Gastro-oesophageal reflux occurs when gastric contents flow back into the oesophagus. Gastro-oesophageal reflux disease (GERD) is defined when reflux causes troublesome symptoms and/or complications(Reference Vakil, van Zanten and Kahrilas1). Common symptoms of GERD include heartburn, a burning sensation in the retrosternal area, and regurgitation, a perception of flow of refluxed gastric contents into the mouth or hypopharynx, together known as the typical reflux syndrome(Reference Vakil, van Zanten and Kahrilas1). Reflux symptoms occurring 2 or more days/week or severe symptoms 1 or more day/week are usually considered troublesome(Reference Vakil, van Zanten and Kahrilas1). Gastro-oesophageal reflux can also have extraoesophageal manifestations such as dental erosion, laryngitis and asthma(Reference Vakil2). If left untreated, GERD is associated with clinically significant impairment in physical and psychosocial functioning(Reference Revicki, Wood and Maton3,Reference Ronkainen, Aro and Storskrubb4) and predisposes individuals to complications such as Barrett’s oesophagus and adenocarcinoma of the oesophagus(Reference Coleman, Xie and Lagergren5).
A global pooled prevalence study estimated that 14 % of the population experienced at least weekly reflux symptoms(Reference Eusebi, Ratnakumaran and Yuan6). Proton pump inhibitors, a class of drug commonly prescribed for managing GERD symptoms, have been among the top five most subsidised prescription drugs in Australia for at least the last decade(7). In the USA, GERD costs approximately $15–20 billion dollars per year, arising from both direct and indirect costs such as work absenteeism and loss of productivity(Reference Shaheen, Hansen and Morgan8).
Since the 1970s, there has been a sharp increase in incidence of oesophageal adenocarcinoma in Western populations(Reference Coleman, Xie and Lagergren5,Reference Napier, Scheerer and Misra9,Reference Schneider and Corley10) . This warrants further study of its modifiable risk factors, including GERD. In contrast to the extensive evidence on dietary triggers of GERD symptoms, evidence on the role of diet in developing GERD is scant. To our knowledge, only one cohort study, from the Swedish Twin Registry, has examined dietary factors(Reference Zheng, Nordenstedt and Pedersen11). None of the food items examined, including vegetables, fruits, fish, meat, rice, flour-based foods, milk, sandwiches, potatoes and grilled and fried food, was associated with the risk of developing GERD.
We conducted a study of the incidence of GERD within the Melbourne Collaborative Cohort Study. Here, we report on diet in relation to the risk of GERD.
Methods
The Melbourne Collaborative Cohort Study
Between 1990 and 1994, 41 513 participants (59 % women) aged 40–69 years were recruited(Reference Milne, Fletcher and MacInnis12). Italian- and Greek-born participants were targeted to extend the range of dietary and lifestyle exposures. This analysis was restricted to participants aged 40–59 years at baseline who had no history of cancer (except keratinocyte skin cancers), diabetes mellitus or CVD (including heart attack, stroke and angina) at baseline (Fig. 1). Participants whose total energy intake was in the top or bottom 1 % of total energy intake, or who had missing data for identified confounders, were ineligible, as were Greek-born participants due to their low participation in the wave of follow-up when information on GERD was obtained. A post hoc exclusion was applied to those whose GERD symptoms began before or within 2 years after baseline. This exclusion was based on time of symptom onset reported at follow-up, as no information on GERD symptoms was collected at baseline.

Fig. 1 Participants flow diagram. MCCS, Melbourne Collaborative Cohort Study; GERD, gastro-oesophageal reflux disease; F, female
Measurement of baseline diet and characteristics
Demographic and lifestyle information was collected via structured interviews at baseline. Baseline characteristics of interest include age, sex, country of birth (Southern Europe or others), socio-economic position, educational attainment (primary or less, some high school, completed high/technical school or completed degree or diploma), smoking status (never, former or current), alcohol consumption (lifelong abstinence, former or current <20, 20–39 or ≥40 g/d), leisure-time physical activity (a relative physical activity score based on frequency of vigorous exercise, moderate exercise and walking per week over the last 6 months) and occupational physical activity (moderate-heavy physical exertion at work or no). Socio-economic position was measured using an area-based measure of relative socio-economic disadvantage (the Index of Relative Socioeconomic Disadvantage from the Socio-economic Indexes for Areas), which was calculated from census data and based on a list of indicators for disadvantage such as proportion of household or resident with low annual income, low skilled occupations or unemployment(13). Anthropometric measurements were collected by trained staff, and BMI was calculated.
Dietary data were collected using a self-administered 121-item FFQ, from which total energy intake (kJ/d) and nutrient intakes (g/d) were calculated. We selected thirty-two dietary variables for analysis based on dietary components, including beverages, that have been cited as triggers of reflux symptoms(Reference Hunt, Armstrong and Katelaris14) or associated with risk of Barrett’s oesophagus or oesophageal adenocarcinoma(Reference Schneider and Corley10). We also examined the Mediterranean Dietary Score (MDS) and the Alternate Heathy Eating Index (AHEI-2010). The MDS was modified from Trichopoulou et al. (Reference Trichopoulou, Costacou and Bamia15). A score of nine indicates the highest degree of adherence; further details are described elsewhere(Reference Hodge, English and Itsiopoulos16). The AHEI-2010 assesses diet based on foods and nutrients that are predictive of chronic disease risk; a higher score predicts lower risk(Reference Chiuve, Fung and Rimm17).
Data on past Helicobacter pylori (H. pylori) infection were available for randomly chosen subsets of participants, 583 with daily GERD symptoms and 588 with symptoms <1 d/week. In 2012 and 2019, H. pylori antibodies were measured in baseline plasma that had been stored in liquid N2, using a commercially available immunoblotting kit (Helicoblot 2.1; Genelabs Diagnostics).
Definition and ascertainment of gastro-oesophageal reflux disease
Information on GERD and use of acid-suppressant medications was collected via computer-assisted telephone interviews between 2007 and 2010. Participants were asked whether they had ever experienced heartburn or regurgitation at least 1 d/week, and if so, the maximum symptom frequency and the time of onset (reported as age at onset, number of years since onset or year of onset). For this analysis, we compared participants with current or past daily heartburn and/or regurgitation (cases) with participants who had symptoms <1 d/week or symptoms only during pregnancy (non-cases). Participants with symptoms 1–6 d/week were excluded from analysis to reduce misclassification of the outcome.
Statistical analyses
Logistic regression was used to estimate the OR and 95 % CI for GERD in relation to the dietary variables. Given that fewer than 10 % of participants had daily GERD symptoms, the OR is a good approximation to the risk ratio(Reference Rothman, Greenland and Lash18). In order to address potential selection bias due to missing outcome data (see online Supplemental Table 1), a weighted analysis was performed using the inverse probability weighting method with se estimated using a robust variance estimator(Reference Seaman and White19). Participants were weighted by the inverse of their probability of providing complete GERD data, which was estimated from a logistic prediction model. Participants who completed follow-up and answered all relevant questions on GERD were defined as having provided complete GERD data (Fig. 1). The prediction model was trained using lasso penalised regression(Reference Tibshirani20) with 60 % of the data from eligible participants, and validated using the remaining 40 %. Age, sex, country of birth, socio-economic index for area, education, smoking status and alcohol consumption were pre-specified to be included in the prediction model, as their distribution was different for participants with and without missing outcome (see online Supplemental Table 1) and was identified as confounders (see online Supplemental Material 1). The Hosmer–Lemeshow test(Reference Seaman and White19) was performed to assess stability of the weights. The prediction model was trained and validated using the statistical software R(21). All other analyses were performed using Stata version 14(22).
Nutrient intakes were energy-adjusted using the residual method(Reference Willett23). For example, the energy-adjusted fat intake is the residuals from a regression of fat intake on total energy intake. Increments reported are based on approximately 1 sd of intake. In cross-sectional studies, only saturated fat intake has been reported to be associated with GERD(Reference El-Serag, Satia and Rabeneck24,Reference Shapiro, Green and Bautista25) . To investigate the potential effects of intake of different types of fat (saturated, monounsaturated, polyunsaturated) on the risk of GERD, we used Willett’s substitution models(Reference Willett23). The first model included energy-adjusted saturated, monounsaturated and polyunsaturated fat and total energy. This assesses whether each type of fat was ‘independently’ associated with risk of GERD; for intake of one type of fat to vary requires isoenergetic substitution by energy sources other than fat. The second model estimated the potential effect of substituting one type of unsaturated fat with saturated fat while holding the other unsaturated fat intake constant. For instance, to examine the effect of substituting monounsaturated fat with saturated fat, the model included total fat intake, total energy intake, total-fat-adjusted saturated fat and total-fat-adjusted polyunsaturated fat intakes, while monounsaturated fat intake was not included and thus allowed to vary.
Food groups and food items were analysed as approximate quintiles of frequency (times/week) using the lowest quintile as the reference group, and tests for linear trend were performed using the median in each quintile. The MDS and AHEI-2010 were analysed as ordinal variables, using the least adherent category as the reference.
Sex-specific analyses were performed for all dietary exposures. Previous studies have reported that the associations between adiposity and risk of GERD differed between men and women(Reference Zheng, Nordenstedt and Pedersen11,Reference Nilsson, Johnsen and Ye26) and that oestrogen might be involved in the pathogenesis of GERD and oesophagitis in obese women(Reference Nilsson, Lundegårdh and Carling27). Given that adiposity is a potential intermediate on the causal pathway for the effect of diet on risk of GERD, the association could also be modified by sex. We further examined whether adiposity modifies the association between diet and risk of GERD. Tests for interaction between dietary variables and waist circumference were performed using the likelihood ratio test.
Confounders
Pre-exposure confounders measured at baseline were identified from the literature and included in a causal diagram (see online Supplemental Material 1). Sex, country of birth, education, socio-economic index for area, occupational and leisure-time physical activity, cigarette smoking and alcohol consumption were included as categorical variables. Age and total energy intake at baseline were included as continuous variables. Dietary confounders specific to certain analyses were also included (Fig. 2 and Table 3). Pearson correlation coefficients were used to examine correlations between dietary exposures. The maximum correlation between variables simultaneously included in regression models was between total fat and total fibre intake (r = −0·51).

Fig. 2 Estimated OR for nutrient intakes and glycaemic index comparing cases (daily gastro-oesophageal reflux disease (GERD) symptoms) with non-cases (symptoms <1 d/week) by sex. Regression models adjusted for age, total energy intake, education, Socio-economic Indexes for Areas, occupational and leisure physical activity, cigarette smoking, alcohol consumption, country of birth and dietary variables. For the analyses of fat, carbohydrate, protein intake and glycaemic index, the models included total fruit, cruciferous vegetables and leafy vegetables. For the analyses of fibre, the models included total fat
Sensitivity analyses
We performed sensitivity analyses further adjusted for adiposity (measured using waist circumference), asthma or H. pylori infection separately to examine whether results were robust against the three key assumptions shown in the causal diagram (see online Supplemental Material 1). First, the primary analysis assumed adiposity is an intermediate on the causal pathway, instead of a confounder. While it is more likely that diet influences adiposity, it is plausible that in midlife, adiposity affects diet. Second, the primary analysis assumed GERD preceded asthma. There is strong evidence that GERD is an aggravating cofactor that could contribute to asthma development(Reference Vakil, van Zanten and Kahrilas1,Reference Pace, Porro, Granderath, Kamolz and Pointner28) , but the use of asthma medication has also been associated with increased risk of GERD(Reference Crowell, Zayat and Lacy29). Third, the primary analysis assumed prior H. pylori infection did not affect diet at baseline. However, because prior H. pylori infection might influence diet, we adjusted for it in the subset of participants with H. pylori assessment.
In addition, we performed analyses aimed at minimising any reverse causation associated with uncertainty in the date of GERD onset and changed diets at baseline in response to non-specific gastric symptoms: (i) by excluding GERD cases with onset within 5 years from baseline; and (ii) by excluding participants who reported following a special diet at baseline.
An analysis with GERD cases redefined as having symptoms ≥1 d/week was performed in order to compare results with the only other cohort study of diet and risk of GERD, which used this definition(Reference Zheng, Nordenstedt and Pedersen11). Last, a complete case (unweighted) analysis was performed.
Results
Of the 20 926 initially eligible participants, 16 629 (79 %) were interviewed for GERD and 15 597 (75 %) provided complete outcome data (Fig. 1). The follow-up time ranged from 12·7 to 19·9 years, with a median of 15·8 years. Response was similar for men and women (see online Supplemental Table 1). Southern Europe-born participants, those with less educational attainment, those who lived in more socio-economically disadvantaged areas and current smokers at baseline, were less likely to provide complete GERD data (see online Supplemental Table 1). Baseline age, total energy intake, waist circumference, BMI and all dietary exposures were similar for those with and without missing GERD data (see online Supplemental Tables 1, 2).
Of the 15 597 participants with complete data, 1407 who reported onset of GERD symptoms before or within 2 years from baseline were excluded (Fig. 1). Daily symptoms were reported by 309 men and 764 women, whereas symptoms <1 d/week (including symptoms during pregnancy only) were reported by 4331 men and 7091 women (Fig. 1). Daily use of acid-suppressant prescription medication was reported by 60 % of participants who reported daily symptoms, 43 % of participants who reported symptoms 2–6 d/week, 27 % of participants who reported symptoms 1 d/week and 0·06 % of participants who reported symptoms <1 d/week.
Women and participants born in Australia, New Zealand or Northern Europe were more likely to develop daily symptoms than those born in Italy (Table 1). For men, those who had lower educational attainment, were former smokers at baseline, reported light, former or no alcohol consumption, were more physically active for leisure or reported physical exertion at work were more likely to develop daily symptoms. Male cases and non-cases had similar waist circumference and BMI, whereas total energy intake was higher for cases. For women, those who had lower educational attainment, were current smokers at baseline, reported moderate, light, former or no alcohol consumption, were less physically active for leisure or reported physical exertion at work were more likely to develop daily symptoms. Female cases had higher total energy intake, waist circumference and BMI compared with non-cases. The median age at baseline was 48 years for both male and female cases. The median age at symptom onset was 58 years for men and 59 years for women.
Table 1 Baseline characteristics of eligible participants

GERD, gastro-oesophageal reflux disease; SEIFA, socio-economic index for area.
For men, cases had higher intakes of fat, meat and dairy, and lower intakes of carbohydrate, fibre and citrus compared with non-cases (Table 2). For women, cases had higher consumption of tea and lower consumption of citrus and coffee compared with non-cases (Table 2). For both men and women, degree of adherence to the MDS and AHEI-2010 was similar for cases and non-cases.
Table 2 Diet at baseline for gastro-oesophageal reflux disease (GERD) cases (daily symptoms) and non-cases (symptoms <1 d/week)
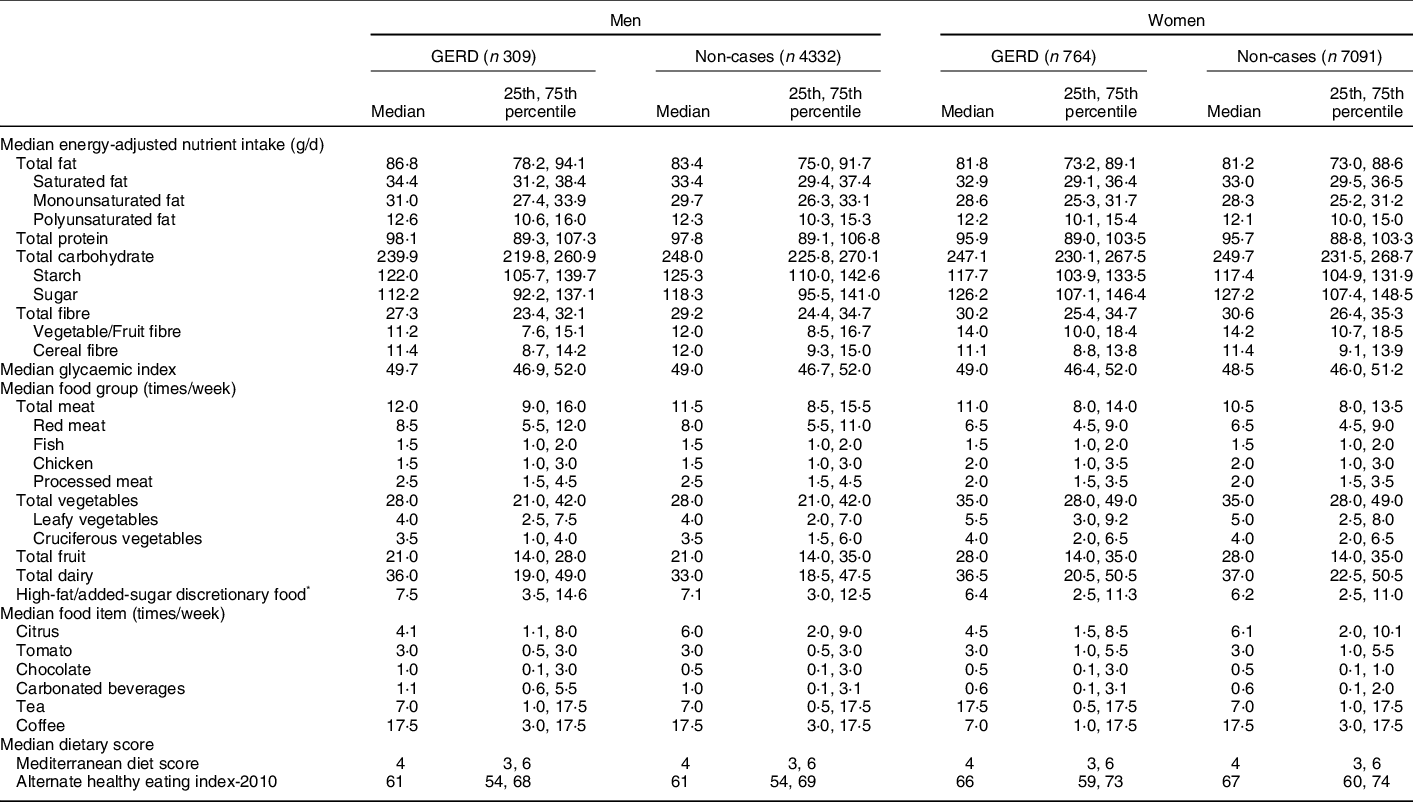
* High-fat added-sugars discretionary food included ice cream, sweet biscuits, cakes or sweet pastries, puddings and chocolate confectionary.
Nutrient intakes
For men, total fat intake was associated with increased risk of GERD (OR 1·05 per 5 g/d; 95 % CI 1·01, 1·09; P = 0·016) and an association was observed for all three types of fat (Fig. 2). Increasing intakes of total carbohydrate (OR 0·89 per 30 g/d; 95 % CI 0·82, 0·98; P = 0·010) and starch (OR 0·84 per 30 g/d; 95 % CI 0·75, 0·94; P = 0·005), but not sugar or glycaemic index, was associated with reduced risk of GERD. It was impossible to separate the associations for fat and carbohydrate intake, as these nutrients were strongly inversely correlated (r = -0·90). Fibre intake was also weakly inversely associated with risk (OR 0·93 per 5 g/d; 95 % CI 0·86, 1·01; P = 0·099). For women, there was little evidence of association between intake of any nutrient and risk of GERD (Fig. 2).
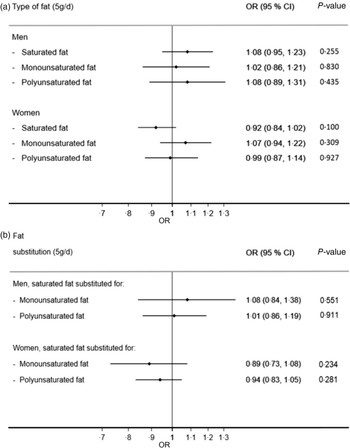
Fat substitutions
For both men and women, there was no evidence that substituting other sources of energy with individual types of fat (saturated fat, monounsaturated fat or polyunsaturated fat) was associated with risk of GERD (Fig. 3a). This is reflected in the fat substitution model, where substituting monounsaturated fat or polyunsaturated fat with saturated fat did not result in change in GERD risk (Fig. 3b).

Fig. 3 Estimated OR comparing cases (daily gastro-oesophageal reflux disease (GERD) symptoms) with non-cases (symptoms <1 d/week) by sex when (a) each type of fat was substituted for other sources of energy and when (b) saturated fat was substituted for monounsaturated or polyunsaturated fat. Estimates are adjusted for non-dietary and dietary confounders as per Figure 2
Food groups, food items and dietary patterns
The results from sex-specific analysis for foods and dietary patterns did not differ substantially from those combining the sexes (see online Supplemental Table 3), and thus combined results are presented here. More frequent consumption of fish (OR 1·09 per time/week; 95 % CI 1·03, 1·15; trend P = 0·002) and chicken (OR 1·08 per time/week; 95 % CI 1·03, 1·14; trend P = 0·002) was associated with increased risk of GERD, whereas total meat, red meat and processed meat intakes were not (Table 3). Total vegetable consumption and leafy vegetable consumption were not associated with GERD, whereas consumption of cruciferous vegetables was associated with increased risk (OR 1·04 per time/week; 95 % CI 1·02, 1·06; trend P < 0·001). For total fruit intake, reduced risk was observed across all quintiles compared with the lowest quintile of consumption frequency (Q5 v. Q1: OR 0·73; 95 % CI 0·58, 0·92; trend P = 0·012). Citrus fruit consumption was associated with reduced risk of GERD (OR 0·97 per time/week; 95 % CI 0·95, 0·98; trend P < 0·001). Carbonated beverages were associated with increased risk (OR 1·06 per time/week, 95 % CI 1·03, 1·08; trend P < 0·001). There was no evidence that consumption of dairy, tea, coffee, chocolate, high-fat added-sugar discretionary food or tomato was associated with the risk of GERD. Neither the MDS nor the AHEI-2010 was associated with risk.
Table 3 OR for food groups, food items and dietary patterns, comparing cases (daily GERD symptoms) with non-cases (symptoms <1 d/week)

MDS, Mediterranean Diet Score; AHEI-2010, Alternate Healthy Eating Index-2010.
* Estimated OR comparing each quintile of consumption frequency with the lowest quintile. Regression models adjusted for age, total energy intake, education, Socio-economic Indexes for Areas, occupational and leisure physical activity, cigarette smoking, alcohol consumption, country of birth and dietary variables. For the analyses of meat, dairy, high-fat added-sugar discretionary food, chocolate, carbonated beverages, tea, coffee and alcohol intake, the models included total fruit, cruciferous vegetables and leafy vegetables. For the analyses of vegetable and fruit intake, the models included total fat.
Dietary factors and adiposity
There was no evidence that the association between dietary factors and risk of GERD was modified by waist circumference (see online Supplemental Table 4).
Sensitivity analyses
Further adjustment for waist circumference or asthma had no influence on the results (see online Supplemental Tables 5 and 6). In the subset of participants with H. pylori measurements, adjustment for H. pylori status also had no influence (see online Supplemental Table 7). Excluding participants who reported GERD onset within 5 years from baseline did not change the study results (see online Supplemental Table 8). When analysis was restricted to people who reported not following a special diet at baseline, the association for fish, chicken and total fruit was attenuated (see online Supplemental Table 9). Results from the complete case (unweighted) analysis (see online Supplemental Table 10) did not differ from the analysis using the inverse probability weighting method, suggesting that missing outcome data did not have a material effect on the results. When the case definition was redefined as symptoms ≥1 d/week, all observed associations, except for chicken, weakened; the greatest attenuation was observed for fat and carbohydrate intake in men, although starch and cereal fibre remained inversely associated with GERD (see online Supplemental Table 11).
Discussion
For men, fat intake was associated with increased GERD risk, while carbohydrate intake, specifically intake of starch, was associated with reduced risk. The strong negative correlation between fat and carbohydrate intakes did not allow attributing the association to either macronutrient. No nutrient was associated with risk for women. For both sexes, substituting monounsaturated or polyunsaturated fat with saturated fat did not change GERD risk. For both sexes combined, higher frequencies of consuming fish, chicken, cruciferous vegetables and carbonated beverages were associated with increased risk of GERD, while total fruit and citrus were inversely associated with risk. There was no evidence of association with MDS or AHEI-2010. There was no evidence of interaction between dietary factors and adiposity.
Comprehensive data on demographic and lifestyle factors allowed adjustment for pre-exposure confounders, although residual confounding due to unmeasured or poorly measured variables is possible. Complete data on GERD were missing for 25 % of eligible participants, but the results from inverse probability weighted and unweighted analyses were similar. The inverse probability weighting method assumes data were missing at random (i.e. the probability that data are missing depends only on observed data). We cannot rule out that there might be unobserved variables that are predictive of missingness. Although the assumption is unverifiable, effort was made to include key variables in the prediction model. While our study was not strictly prospective because information on GERD was obtained after baseline, exclusion of participants whose symptoms began before or within 5 years after measurement of diet suggested that reverse causality due to pre-baseline GERD, a feature of cross-sectional studies, is unlikely to explain the associations.
The large sample size permitted estimation of sex-specific associations and allowed us to contrast the maximum (daily) and minimum (<1 d/week) symptom frequency to improve the specificity of our outcome definition. Cross-checking with daily use of acid-suppressant medication (60 % for cases and 0·06 % for non-cases) demonstrated that the degree of contamination between cases and non-cases was likely to be minimal. The only other cohort study of diet and risk of GERD(Reference Zheng, Nordenstedt and Pedersen11) compared symptoms 1 or more days per week with less frequent symptoms and found no association for any dietary exposures, possibly due to misclassification of the outcome. In our sensitivity analysis in which the case definition was relaxed to symptoms on ≥1 d/week, the observed associations were attenuated.
A limitation of the study is the overlap in symptoms between GERD and other gastrointestinal conditions(Reference de Bortoli, Tolone and Frazzoni30), which could explain some of the associations observed. For example, functional dyspepsia is characterised by both non-GERD-related (e.g. fullness and bloating) and GERD-related symptoms(Reference Feinle-Bisset and Azpiroz31). Red meat has been associated with symptoms of fullness but not with other dyspeptic symptoms(Reference Filipović, Randjelovic and Kovacevic32,Reference Carvalho, Lorena and de Souza Almeida33) and was avoided by patients in a case–control study of functional dyspepsia(Reference Carvalho, Lorena and de Souza Almeida33). It is plausible that these people have higher chicken and seafood consumption. Eosinophilic oesophagitis could also exhibit GERD-related symptoms, and dietary interventions such as the six-food elimination diet (milk, eggs, nuts, seafood, soy and wheat) have shown to be effective for disease remission(Reference Arias, González-Cervera and Tenias34). In our study, no evidence for association was observed for total, red and processed meat, whereas fish and chicken were associated with increased risk of GERD. These associations were attenuated after excluding participants who reported following a special diet at baseline. Symptom overlaps between GERD and irritable bowel syndrome could partly explain the association observed for cruciferous vegetables. An estimated 5–25 % of people worldwide suffer from irritable bowel syndrome, which overlaps with GERD in 5–30 % of community-based individuals(Reference de Bortoli, Tolone and Frazzoni30). Some cruciferous vegetables contain high levels of Fermentable Oligosaccharides, Disaccharides, Monosaccharides and Polyols, which are known to trigger irritable bowel syndrome(Reference Muir, Rose and Rosella35).
Our findings are applicable to populations with similar Western-style diets to participants of the Melbourne Collaborative Cohort Study. Findings may be less applicable to populations where the Western-style diet is not as common, although diet is becoming more ‘westernized’ globally. We examined the associations in middle-age people, which might limit generalisability to other age groups.
Sex-specific associations
Women (5·9 %) were more likely to develop daily symptoms than men (3·9 %); however, nutrient intakes showed no associations for women. A proposed mechanism for GERD involves increased episodes of inappropriate transient relaxation of the lower oesophageal sphincter (LES) accompanied by reflux of gastric contents(Reference Mikami and Murayama36). Oestrogen might contribute to reflux symptoms by lowering the LES tone and inhibiting gastric emptying(Reference Fisher, Roberts and Grabowski37–Reference Hutson, Roehrkasse and Wald39). Thus, while increased fat or decreased starch intake might have contributed to the pathogenesis of GERD in men, it might not contribute to the already elevated risk for women. Effect modification by sex might explain conflicting findings on the effect of fat on the LES. A trial with ten healthy men found LES pressure was lower after ingestion of a high-fat meal(Reference Nebel and Castell40), whereas two other small trials with approximately equal numbers of men and women found no significant difference in LES pressure after fat ingestion(Reference Penagini, Mangano and Bianchi41,Reference Pehl, Waizenhoefer and Wendl42) .
Types of fat intake
All types of fat intake were associated with risk of GERD for men, whereas in cross-sectional studies, only saturated fat intake was associated with reflux symptoms(Reference El-Serag, Satia and Rabeneck24,Reference Shapiro, Green and Bautista25) . This suggests that while saturated fat in particular may trigger symptoms in established GERD, any type of fat could contribute to the development of GERD in men.
Diet as trigger v. risk factor
Our findings highlight that food commonly considered as triggers of GERD symptoms might not necessarily contribute to disease development. Citrus, tomato and coffee are considered triggers of symptoms(Reference Sethi and Richter43,Reference Feldman and Barnett44) . In our study, tomato and coffee were not associated with the risk of developing GERD, while citrus had an inverse association. A trial that investigated heartburn symptoms following infusion of orange juice, tomato juice and coffee demonstrated that patients experienced symptoms even when the acidity of the solution was altered to neutral (pH 7), suggesting that the acidity of these items was not a cause of symptoms(Reference Price, Smithson and Castell45).
Carbonated beverages were associated with increased risk of GERD and are a commonly cited trigger for GERD symptoms(Reference Song, Chung and Lee46,Reference Fass, Quan and O’Connor47) . This could be explained by the carbon dioxide present in these beverages, which has been linked to increased belching and inappropriate LES relaxation(Reference Hamoui, Lord and Hagen48). It was unclear whether these beverages were caffeinated. However, given the lack of association observed for coffee, it is unlikely that caffeine would explain the association observed for carbonated beverages.
Dietary patterns
We found no evidence of association between the MDS or the AHEI-2010 and risk of GERD. A cross-sectional study in a south-eastern European population found an inverse association between Mediterranean diet and the presence of GERD(Reference Mone, Kraja and Bregu49). The lack of association in our study could be due to the contrasting association between components of the dietary scores and GERD, which offset each other. For instance, fish was associated with increased risk of GERD, but is a ‘beneficial’ component in the MDS, whereas dairy was associated with reduced risk of GERD but is considered a ‘harmful’ component in the MDS(Reference Trichopoulou, Costacou and Bamia15).
Conclusion
In conclusion, our prospective analysis suggests that some dietary components are possible risk factors for GERD, but diets conventionally considered healthy appear unlikely to reduce its risk. Food commonly considered as triggers of GERD symptoms might not necessarily contribute to the development of the disease. The potential differential associations for men and women warrant further investigation.
Acknowledgements
Acknowledgements: We thank David Whiteman for assistance with developing the questionnaire. Financial support: The Melbourne Collaborative Cohort Study (MCCS) cohort recruitment was funded by VicHealth and Cancer Council Victoria. The MCCS was further augmented by Australian National Health and Medical Research Council grants 209057, 396414 and 1074383 and by infrastructure provided by Cancer Council Victoria. Cancer cases and vital status of participants were ascertained through the Victorian Cancer Registry and the Australian Institute of Health and Welfare, including the National Death Index and the Australian Cancer Database. The study of gastro-oesophageal reflux was funded by NHMRC project grant (504708). S.W. is supported by an Australian Government Research Training Program Scholarship. Conflict of interest: The authors have no conflict of interest to declare. Authorship: S.E.W., A.M.H. and S.G.D. were involved in research question formulation, data analysis, results interpretation and article revision; S.C.D.-S. and B.J.K. were involved in results interpretation and article revision; H.M. was involved in data acquisition; R.J.S.T., A.B. and A.M.H. were involved in obtaining financial support and article revision; E.M.W. and R.L.M. revised the article; E.M. was involved in data analysis and article revision; G.G.G. was involved in obtaining financial support and article revision; D.R.E. was involved in research question formulation, financial support and data acquisition, data analysis, results interpretation and article revision. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving study participants were approved by the Human Research Ethics Committee at Cancer Council Victoria. Written informed consent was obtained from all participants.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980021000197