Breast cancer is the most common type of cancer and the leading cause of cancer mortality among women worldwide( Reference Edefonti, Randi and La Vecchia 1 ). Although the incidence of female breast cancer in most Asian countries is much lower than that in Western societies( Reference Hirose, Matsuo and Iwata 2 ), the rate has increased steadily in recent years( Reference Hirose, Matsuo and Iwata 2 – Reference Adebamowo, Hu and Cho 7 ). In Iran, the age-adjusted incidence rate for breast cancer is 24·42 per 100 000 women per year (standardized on the world population).
Although a number of risk factors have been identified for breast cancer, some are difficult to modify (e.g. genetics) while dietary pattern is a modifiable risk factor which could be targeted to prevent and treat breast cancer( Reference Hirose, Matsuo and Iwata 2 , Reference Fung, Hu and Holmes 8 , Reference Buck, Vrieling and Flesch-Janys 9 ). Although several studies have examined the role of single micronutrients, macronutrients and food items in the pathogenesis of breast cancer, most of the relationship remains controversial as the evidence from these studies does not take into account the complexity of the dietary pattern as a whole( Reference Edefonti, Randi and La Vecchia 1 , Reference Mannisto, Dixon and Balder 6 ). Diet is a complex combination of nutrients with synergic interactions which should be considered when analysing diet–disease relationships( Reference Velie, Schairer and Flood 10 ). During the past decade interest has shifted to dietary pattern analysis, as it can accommodate the complex interplay of nutrients within a diet and identify patterns in dietary behaviours by representing a broad picture of food and nutrient intakes( Reference Cottet, Touvier and Fournier 4 , Reference Buck, Vrieling and Flesch-Janys 9 , Reference Wu, Yu and Tseng 11 , Reference Sieri, Krogh and Pala 12 ). In addition, dietary patterns form intuitively practical tools for determining public health recommendations( Reference Edefonti, Randi and La Vecchia 1 , Reference Cottet, Touvier and Fournier 4 , Reference Mannisto, Dixon and Balder 6 , Reference Fung, Hu and Holmes 8 , Reference Velie, Schairer and Flood 10 ).
Dietary patterns have previously been assessed in relation to breast cancer risk in several studies conducted in the USA( Reference Adebamowo, Hu and Cho 7 , Reference Fung, Hu and Holmes 8 , Reference Velie, Schairer and Flood 10 , Reference Wu, Yu and Tseng 11 , Reference Agurs-Collins, Rosenberg and Makambi 13 , Reference Murtaugh, Sweeney and Giuliano 14 ), Europe( Reference Cottet, Touvier and Fournier 4 , Reference Mannisto, Dixon and Balder 6 , Reference Buck, Vrieling and Flesch-Janys 9 , Reference Sieri, Krogh and Pala 12 , Reference Edefonti, Decarli and La Vecchia 15 , Reference Sant, Allemani and Sieri 16 ), Uruguay( Reference De Stefani, Deneo-Pellegrini and Boffetta 17 , Reference Ronco, De Stefani and Boffetta 18 ), Japan( Reference Hirose, Matsuo and Iwata 2 ) and China( Reference Zhang, Ho and Fu 3 , Reference Cui, Dai and Tseng 5 ), and a reduced risk has been reported among those following a ‘prudent’ dietary pattern( Reference Hirose, Matsuo and Iwata 2 , Reference Agurs-Collins, Rosenberg and Makambi 13 , Reference De Stefani, Deneo-Pellegrini and Boffetta 17 ), a ‘Mediterranean’ pattern( Reference Murtaugh, Sweeney and Giuliano 14 ), a ‘traditional Southern’ dietary pattern( Reference Velie, Schairer and Flood 10 ), ‘stew’ and ‘traditional’ dietary patterns( Reference Ronco, De Stefani and Boffetta 18 ), a ‘salad vegetable’ pattern( Reference Sieri, Krogh and Pala 12 , Reference Sant, Allemani and Sieri 16 ), a ‘vegetable–soy’ pattern( Reference Wu, Yu and Tseng 11 ) and a ‘vegetables–fruit–soy–milk–poultry–fish’ dietary pattern( Reference Zhang, Ho and Fu 3 ). Compared with the typical ‘Western’ diet, all of these dietary patterns were higher in fruits and vegetables and lower in animal fats. In contrast, an increased risk of breast cancer has been associated with a ‘Western’ dietary pattern( Reference Murtaugh, Sweeney and Giuliano 14 , Reference De Stefani, Deneo-Pellegrini and Boffetta 17 , Reference Ronco, De Stefani and Boffetta 18 ), a ‘starch rich’ pattern( Reference Edefonti, Decarli and La Vecchia 15 ), an ‘ethnic-meat/starch’ pattern( Reference Wu, Yu and Tseng 11 ), a ‘refined grain–meat–pickle’ pattern( Reference Edefonti, Randi and La Vecchia 1 ), a ‘meat–sweet’ pattern( Reference Cui, Dai and Tseng 5 ), a ‘Western-alcohol’ pattern( Reference Cottet, Touvier and Fournier 4 ) and a ‘drinker’ pattern characterized by high intakes of alcoholic beverages( Reference De Stefani, Deneo-Pellegrini and Boffetta 17 ). Furthermore, adherence to a ‘prudent’ dietary pattern( Reference Murtaugh, Sweeney and Giuliano 14 ) and an ‘animal products’ pattern( Reference Edefonti, Decarli and La Vecchia 15 ) has been positively associated with breast cancer risk, while a ‘pork, processed meat and potato’ pattern has shown an inverse association( Reference Mannisto, Dixon and Balder 6 ).
To our knowledge, no previous studies have evaluated the association between dietary patterns and breast cancer risk among Middle-Eastern women. However, dietary patterns vary according to geographic region, socio-economic status, cultural practices and food preferences and availability( Reference Zhang, Ho and Fu 3 ). Specifically, dietary intake of the Middle-Eastern population has its own unique features, being characterized by high intakes of refined grains (white rice and bread) and hydrogenated fats and a greater percentage of energy from carbohydrates( Reference Esmaillzadeh and Azadbakht 19 ). With these features, factor analysis may result in different dietary patterns in this region compared with other parts of the world. The aims of the present hospital-based case–control study were therefore to identify major dietary patterns among Iranian women and to examine the relationship between these dietary patterns and breast cancer risk.
Materials and methods
The present case–control study included 100 female cases aged 30–65 years who were admitted to the major general hospitals in Tehran province, Iran. Cases were diagnosed with incident, histologically confirmed breast cancer within the past 5 months, and they did not have a history of cancers at other sites or hormone replacement therapy. Controls (n 184) were selected from female patients admitted to the same hospital as the cases for a variety of acute, non-neoplastic conditions unrelated to long-term modification of diet. Controls and cases were frequency-matched by age (5-year groups). Nine controls withdrew during the research and one was excluded due to having incomplete dietary records. Moreover, dietary records with reported energy intakes of ≤2100 kJ/d or ≥21 000 kJ/d and those with fifty or more skipped food items were considered invalid and were excluded from all analyses. This reduced our sample size to 100 cases and 174 controls (response rate = 96·5 %).
Dietary assessment
The study data were collected by specifically trained professional interviewers through private face-to-face interviews. Trained dietitians collected the dietary data by means of a validated 168-item semi-quantitative FFQ that was modified to include Iranian food items( Reference Mirmiran, Esfahani and Mehrabi 20 ). This FFQ has previously shown relative validity and reproducibility for assessing food and nutrient intakes among Iranian adults and it is an acceptable tool for use in this population( Reference Mirmiran, Esfahani and Mehrabi 20 ). Dietary habits of the cases in the year prior to diagnosis and of the controls in the year before the interview were collected. Using this FFQ, the consumption frequency of each food item was evaluated on a daily, weekly or monthly basis, and the portion sizes of consumed foods were specified according the US Department of Agriculture portion sizes (e.g. apple, one medium; bread, one slice; dairy, one cup). Whenever use of the US Department of Agriculture portion sizes was not possible, household measures were used alternatively (e.g. beans, one tablespoon; chicken meat, one leg or wing; rice, one large, medium or small plate).
Intakes of the 168 FFQ food items were reclassified into twenty-six predefined food groups according to the similarity of their nutrient contents (see Appendix)( Reference Zhang, Ho and Fu 3 , Reference Buck, Vrieling and Flesch-Janys 9 , Reference Sant, Allemani and Sieri 16 ). For each participant, the average daily intake of each food group was calculated by summing the intakes of individual food items within that group.
In addition, the relative accuracy of reported energy intakes was assessed by dividing energy intake by BMR (EI:BMR) in order to control for its confounding effect in the analyses. BMR was calculated according to the Schofield equation using participants’ gender, age, weight and height( Reference Goldberg, Black and Jebb 21 ). An EI:BMR value of <1·35 indicated under-reporting, while a value in the range 1·35–2·39 indicated normal reporting and a value ≥2·40 indicated over-reporting of energy intake( Reference Johansson, Solvoll and Bjorneboe 22 ).
Other measures
Weight was measured on a flat, uncarpeted surface using a digital scale (model 803; Seca, Hamburg, Germany) and was recorded to the nearest 0·1 kg. Height was measured using a standard stadiometer (model 206 Portable Body Meter Measuring Device; Seca) with the participant's head in the Frankfort horizontal plane, and it was recorded to the nearest 0·5 cm. BMI was calculated by dividing weight by the square of height (kg/m2).
During the face-to-face interviews, physical activity level was assessed using a pre-tested questionnaire and data were expressed as metabolic equivalent hours per day (MET-h/d)( Reference Aaron, Kriska and Dearwater 23 ). Additional information on participants’ demographic characteristics, smoking history, alcohol consumption, medical history and prescription drug use was also collected using a questionnaire.
Statistical analysis
Data were analysed using the statistical software package SPSS version 16·0. Factor analysis (principal component analysis) was used to identify major dietary patterns based on the twenty-six predefined food groups (Appendix) and two interpretable factors were retained based on the scree test( Reference Rezazadeh, Rashidkhani and Omidvar 24 ). An orthogonal rotation procedure (varimax rotation) was then applied to simplify the factor structure and render it more easily interpretable. The derived factors were labelled based on their interpretability and review of the literature. The factor score for each pattern was calculated by summing intakes of food groups weighted by factor loadings, and each participant was then assigned a score for each of the identified patterns. A factor loading of >0·3 was used as a cut-off point to identify the primary factors on which the items were loaded. For further analyses, factor scores were categorized into tertiles of the control group.
To compare general characteristics across the tertile categories of dietary pattern scores, ANOVA and χ 2 tests were used as appropriate. The relationship between major dietary patterns and breast cancer risk was assessed using logistic regression analysis in different models, controlling for age (continuous) and menopausal status (postmenopause/premenopause) in model I and for age (continuous), menopausal status (postmenopause/premenopause), age at menarche (continuous), age at first full-term pregnancy (FFTP; continuous), smoking status (yes/no), oral contraceptive drug use (yes/no), BMI (continuous), relative accuracy of energy reporting (under-reporting/accurate reporting/over-reporting of energy intake), physical activity (continuous) and family history of breast cancer in a first- or second-degree relative (yes/no) in model II. Results are presented as odds ratios and 95 % confidence intervals. Generally, due to the small sample size and for maintaining the statistical power, all individuals with missing answers to any of the confounders were included in a separate ‘missing’ category (missing indicator method)( Reference Rashidkhani, Akesson and Lindblad 25 ). Thus the same number of women was included in all models, making the comparison possible.
As a basis for trend analyses, ordinal scores were constructed from the categorized variables and were placed into the model as successive integers. All statistical tests were two-sided and α level was set at P < 0·05.
Ethical approval
The study was conducted based on the Declaration of Helsinki guidelines and all procedures were approved by the ethics board of the Faculty of Nutrition Sciences and Food Technology, National Nutrition and Food Technology Research Institute (WHO Collaborating Center), Shahid Beheshti University of Medical Sciences, Tehran, Iran. Written informed consent was obtained from all participants before data collection began.
Results
As presented in Table 1, two major dietary patterns were extracted using factor analysis: a ‘healthy’ dietary pattern (high consumption of vegetables, fruits, low-fat dairy products, legumes, olive and vegetable oils, fish, condiments, organ meat, poultry, pickles, soya and whole grains) and an ‘unhealthy’ dietary pattern (high in soft drinks, sugars, tea and coffee, French fries and potato chips, salt, sweets and desserts, hydrogenated fats, nuts, industrial juice, refined grains, and red and processed meat). Overall, these factors explained 24·31 % of the entire variance.
Table 1 Factor-loading matrix for the two major dietary patterns

Selected characteristics of participants within tertiles of dietary pattern scores are shown in Table 2. In comparison with participants in the lowest tertile of the ‘healthy’ dietary pattern score, those in the highest were significantly younger at FFTP (P = 0·03), had higher energy intake (P < 0·001), were more physically active (P < 0·01), were less likely to have used oral contraceptive drugs (P < 0·01) and had higher over-reporting of energy intake (P < 0·001). In contrast, compared with women in the lowest tertile of the ‘unhealthy’ dietary pattern, those in the highest tertile were less likely to be postmenopausal (P < 0·001), had higher energy intake (P < 0·001), were more likely to have used oral contraceptive drugs (P = 0·03) and had higher over-reporting of energy intake (P < 0·001). Generally, about 1 % of women consumed alcoholic beverages and 45·2 % of women used supplements in this population.
Table 2 Participants’ characteristics in tertiles of dietary pattern scores: 100 patients aged 30–65 years with breast cancer (cases) and 174 hospital controls, Tehran, Iran

T1, first tertile (lowest); T2, second tertile; T3, third tertile (highest); FFTP, first full-term pregnancy; MET, metabolic equivalents; EI, energy intake.
*ANOVA for quantitative variables and χ 2 test for qualitative variables.
†Family history of breast cancer in a first- or second-degree relative.
‡EI:BMR < 1·35.
§1·35 ≤ EI:BMR ≤ 2·39.
∥EI:BMR ≥ 2·40.
Table 3 presents the odds ratio for breast cancer risk across tertiles of dietary pattern scores. After multivariable adjustment for age and menopausal status (model I), no significant association was observed between the ‘healthy’ dietary pattern and breast cancer risk (P for trend =0·10), while the highest tertile of the ‘unhealthy’ dietary pattern was associated with a 5·94-fold increase in breast cancer risk (P for trend <0·0 0 1) compared with the lowest tertile. After further adjustment for all confounders in model II (age, menopausal status, age at menarche, age at FFTP, smoking status, oral contraceptive drug use, BMI, relative accuracy of energy reporting, physical activity and family history of breast cancer in a first- or second-degree relative), women in the highest tertile of the ‘healthy’ dietary pattern had 75 % decreased risk of breast cancer (P for trend = 0·02) while those in the highest tertile of the ‘unhealthy’ pattern had 7·78-fold increased risk of breast cancer (P for trend = 0·001) compared with those in the lowest.
Table 3 Odds ratios (95 % confidence intervals) for breast cancer risk across tertiles of dietary pattern scores*: 100 patients aged 30–65 years with breast cancer (cases) and 174 hospital controls, Tehran, Iran
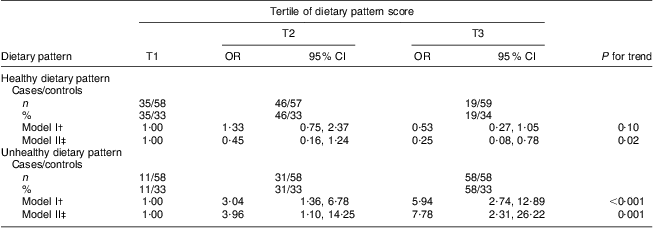
T1, first tertile (lowest); T2, second tertile; T3, third tertile (highest).
*OR were calculated using logistic regression analysis.
†Adjusted for age and menopausal status.
‡Additionally adjusted for age at menarche, age at first full-term pregnancy, smoking status, oral contraceptive drug use, BMI, physical activity, family history of breast cancer and relative accuracy of energy reporting.
Further effect modification by menopausal status revealed that breast cancer risk was not significantly different between premenopausal and postmenopausal women (P = 0·99). Table 4 presents the odds ratio for breast cancer risk across tertiles of dietary pattern scores based on menopausal status. After multivariable adjustment (age, menopausal status, age at menarche, age at FFTP, smoking status, oral contraceptive drug use, BMI, energy intake, physical activity and family history of breast cancer in a first- or second-degree relative), the P value for the interaction term suggested that there was no significant difference in dietary patterns of menopausal and non-menopausal women in relation to risk of breast cancer (P for interaction (‘healthy’ dietary pattern) = 0·23, P for interaction (‘unhealthy’ dietary pattern) = 0·14).
Table 4 Odds ratios (95 % confidence intervals) for breast cancer risk across tertile categories of dietary pattern scores by menopausal statusFootnote *: 100 patients aged 30–65 years with breast cancer (cases) and 174 hospital controls, Tehran, Iran

T1, first tertile (lowest); T2, second tertile; T3, third tertile (highest).
* All OR were calculated from logistic regression analysis.
† Adjusted for age, age at menarche, age at first full-term pregnancy, smoking status, oral contraceptive drug use, BMI, energy intake, physical activity and family history of breast cancer.
Discussion
Two major dietary patterns were identified in this population of Iranian women that together explained 24·31 % of the variance in dietary intakes as measured by the FFQ. These patterns were similar to the healthy and unhealthy dietary patterns found in other studies conducted in Iran which have used the same FFQ( Reference Rezazadeh, Rashidkhani and Omidvar 24 , Reference Hajizadeh, Rashidkhani and Rad 26 ). After adjusting for potential confounders (age, menopausal status, age at menarche, age at FFTP, smoking status, oral contraceptive drug use, BMI, relative accuracy of energy reporting, physical activity and family history of breast cancer in a first- or second-degree relative), those in the highest tertile of the ‘healthy’ dietary pattern had 75 % lower risk of breast cancer, while being in the highest tertile of the ‘unhealthy’ pattern was linked to 7·78-fold increased risk for breast cancer, compared with being in the lowest tertile. The odds for the ‘unhealthy’ dietary pattern increased from OR = 5·94 (95 % CI 2·74, 12·89) to OR = 7·78 (95 % CI 2·31, 26·22) from model I to model II, respectively, and the 95 % confidence intervals were wide, which may be due to the small sample size and use of a large number of adjusting variables in model II.
Generally, findings from previous studies have been inconclusive. Some case–control studies have found an inverse association between breast cancer risk and a healthy dietary pattern or patterns similar to a healthy pattern( Reference Hirose, Matsuo and Iwata 2 , Reference Zhang, Ho and Fu 3 , Reference Wu, Yu and Tseng 11 , Reference Murtaugh, Sweeney and Giuliano 14 , Reference De Stefani, Deneo-Pellegrini and Boffetta 17 , Reference Ronco, De Stefani and Boffetta 18 ) and a positive association has been reported between Western or Western-like dietary patterns and breast cancer risk( Reference Cui, Dai and Tseng 5 , Reference Wu, Yu and Tseng 11 , Reference Murtaugh, Sweeney and Giuliano 14 , Reference Edefonti, Decarli and La Vecchia 15 , Reference De Stefani, Deneo-Pellegrini and Boffetta 17 , Reference Ronco, De Stefani and Boffetta 18 ). Higher scores on the ‘vegetable–fruit–soy–milk–poultry–fish’ dietary pattern were associated with a decreased breast cancer risk among Chinese women (OR = 0·26, 95 % CI 0·17, 0·42)( Reference Zhang, Ho and Fu 3 ). In addition, being in the highest quartile of a ‘prudent’ dietary pattern, defined as high consumption of vegetables, fruits and fish, was shown likely to decrease the breast cancer risk by 27 % among Japanese women (OR = 0·73, 95 % CI 0·63, 0·84)( Reference Hirose, Matsuo and Iwata 2 ). Among Uruguay women, a ‘healthy’ dietary pattern characterized by high loadings of raw vegetables, cooked vegetables, total fruits, poultry and fish was inversely related to breast cancer risk (OR = 0·46, 95 % CI 0·31, 0·69)( Reference Ronco, De Stefani and Boffetta 18 ). A prospective cohort study in the USA showed that the ‘traditional Southern’ pattern characterized by high intakes of cooked greens, beans, legumes, cabbage, sweet potatoes and cornbread may reduce the risk of invasive breast cancer significantly (relative risk = 0·78, 95 % CI 0·65, 0·95)( Reference Velie, Schairer and Flood 10 ). In addition, another cohort study in Australia supported the hypothesis that a dietary pattern rich in fruit and salad might protect against invasive breast cancer and that the effect might be stronger for oestrogen receptor- and progesterone receptor-negative tumours (hazard ratio = 0·48, 95 % CI 0·26, 0·86)( Reference Baglietto, Krishnan and Severi 27 ). Similarly, adherence to the ‘vegetable–soy’ pattern/'prudent’ pattern/healthy ‘Mediterranean’ pattern has been inversely linked with the breast cancer risk in Asian American women (P for trend =0·013, case–control study)( Reference Wu, Yu and Tseng 11 ), African American women (P for trend = 0·06, cohort study)( Reference Agurs-Collins, Rosenberg and Makambi 13 ) and French women (P for trend = 0·003, cohort study)( Reference Cottet, Touvier and Fournier 4 ).
Overall, a recent meta-analysis of sixteen studies examining the association of dietary patterns and breast cancer risk concluded that being in the highest categories of the ‘prudent’/'healthy’ dietary patterns reduces the breast cancer risk significantly (OR = 0·89, 95 % CI 0·82, 0·99)( Reference Brennan, Cantwell and Cardwell 28 ). However, a ‘prudent’ dietary pattern characterized by high intakes of low-fat dairy products, fruits and vegetables, whole grains, legumes and soups has been associated with an increased breast cancer risk among American women in a case–control study( Reference Murtaugh, Sweeney and Giuliano 14 ). In addition, some case–control( Reference Cui, Dai and Tseng 5 ) and prospective cohort( Reference Mannisto, Dixon and Balder 6 , Reference Adebamowo, Hu and Cho 7 , Reference Sant, Allemani and Sieri 16 ) studies have not found any significant associations between healthy dietary patterns and breast cancer risk. The cancer-protective effects of a healthy dietary pattern may be related, in part, to the higher dietary fibre and antioxidant vitamins included in these patterns. Specifically, it has been suggested that inadequate intakes of green vegetables could result in folic acid deficiency in unhealthy dietary patterns which could reduce the availability of S-adenosyl methionine for DNA methylation and thereby influence gene expression( Reference Hirose, Matsuo and Iwata 2 , Reference Cottet, Touvier and Fournier 4 , Reference Velie, Schairer and Flood 10 , Reference Agurs-Collins, Rosenberg and Makambi 13 ).
Case–control studies among Chinese women have found that a ‘refined grain–meat–pickle’ pattern (OR = 2·58, 95 % CI 1·53, 4·34)( Reference Zhang, Ho and Fu 3 ) and a ‘meat–sweet’ pattern (OR = 1·3, 95 % CI 1·0, 1·7)( Reference Cui, Dai and Tseng 5 ) could increase the breast cancer risk significantly. In addition, a ‘Western’ pattern characterized by high consumption of fried, barbecued and processed meat has been positively associated with breast cancer risk (OR = 2·16, 95 % CI 1·46, 3·29) in Uruguay( Reference Ronco, De Stefani and Boffetta 18 ). A study among Italian women has found an increased breast cancer risk among women with a ‘starch rich’ dietary pattern (high in bread, pasta dishes, cakes and desserts; OR = 1·34 95 % CI 1·10, 1·65)( Reference Edefonti, Decarli and La Vecchia 15 ). Similarly, a ‘Western’ dietary pattern characterized by higher intakes of processed and red meats, refined grains, sweets and desserts has been associated with increased breast cancer risk among smokers in the USA in a cohort study (relative risk = 1·44, 95 % CI 1·02, 2·03)( Reference Fung, Hu and Holmes 8 ). The E3N-EPIC prospective cohort study has also found that a ‘Western-alcohol’ pattern characterized by meat products, French fries, appetizers, rice/pasta, potatoes and canned fish may be positively associated with breast cancer risk (hazard ratio = 1·20, 95 % CI 1·03, 1·38) among French women( Reference Cottet, Touvier and Fournier 4 ). Only two studies have found that a ‘pork, processed meat and potato’ dietary pattern (relative risk = 0·69, 95 % CI 0·52, 0·92)( Reference Mannisto, Dixon and Balder 6 ) and an ‘animal products’ pattern (OR = 0·74, 95 % CI 0·61, 0·91)( Reference Edefonti, Decarli and La Vecchia 15 ) have negative associations with breast cancer risk, while other cohort studies have failed to show any significant relationship( Reference Adebamowo, Hu and Cho 7 , Reference Velie, Schairer and Flood 10 – Reference Agurs-Collins, Rosenberg and Makambi 13 , Reference Sant, Allemani and Sieri 16 ). Several biological mechanisms may explain the positive association between unhealthy dietary patterns and breast cancer risk. Processed meat is a source of carcinogens such as heterocyclic amines, N-nitroso compounds and polycyclic aromatic hydrocarbons that increase mammary tumours in animal models and are hypothesized to increase breast cancer risk in human subjects( Reference Zhang, Ho and Fu 3 ). In addition, heterocyclic amines and polycyclic aromatic hydrocarbons are not restricted to processed meats; these are largely formed in unprocessed meats by high-temperature cooking.
To our knowledge, the present study is the first one in a Middle-Eastern country to report the association between major dietary patterns and breast cancer risk. The study has several strengths; the first one being the high participation rate, as 100 % of cases and 94·6 % of controls who were initially invited to participate in the research were retained in the final analyses. In addition, using a valid and reliable FFQ for evaluating the dietary intakes increased the data quality( Reference Mirmiran, Esfahani and Mehrabi 20 ). One other strength is that the study was conducted in a province with a very high point prevalence of breast cancer where the risk factors for this malignancy are not yet fully known. Findings from the present research could potentially be used in designing interventional strategies targeting dietary intake modifications in order to decrease the breast cancer risk. Studies in developing countries can provide unique opportunities to test the association between dietary patterns and cancer risk( Reference Hajizadeh, Rashidkhani and Rad 26 ). Generally, where economic resources are severely restricted, food intake is strongly linked to income, so that even small economic differences are directly reflected in dietary intakes. This linkage would tend to increase the between-person variation. Furthermore, in developing countries, socio-economic backgrounds are quite different from those in the Western world (mainly the status of women, parity, autonomy, work participation, family size and access to the health-care system), which might influence the relationship between dietary patterns and disease outcomes observed in these countries.
Before the implications of the present study are discussed, it is necessary to consider potential limitations. First, since dietary intakes were assessed using an FFQ, measurement errors were inevitable, which may have led to underestimation of some associations. However, we compensated for this limitation by using a validated FFQ and excluding over- and under-reporters of energy intake. Second, controls were selected from patients with other diseases (hospital-based case–control design), which is a weakness since their exposure may not be representative of that in members of the study population who are at risk of becoming cases. Third, we cannot entirely rule out the likelihood of residual confounding due to imprecise measurement of important covariates. Being a case–control study, recall bias was also inevitable as patients may recall their diets differently after a cancer diagnosis and there is the possibility that cases who are aware of any possible associations between diet and breast cancer recall exposure more than non-cases. However, we tried to reduce the recall bias through recruiting hospital controls whose medical conditions were unrelated to diet or other major risk factors of breast cancer. In addition, incident cases were registered at a median of 2·5 months after diagnosis and all FFQ were administered by trained dietitians to reduce the recall bias. Moreover, since participants were selected from a population that was readily accessible and convenient through a non-probability sampling strategy, findings may not be generalizable to the entire population. However, recruiting hospital controls using the same convenient sampling strategy is likely to have reduced the impact of non-random sampling. Another limitation of the current study is the presence of missing values for several variables, which may have influenced the results in the multivariate analyses. In addition, our study had mainly the character of a pilot hypothesis-generating study, which allows a further search for breast cancer risk factors. Another limitation stems from the several subjective or arbitrary decisions in the use of factor analysis as the investigator is forced to specify the number of factors. Although eigenvalues, scree plots and interpretability are used to guide the investigator in determining the best factor solution, ultimately such a decision is subjective( Reference Rezazadeh, Rashidkhani and Omidvar 24 ).
Overall, some of the differences in the association of dietary patterns and breast cancer risk reported in previous studies may be related to participants’ characteristics. Nevertheless, evaluation of the diet–cancer relationship is complex and requires stratification by multiple characteristics.
Conclusion
Our findings suggest that a ‘healthy’ dietary pattern, characterized by high consumption of vegetables, fruits, low-fat dairy products, legumes, olive and vegetable oils, fish, condiments, organ meat, poultry, pickles, soya and whole grains, may protect against risk of breast cancer, while an ‘unhealthy’ dietary pattern with high consumption of soft drinks, sugars, tea and coffee, French fries and potato chips, salt, sweets and desserts, hydrogenated fats, nuts, industrial juice, refined grains, and red and processed meat might be associated with higher risk of breast cancer. However, these findings await replication in large-scale longitudinal studies to control for potential biases associated with small sample size and wide confidence intervals in the present research.
Acknowledgements
Sources of funding: This study was funded by the National Nutrition and Food Technology Research Institute, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Conflicts of interest: The authors have no conflicts of interest. Authors’ contributions: Z.K. and B.R. designed the study. A.H. and H.-R.M. helped in data collection. M.J. revised the manuscript, provided input and consultation, and finalized the data and manuscript. Acknowledgements: The authors wish to thank all of the women who participated in the current study.
Food groupings used in the dietary pattern analyses







