developing type 2 diabetes during child- and adulthood(Reference Ip, Chung and Raman14, Reference Horta, Bahl and Martines15). This suggests that postnatal programming in infancy has an effect on feeding preferences and practices, which may subsequently affect health outcomes during later life.
A healthy diet is important for the prevention and management of a number of chronic diseases(Reference Hu and Willett16). However, it is of concern that, in young children, nutrient intakes, particularly Ca, folate, K and Zn(Reference Coppinger, Jeanes and Dobinett17–Reference Jennings, Davies and Costarelli19), and intakes of fruit and vegetables(Reference Jones, Steer and Rogers20, Reference Magarey, Daniels and Smith21) have been reported to be low, whereas intakes of take-away foods and soft drinks have been reported to be high(Reference Babey, Jones and Yu22). Some studies have reported associations between unhealthier dietary patterns of children and various factors such as fat mass(Reference Wosje, Khoury and Claytor23), sociodemographic status(Reference Aranceta, Perez-Rodrigo and Ribas24–Reference North and Emmett26) and lower levels of education of the main food provider(Reference Craig, McNeill and Macdiarmid25).
There appears to be a link between breast-feeding and diet in infants; however, it is not known whether breast-feeding is associated with food choices and dietary patterns during later childhood. Therefore, the aims of the present study were to determine the dietary patterns of a national sample of 2–8-year-old Australian children and to establish whether breast-feeding is associated with dietary patterns in this age group.
Methods
Study population and procedures
The 2007 Australian National Children's Nutrition and Physical Activity Survey was a national survey involving 4487 children aged between 2 and 16 years. Children and their parents were interviewed in their home during which time the children's food and beverage intakes and use of supplements were assessed using a 24 h food recall and a food habits survey. At this time, their height, weight and waist circumference were measured and further information was collected on their level of physical activity and demographic characteristics.
Food and nutrient intake data were collected on two occasions using a 24 h food recall. A computer-assisted personal interview (CAPI) was conducted in the child's home and was followed 7–21 d later by a computer-assisted telephonic interview (CATI). All field interviewers were trained in recruitment procedures and in dietary assessment, physical activity and anthropometry measures. Age- and sex-specific BMI cut-offs for normal weight, overweight and obese children and for adolescents were applied to the data(Reference Cole, Bellizzi and Flegal27). For underweight participants, grade-3 thinness (corresponding to an adult BMI of 18·5 kg/m2) was used as the cut-off point(Reference Cole, Flegal and Nicholls28).
Sample selection
The survey was conducted using a quota-sampling scheme stratified by state/territory and by capital city statistical division or by the rest of the state/territory. Postcodes were used as the primary sampling units in each state, allocated to a stratum using the Australian Bureau of Statistics (ABS) postal area-to-statistical local area concordance. Random digit dialling was used to recruit households (private dwellings) from selected postcodes to the survey. The telephone number prefix acted as a ‘geographical indicator’ that corresponded to the postcode. Households with children aged between 2 and 16 years were identified and were asked to participate. One child within the household was selected as the ‘study child’ using a defined method for children's selection. For example, using a predefined Kish table (refer to user guide for additional details), the parent or caregiver was asked for the name, gender and age in years of each child in the household aged 2–16 years, arranging the children by age (from the oldest to the youngest). As the number of children required in the sample for each of the age cohorts was not proportional to the number of children in each of the age cohorts in the population, children aged 2–3 or 14–16 years had a higher probability of selection compared with children aged 4–13 years in any one household. Therefore, the Kish table showed a bias towards selecting children who were aged 14–16 years, followed by children aged 2–3 years and finally children aged 4–13 years. In cases where the age and gender quota for a particular location had already been filled, recruitment of the study child did not proceed. Some postcodes were excluded if they were in very remote areas, having few in-scope children according to the 2001 ABS Census.
Further details of the survey methodology and sample design can be obtained from the 2007 Australian National Children's Nutrition and Physical Activity Survey Users Guide available at http://www.health.gov.au/internet/main/publishing.nsf/Content/AC3F256C715674D5CA2574D60000237D/$File/user-guide-v2.pdf.
The current analysis was focused on 2–8-year-old children using the 24 h dietary food recall data collected through the CAPI from the primary caregiver of the child.
Food group consumption
Dietary intake information collected from the CAPI 24 h food recall generated a large number of foods. In order to process and clean the data and identify meaningful dietary patterns, a number of foods were eliminated that were considered unlikely to contribute to a dietary pattern according to the following criteria. Food items that were not included in the analysis as they contributed to a cumulative <10 % frequency of all individual food items consumed were: dietary/multivitamin and oil supplements; gelatin; meat substitutes; vegetable-based chutneys/dressings/relishes; energy drinks; essences/baking powder; flour; yeast; stuffing; alcohol; chewing gum; soya beverages; infant drinks/formulae; pretzels; unspecified fats; mature legumes and pulses; chives; garlic; ginger; electrolyte and fortified drinks; extruded or reformed snacks; and batter-based products. A frequency of the ‘gram intake’ for each food was calculated. Individual foods were eliminated from the analysis when the amount consumed was <3rd percentile of the study population (e.g. <20 g for meat/poultry or <5 g for vegetables). It was believed that intakes in these amounts were unlikely to make a significant contribution to a dietary pattern.
A total of forty-five food groups were created that were mutually exclusive and were included in the analysis (Table 1). Frequency of consumption was binary coded to 0 (not consumed) or 1 (consumed).
Table 1 Food groups and the description of foods used in the factor analysis

NFS, not further specified.
Factor analysis
Dietary patterns were derived using factor analysis with factor loadings extracted using the principal component method and Varimax/orthogonal rotation. Factor loadings for binary variables are usually underestimated and therefore tetrachoric correlation coefficients were calculated to estimate the correlation between the continuous versions of the variables. The number of dietary patterns identified was based on eigenvalues > 1·5 on identification of a break point in the scree plot and on interpretability(Reference Schulze, Hoffmann and Kroke29). Items were loaded on a factor if they had a correlation >0·25 with that factor. These items represent the foods most highly related to the identified factor(Reference McCann, Marshall and Brasure30, Reference Slattery, Boucher and Caan31). Foods that cross-loaded on several factors were also retained. Inter-item reliability for each factor was assessed using Cronbach's α coefficients.
Initially, a factor analysis was conducted separately for the 2–3-year-olds and 4–8-year-olds; however, food groups that loaded on the patterns were similar, and therefore the age groups were combined and the analysis re-run. Three distinct dietary patterns emerged and all children received a dietary pattern score for each of the identified patterns. A confirmatory factor analysis was also conducted using a second 24 h recall from the CATI data in which similar food groups loaded on a three-factor solution (data not shown).
Food habits survey
As part of the interview process, a ‘food habits’ survey was completed by the child (if aged >9 years) or by a parent/caregiver during the CAPI. The survey included questions related to diet and food habits (e.g. type of milk used, servings of fruit/vegetables normally consumed, who prepares meals, is salt added to meals) with a series of answers pertaining to each question that the parent/caregiver could choose from. Four questions were included on breast-feeding, such as whether the child had ever been breast-fed; duration of breast-feeding; age of introduction of solids; and if, and at what age, the child received formula. For the current data analysis, breast-feeding was categorised into a dichotomous variable (‘ever’ or ‘never’ breast-fed).
Socio-economic Indexes for Areas
The Socio-economic Indexes for Areas (SEIFA) ranks geographical areas across Australia according to a ‘score’ that is created for the area on the basis of the characteristics of people, families and dwellings within that area(32). Every geographical area in Australia is given a SEIFA score for each index, which shows how disadvantaged that area is compared with other areas in Australia. Lower scores indicate greater disadvantage(32). The SEIFA index was categorised into tertiles.
Statistical analyses
All analyses were performed using the STATA statistical software package for Windows version 10·1 (StataCorp., College Station, TX, USA) with population weights applied. Continuous data were assessed for normality and, if required, normalised with natural log transformation. Back transformations from the logarithmic scale to the original scale were undertaken in the multivariate regression models. Data are reported as OR and 95 % CI. Only significant covariates in the final regression models are reported. The α level for significance was set at P < 0·05.
Results
A total of 2287 children were included in the analysis (51 % male). Selected characteristics (mean and sem) of the children are reported in Table 2. The median duration of breast-feeding and the interquartile range were 30·0 and 8·2–51·6 weeks, respectively; 223 children (10·7 %) were not breast-fed.
Table 2 Characteristics of the children
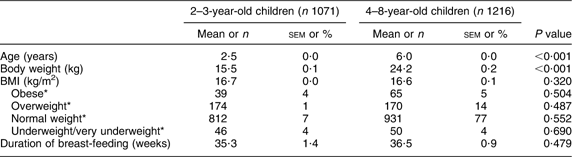
*Data are presented as n and %.
Factor analysis
The dietary pattern analysis revealed three distinct patterns that explained 4·2 %, 4·1 % and 3·7 % of the variance for each respective pattern. The first pattern was labelled ‘Non-core food groups’, with food groups such as whole-fat dairy products, cheese, luncheon and deli-type meats, medium–high sugar-sweetened breakfast cereals and sweet biscuits loading on this pattern (Table 3). Some food groups that were inversely associated with this pattern were reduced/low-fat dairy products, wholegrain/wholemeal breads and non-carbonated, non-nutritive beverages. The second pattern was labelled ‘Healthy, meat and vegetable’ as it included two types of vegetables, red meat, fruit and wholegrain/wholemeal breads. Food groups that were inversely associated with this pattern were potatoes from take-away outlets, carbonated sugar-sweetened beverages, take-away foods and added sugar. The final dietary pattern was loaded with the greatest number of food groups with similar food group loadings and was labelled ‘Combination’. This pattern did not include any fruit or vegetables but included candy (not chocolate based), other dairy products (e.g. ice cream, custard), pasta/rice products, reduced/low-fat milk, nuts/seeds, cakes and chocolate.
Table 3 Whole-day dietary patterns and food group loadings for 2–8-year-old children

Cronbach's α for inter-item reliability was 0·78 for the non-core food groups pattern, 0·74 for the healthy, meat and vegetable pattern and 0·71 for the combination pattern.
Breast-feeding and dietary patterns
Children were classified as ‘breast-fed’ (n 2064, 89·3 %) or ‘never breast-fed’ (n 223, 10·7 %). After adjusting for a number of potential confounders, i.e. age, gender, energy intake, BMI, SEIFA, number of adults in the household, number of children in the household, birth weight, waist girth, age when the child started solids (if formula fed), age (weeks) at which the child received infant formula for the first time and the highest completed level of education/qualification by the primary caregiver and their partner, the results of the final models for breast-fed children compared with children who were ‘never breast-fed’ are shown in Table 4.
Table 4 Multivariate linear regression coefficients (β) associating factor scores with breast-feeding (not breast-fed v. ever breast-fed)

SEIFA, Socio-economic Indexes for Areas.
Final models with significant covariates.
*Higher values represent lower educational level.
There was no association between breast-feeding and the non-core food groups pattern or the combination pattern (Table 4). Higher scores on the non-core food groups pattern were associated with the following: earlier introduction to solid foods; more children in the household; and greater likelihood of being in the disadvantaged SEIFA category. Higher scores on the combination pattern were associated with: being older; greater likelihood of being a girl; less likelihood of having a lower educational level; and lower SEIFA category (indicating more disadvantaged).
There was a positive association between breast-feeding and the healthy, meat and vegetable pattern (Table 4). Scores on the healthy, meat and vegetable pattern were 26 % higher for children who were breast-fed compared with children who were not breast-fed. Higher scores on this pattern were also associated with younger age, lower BMI, higher birth weight, greater likelihood of being in the less-disadvantaged SEIFA category and less likelihood of the children's parents having lower educational level.
Discussion
The present study is the first in Australia to conduct a factor analysis on a national sample of 2–8-year-old children and to associate the resulting dietary patterns with prior breast-feeding.
A positive association was found between the healthy, meat and vegetable dietary pattern and breast-feeding. Higher factor scores indicate more food groups loading on this pattern. Therefore, compared with children who were not breast-fed, children who were breast-fed were more likely to consume healthy foods such as vegetables, fruit, wholegrain bread and red meat, which were retained in this pattern. Only one study has examined the relationship between breast-feeding and dietary patterns; however, this was conducted in infants at 6 months and 1 year of age(Reference Robinson, Marriott and Poole33). In that study, a dietary pattern characterised by a higher frequency of consumption of vegetables, fruit, meat and fish, in addition to high consumption of breast milk, emerged in infants at 6 months of age and again at 1 year of age(Reference Robinson, Marriott and Poole33). The present study extends these findings in infants to an older age group of Australian children, indicating that breast-feeding appears to be associated with an overall healthy eating pattern, and tracks throughout childhood.
The mechanism behind this relationship may relate to findings from earlier studies in animals(Reference Galef and Henderson34–Reference Campbell37) and to subsequent human studies that revealed that breast-feeding was associated with greater acceptability to new foods and flavours(Reference Mennella, Forestell and Morgan7, Reference Mennella and Beauchamp11, Reference Mennella, Griffin and Beauchamp38). Moreover, it has been shown that flavours from the mothers’ diet are transmitted through amniotic fluid(Reference Mennella, Johnson and Beauchamp39) and later though breast milk(Reference Desage, Schaal and Soubeyrand40, Reference Mennella and Beauchamp41). Therefore, flavours and components consumed in the mothers’ diet are associated with, and may have a significant effect on, feeding preferences and practices in later life.
There is also some, albeit limited, evidence linking breast-feeding to improved cardiovascular risk factors such as blood pressure and lipids(Reference Singhal, Cole and Fewtrell42, Reference Singhal, Cole and Lucas43), type 2 diabetes(Reference Owen, Martin and Whincup44) and risk for obesity(Reference Owen, Martin and Whincup45). All these outcomes are also affected by dietary intakes. Therefore, if at the onset of life positive breast-feeding practices influence childhood and adolescent food choices and dietary intakes, it is imperative that the benefits of breast-feeding be promoted and clearly understood by new mothers.
The WHO recommends that all infants be exclusively breast-fed for the first 6 months of life and that breast-feeding continues into the second year of life(46). This is not currently achieved, as data from the 2004–2005 Australian National Health Survey reported that, although 88 % of mothers initiated breast-feeding, only 50 % of infants were still breast-fed at 6 months and 23 % were breast-fed at 12 months(Reference Amir and Donath47). This further suggests the need to educate and promote breast-feeding and inform mothers on possible health benefits and its associated outcomes in young children.
There was no association between the non-core food groups pattern and breast-feeding. However, this pattern was associated with earlier introduction to solids, to the presence of more children in the household, to a greater likelihood of being in the more disadvantaged SEIFA categories and to higher energy intakes. This finding is supported by the results of another Australian study which reported that infants were more likely to have received non-core foods if they received solids for the first time before 17 weeks and if they had two or more older siblings(Reference Koh, Scott and Oddy48). No association was found between the combination dietary pattern and breast-feeding. However, the direction of breast-feeding was negative, suggesting that being breast-fed was less likely to be associated with higher scores on this pattern. This pattern was characterised by a large number of food groups, with many being non-core foods. In the study by Robinson et al.(Reference Robinson, Marriott and Poole33) conducted in infants, the second dietary pattern identified was a pattern containing ‘adult’-type foods such as bread, savoury snacks, breakfast cereals and chips. This pattern was inversely associated with consumption of breast milk and infant-type foods(Reference Robinson, Marriott and Poole33). These food groups were loaded on either the combination or non-core food groups pattern, supporting the fact that poorer food choices and dietary patterns are associated with little or no breast-feeding.
The role of the primary parent's educational level (typically that of the mother) also needs to be highlighted. It is of interest that the association between breast-feeding and the healthy, meat and vegetable pattern was independent of education, which was associated with this pattern as well. Other studies have reported positive associations between breast-feeding and education(Reference Rebhan, Kohlhuber and Schwegler49, Reference Senarath, Dibley and Agho50), and women who breast-fed for >16 weeks were more often highly educated(Reference Scholtens, Brunekreef and Smit51). In the latter study, breast-fed children were less likely to have a low consumption of fruit and vegetables and a high consumption of snacks or an unhealthy diet, independent of maternal education(Reference Scholtens, Brunekreef and Smit51). This earlier study and our data indicate that the association between breast-feeding and diet is independent and that breast-feeding is not just a proxy measure of educational level.
There are some limitations to the present study. This was a cross-sectional study in which only a snapshot of the foods normally consumed by these children was captured. However, the large sample size reduced the estimated variance. Factor analysis is a validated method for analysis of dietary patterns(Reference Hu, Rimm and Smith-Warner52, Reference Khani, Ye and Terry53); however, rather than assigning an individual to a specific dietary pattern, this method produced continuous dietary factor scores for each individual for each pattern. Therefore, it was not possible to determine statistically significant differences between each dietary pattern, and only associations and relationships between continuous variables and dietary pattern scores could be tested. Factor analysis is also sample specific; therefore, the results may not be generalisable to other populations. However, we performed the same factor analysis methodology in this population using the 24 h CATI data, in which three similar factors emerged.
The total variance explained by each factor was also low, compared with previous factor analysis studies conducted in older age groups(Reference Cole, Bellizzi and Flegal27, Reference Slattery, Boucher and Caan31, Reference Ambrosini, Oddy and Robinson54, Reference Hu, Rimm and Stampfer55). However, Cronbach's α coefficients were all >0·7, indicating acceptable inter-item reliability. In addition, the food groups loading on the factors were varied and many were greater than the 0·25 cut-off value. This suggests that our population had a varied diet that was, nevertheless, still specific to the identified factors. As the present study is the first of its kind in this age group, further studies are required to refute or support our findings.
In conclusion, our results provide suggestive evidence that breast-feeding during infancy is associated with a healthy dietary pattern in childhood and offers a likely mechanistic pathway to explain the previously reported association of breast-feeding with chronic disease. A number of health benefits have been associated with breast-feeding and these have also been associated with health outcomes in adulthood. Although we did not find any association between breast-feeding and the unhealthier patterns, it is of concern that these patterns were identified at such a young age, as these may track through adulthood. It is important to inform parents of a healthy diet during pregnancy and of the significance of breast-feeding and how food choices in childhood may track into adulthood. It is also essential to inform young children of the importance of healthy eating.
Further studies investigating dietary patterns and duration of breast-feeding are warranted to supplement the limited existing literature on the significance of breast-feeding and how it may relate to children's diet and to later health outcomes.
Acknowledgements
The present study was supported by the Meat and Livestock Association but the Association had no input into the results presented in the current report. The authors have no conflict of interest to declare. J.A.G. was responsible for analysing and interpreting the data and for writing the manuscript; L.C. and J.S. were responsible for the study design and for reading and editing the drafts. The authors acknowledge the assistance of Dr Richard Woodman who provided invaluable statistical assistance.






