Elevated plasma homocysteine is an independent risk factor for CVD(1,Reference Wald, Morris and Wald2) and has also been associated with vascular-related complications in pregnancy(Reference Vollset, Refsum and Irgens3– Reference Dodds, Fell and Dooley 5 ). These include pre-eclampsia, small for gestational age and foetal demise(Reference Bergen, Jaddoe and Timmermans4– Reference Hogeveen, Blom and den Heijer 7 ).
In studies of the general population and pregnant women, folate intake and serum folate were identified as major nutritional determinants of homocysteine apart from genetic, health and lifestyle factors(Reference Bergen, Jaddoe and Timmermans4,Reference Dodds, Fell and Dooley5,Reference Refsum, Nurk and Smith8,Reference Jacques, Bostom and Wilson9) . Clinical trials have been inconclusive in determining the effectiveness of folic acid supplementation to decrease the risk of vascular conditions, as a consequence of decreased homocysteine concentration(Reference Clarke, Halsey and Lewington10– Reference Sayyah-melli, Ghorbanihaghjo and Alizadeh 12 ). This may be due to the relatively short duration of these trials(Reference Wald, Morris and Wald2,Reference Clarke, Halsey and Lewington10,Reference Holmes, Newcombe and Hubacek13,Reference Clarke, Bennett and Parish14) and due to the post-folic acid fortification era being characterised by folate-replete populations(Reference Shere, Kapur and Koren15).
Homocysteine is an intermediate metabolite formed during the metabolism of the essential amino acid methionine in two main metabolic pathways: trans-sulfuration, in which vitamin B6 acts as an enzyme co-factor, and remethylation, which depends on adequate serum folate and vitamin B12 as an enzyme co-factor(Reference Dasarathy, Gruca and Bennett16,Reference Blom and Smulders17) . Remethylation also requires a methyl group donated by 5-methyl tetrahydrofolate, a form of folate (vitamin B9) that is formed from a reaction involving the enzyme methylenetetrahydrofolate reductase (MTHFR)(Reference Salway18). A common SNP substitutes a T for a C nucleotide at position 677 of the gene encoding the MTHFR enzyme. The MTHFR 677C>T polymorphism produces an enzyme that is thermolabile—with enzyme activity reduced by half, which can result in moderately elevated homocysteine(Reference Clarke, Bennett and Parish14,Reference Sibani, Leclerc and Weisberg19) .
In pregnancy, homocysteine concentrations decrease as early as the first trimester and are lowest during the second trimester(Reference Murphy, Scott and McPartlin20). Concentrations rise into the third trimester and do not reach pre-pregnancy values until postpartum(Reference Walker, Smith and Perkins21). It is, therefore, essential that any studies investigating homocysteine concentrations during pregnancy account for gestational age at the time of bloodwork. Methods used to account for gestational age-related changes in homocysteine concentration include multivariable regression adjustment for gestational age and dichotomising the outcome relative to percentiles for gestational age at measurement(Reference Bergen, Jaddoe and Timmermans4,Reference Dodds, Fell and Dooley5) .
We aimed to identify factors associated with maternal homocysteine in the early to mid-second trimester of pregnancy, particularly in a folic acid-fortified population. As a secondary objective, we examined the interaction of serum folate and the MTHFR 677C>T polymorphism in determining homocysteine concentration.
Methods
Study design
This study is based on the Ottawa and Kingston (OaK) Birth Cohort, which recruited 8085 participants from 2002 to 2009 at the Ottawa Hospital and Kingston General Hospital in the province of Ontario, Canada. OaK participants were recruited the early second trimester when presenting for prenatal appointments and were followed until delivery. Women were excluded from the analytic dataset of the current study if they were <12 or >20 weeks of gestation, carrying twins or multiples, and if they withdrew, were lost to follow-up or if the pregnancy was terminated.
The OaK Birth Cohort has been described previously(Reference Walker, Finkelstein and Rennicks White22). Briefly, the baseline survey consisted of an interviewer-administered questionnaire on maternal characteristics, bloodwork and chart abstraction. Questionnaire responses were verified from medical records and brief telephone interviews to collect missing information.
Lab investigations
Plasma homocysteine measurement (primary outcome)
The primary outcome of this study was maternal plasma homocysteine concentration in µmol/l. The blood samples obtained for homocysteine measurement were collected in K2EDTA Vacutainer tubes (Becton Dickinson, Lincoln Park, NJ). Samples for homocysteine measurement were immediately placed on ice and within 30 min centrifuged in 4°C at 3000 g for 10 min. Blood plasma was aliquoted and stored at –20°C. Plasma homocysteine (µmol/l) was measured within 1 month in batches on the Abbott AxSYM II Immunoassay System (Abbott Laboratories, Abbott Park, IL) using fluorescence polarisation immunoassay.
Serum folate measurement
Maternal blood samples obtained for folate measurement were collected in serum separator tubes (Becton Dickinson). The sample was left to clot and then centrifuged at 3000 g for 10 min. Serum was aliquoted and stored at –20°C. Serum folate (nmol/l) was measured within 1 month in batches on the Access 2 and UniCel® DxI 800 Immunoassay Systems using manufacturer’s reagents (Beckman Coulter, Brea, CA).
DNA extraction and MTHFR genotyping
The blood samples obtained for MTHFR 677C>T genotyping (in a subset of participants due to logistics) were collected in K2EDTA Vacutainer tubes (Becton Dickinson). DNA was extracted by manual extraction and later switched to automated extraction. In manual extraction, blood samples were centrifuged at 2500 g for 10 min and DNA extracted from the buffy coat using the FlexiGene DNA Kit (QIAGEN, Hilden, Germany). In automated extraction, blood samples were centrifuged at 1100 g and DNA extracted using the BioRobot M48 and MagAttract DNA Blood Midi Kit (QIAGEN). The MTHFR gene segment was amplified using PCR and genotyped using the ABI 3130xl Genetic Analyser and the ABI PRISM SNaPshot Multiplex Kit (Applied Biosystems, Waltham, MA).
Determinants of interest
Factors of interest and interactions were prespecified from a literature review of studies on homocysteine determinants(Reference Bergen, Jaddoe and Timmermans4,Reference Dodds, Fell and Dooley5,Reference Refsum, Nurk and Smith8,Reference Jacques, Bostom and Wilson9,Reference Selhub, Jacques and Rosenberg23) and from studies investigating the role of folates and homocysteine in placenta-mediated (i.e. vascular-related) pregnancy complications(Reference Bergen, Jaddoe and Timmermans4,Reference Dodds, Fell and Dooley5,Reference Refsum, Nurk and Smith8,Reference Kahn, Platt and McNamara24) . We considered the following factors: gestational age at bloodwork (abstracted from medical records), maternal age (years), race (based on self-reported ethnicity in terms of race/origin/ancestry), education (highest completed level), household income, parity, smoking during pregnancy, BMI (kg/m2, from measured height and weight), diabetes (type 1 or 2), use of hormonal birth control prior to conception (i.e., oral, injection, IUD), chronic hypertension, history of a placenta-mediated pregnancy complication (i.e., small for gestational age, pre-eclampsia, placental abruption or pregnancy loss), folic acid supplementation and dose (from current prenatal vitamin brand, multivitamin brand and folic acid supplement), serum folate and the MTHFR 677C>T genotype.
Statistical analyses
Multivariable regression
We conducted multivariable linear regression analyses to examine the association of the identified factors of interest with homocysteine concentration while adjusting for gestational age at bloodwork as a continuous variable. Prior to analysis, a variable clustering algorithm was used to rule out multicollinearity among the prespecified variables of interest. Analyses were performed in RStudio version 0.99.892, R version 3.2.3.
Missing data were handled by multiple imputation using the package mice, which stands for multivariate imputation by chained equations(Reference van Buuren and Groothuis-oudshoorn25). In this approach, missing values are replaced by random draws of predicted values from a series of sequential multivariable models specified according to the type of incomplete variable: predictive mean matching for continuous variables, logistic regression for binary variables, multinomial logit model for categorical variables, and ordered logit model for ordinal variables. The number of imputations was set to 10, with 200 iterations. Ten to twenty imputations are considered adequate for this imputation method.(Reference van Buuren and Groothuis-oudshoorn25)
The regression modelling strategies (rms) package within R was used for multivariable regression analyses(Reference Harrell26). Continuous variables were modelled with a restricted cubic spline function. Knots were set by default to the following quantiles: three knots at the 10th, 50th and 90th quantile, four knots at the 5th, 35th, 65th and 95th quantile, and five knots at the 5th, 27·5th, 50th, 72·5th and 95th quantile. To build the multivariable model, we first entered all prespecified variables into the model(Reference Harrell27). Continuous variables were modelled with five knots, and categorical and ordinal variables retained their original categories. Next, Akaike’s Information Criterion (AIC) and the Bayesian Information Criterion (BIC) were used to examine both the goodness of fit of the multivariable model by altering the number of knots for continuous variables, as well as the effect of collapsing categories in categorical variables. AIC and BIC penalise the log likelihood for complexity of the model (i.e., number of parameters) with the aim to avoid over-specifying the model(Reference Harrell27). The final model was then refitted according to the lowest AIC and BIC values for each variable. Rubin’s method was used to combine the results across the ten imputed datasets(Reference van Buuren and Groothuis-oudshoorn25,Reference Harrell27,Reference Harrell28) .
Results are presented using regression coefficients (representing mean differences) with 95 % CIs. For continuous factors modelled with restricted cubic splines, effect estimates (mean differences) are presented for the 75th v. 25th percentiles. Plots of modelled associations were generated to interpret the effects of continuous factors analysed as a restricted cubic spline. Model fit was assessed by plotting residuals against fitted values. Normality was assessed by visual inspection of normal probability plots.
Subgroup analysis
The multivariable analysis was repeated in the subgroup of participants with measured MTHFR 677C>T genotype. These participants had not self-selected into the study and were, therefore, expected to be representative of the entire cohort. The subgroup analysis examined the interaction of serum folate and the MTHFR 677C>T genotype in determining homocysteine concentration. We confirmed Hardy–Weinberg equilibrium of the MTHFR 677C>T genotype(Reference Graffelman29).
Secondary analyses
In addition to the primary analysis adjusting for gestational age at bloodwork as a continuous covariate, we conducted two secondary analyses to examine alternative methods of accounting for gestational age at bloodwork (see online supplementary material). The first method used continuous normalised score (Z-scores) as dependent variable. Z-scores were calculated for each participant by subtracting the mean and dividing by the sd of homocysteine concentration of all participants with the same gestational week at the time of bloodwork. The second method used a dichotomous outcome, with participants classified as having homocysteine concentration greater than the 90th percentile at each gestational week at the time of bloodwork. Similar cut-offs for elevated homocysteine have been used in studies investigating the effect of elevated homocysteine concentration on pregnancy outcomes(Reference Dodds, Fell and Dooley5,Reference Sorensen, Malinow and Williams30– Reference Onalan, Onalan and Gunenc 32 ). The multivariable analysis was repeated for each version of the outcome. Model building followed the same procedures as described for the primary approach.
Results
Participant characteristics
We analysed data of 7587 participants from the OaK Birth Cohort (Fig. 1). Table 1 presents the characteristics of the studied population in terms of demographic characteristics, health indicators and factors associated with homocysteine metabolism. The majority of participants were non-smokers during pregnancy, reported taking folic acid-containing supplements at the time of recruitment, had a normal to overweight BMI and were normotensive and non-diabetic. Most participants were recruited around 12–13 weeks gestation and had a mean homocysteine concentration of 4·8 µmol/l (sd 1·3), with measured values ranging from 1 to 34 µmol/l. The MTHFR 677C>T genotype frequencies were not significantly different from Hardy–Weinberg equilibrium(Reference Graffelman29). Around 12% of participants were homozygous for the TT mutant genotype.

Fig. 1 Participant flow diagram for the analytic dataset
Table 1 Participant characteristics and determinants of interest in the OaK Birth Cohort (n 7587), 2002–2009

* Measured in a subset of participants (n 4006).
Folic acid supplementation dose was categorised as 0–1 or >1 mg. We found a linear relation between serum folate and reported dosage up to approximately 1 mg, corresponding to a serum folate concentration of 45 nmol/l, above which serum folate was less responsive to increasing folic acid dose (Fig. 2). The serum folate distribution was wide and skewed; to normalise the distribution, a ceiling was set to the 90th percentile, ranging from 76 nmol/l at 12 weeks gestation to 48 nmol/l at 20 weeks. Although a serum folate deficiency cut-off in pregnant women has not been established, the WHO sets the cut-off for serum folate deficiency based on homocysteine concentration as a metabolic indicator at 10 nmol/l(33). We, therefore, considered the 10 % of values beyond the 90th percentile cut-points as equally high and differentiating between these high values as unnecessary.
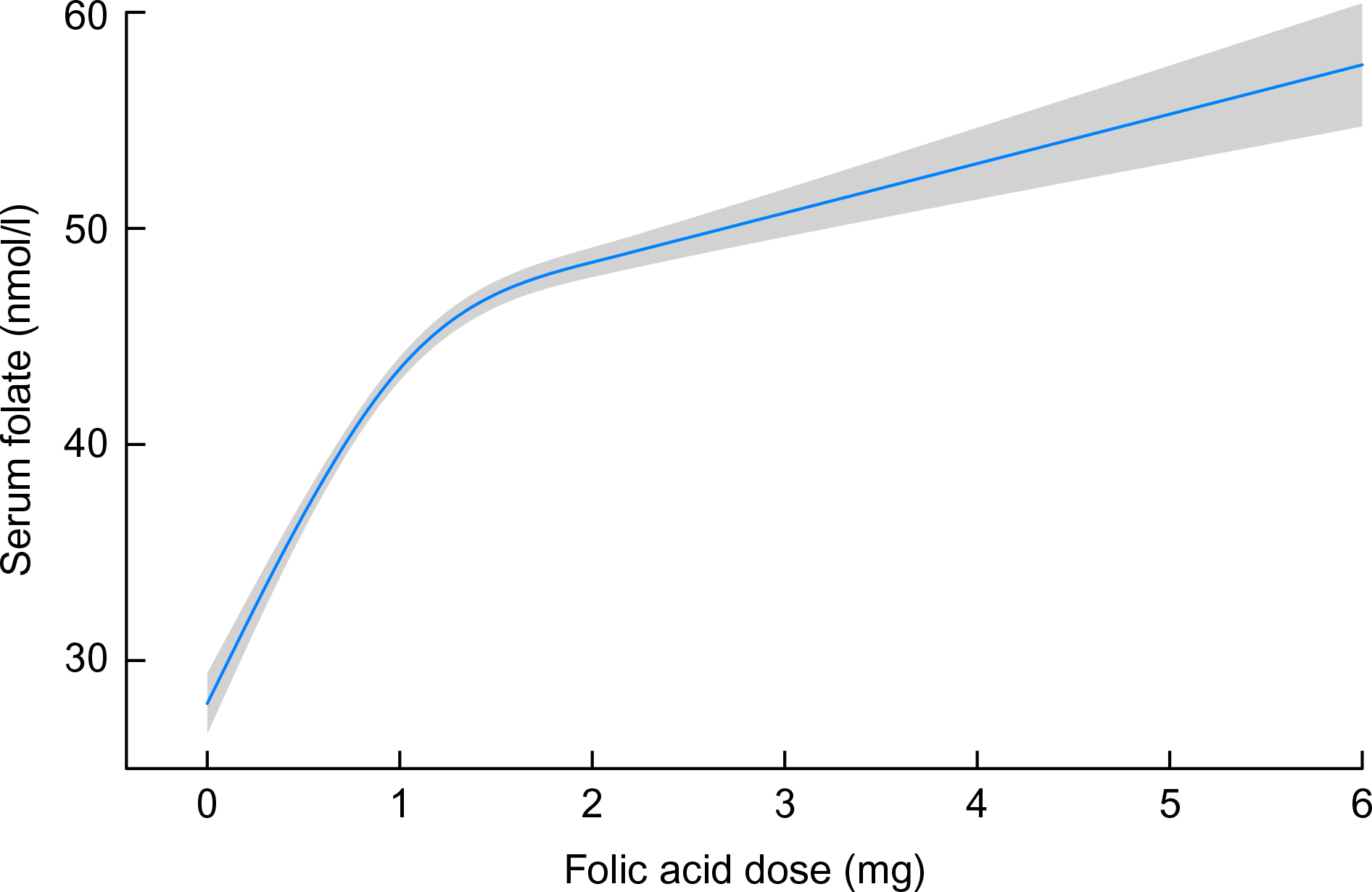
Fig. 2 Modelled association between folic acid supplementation (restricted cubic spline with five knots) and serum folate, adjusting for gestational age at bloodwork and homocysteine. Shaded area represents 95 % CI
Determinants of homocysteine
Multivariable regression analysis
A variable clustering algorithm revealed that maternal education and household income were highly correlated; income was, therefore, dropped from the analysis because education was more strongly associated with homocysteine. The results for the primary multivariable regression analysis are presented in Table 2. All factors were significantly associated with plasma homocysteine concentration, except for maternal education, diabetes and hormonal birth control prior to conception (Table 2). Factors significantly associated with a higher homocysteine concentration were nulliparity, smoking during pregnancy (including exposure to second-hand smoke) and chronic hypertension. Factors significantly associated with lower homocysteine concentration were non-Caucasian race, history of a placenta-mediated (i.e., vascular-related) pregnancy complication and folic acid supplementation (Table 2). Inspection of residuals revealed a symmetrical distribution with two obvious outliers. These outliers were retained in the analysis because excluding them revealed no major differences in Wald tests of most meaningful hypotheses’ P values and the error terms (results not shown).
Table 2 Multivariable linear regression analysis of the determinants of plasma homocysteine, with plasma homocysteine as a continuous dependent variable (n 7587)
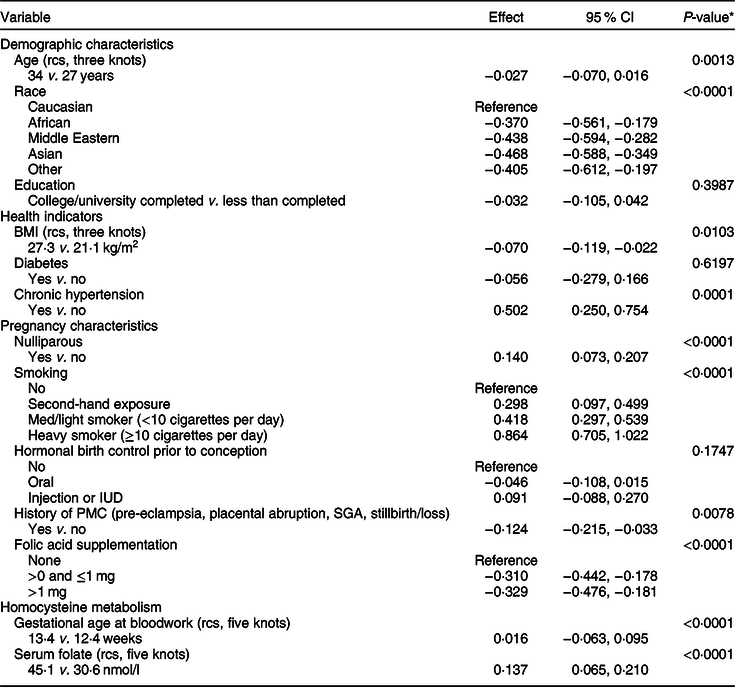
rcs, restricted cubic spline; PMC, placenta-mediated complication.
* Wald test of most meaningful hypotheses, pooled across multiple imputation datasets.
Figure 3 shows plots of associations for continuous factors modelled using a restricted cubic spline function. For gestational age (five knots), the plot of association demonstrated a peak in homocysteine concentration close to 13 weeks gestation and a decrease thereafter. Plots of modelled association for maternal age and BMI (three knots) demonstrated a U-shaped pattern of increasing homocysteine concentration. For serum folate (five knots), the plot of modelled association demonstrated higher homocysteine concentration (i.e., a negative slope) at serum folate concentrations below approximately 30 nmol/l (Fig. 3).

Fig. 3 Association between homocysteine and continuous variables modelled as a restricted cubic spline. (a) Gestational age at bloodwork with five knots at 12·1, 12·4, 12·8, 13·4 and 19; (b) maternal age with three knots at 24, 30 and 37; (c) BMI with three knots at 19·6, 23·5 and 32·2; and (d) serum folate with five knots at 20·7, 32·3, 39·4, 45 and 74·1. Shaded area represents 95 % CI
Subgroup analysis
The subgroup analysis examining the interaction between MTHFR 677C>T genotype and serum folate is presented in Supplemental Table S1. In a subset of 4006 OaK participants with measured genotype, the interaction between MTHFR 677C>T genotype and serum folate was significant (P < 0·0001). For the CC/CT genotypes, there was no association between serum folate and plasma homocysteine, whereas for the TT genotype, the association with homocysteine was a steep negative slope, which levelled off beyond a folate concentration of approximately 30 nmol/l (Fig. 4).

Fig. 4 Modelled association between serum folate (restricted cubic spline) and homocysteine by MTHFR 677C>T genotype. Panels by MTHFR genotype, CC (wild-type) or CT (heterozygous) and TT (homozygous mutant). Folate variable has five knots at 20·7, 32·3, 39·4, 45 and 74·1. Shaded area represents 95 % CI
Folic acid supplementation was not associated with homocysteine in the subgroup analysis (see online supplementary material, Supplemental Table S1). As well, African race (compared to Caucasian) and exposure to second-hand smoke were no longer associated with homocysteine concentration. Plots of association of gestational age at bloodwork, maternal age and BMI modelled using restricted cubic spline functions all demonstrated similar patterns of association with homocysteine concentration, as in the primary multivariable analysis (see online supplementary material, Supplemental Fig. S1).
Secondary analyses
In the analysis with the dependent variable specified as a continuous Z-score (calculated from the mean homocysteine concentration and sd for each gestational week of recruitment), results were similar to those in the primary analysis (see online supplementary material, Supplemental Table S2). In the analysis with the dependent variable specified as a dichotomous variable (homocysteine concentration greater than the 90th percentile for each gestational week of recruitment), there were some differences in results, presented as ORs and 95 % CIs in Supplemental Table S3. Maternal age and history of a placenta-mediated pregnancy complication were no longer associated with homocysteine concentration. Gestational age at bloodwork was significant despite gestational week at bloodwork being factored into the outcome.
Discussion
Using data on 7587 participants from the OaK Birth Cohort, a folic acid-fortified study population, we investigated the determinants of maternal plasma homocysteine concentration in the early second trimester of pregnancy. Factors associated with maternal homocysteine were demographic characteristics (age and race), health indicators (BMI and chronic hypertension), pregnancy characteristics (smoking, history of a placenta-mediated pregnancy complication and folic acid supplementation) and an important factor related to homocysteine metabolism (serum folate). Serum folate modified the effect of the MTHFR 677C>T genotype on plasma homocysteine concentration. The pattern in which these factors were associated with either higher or lower homocysteine concentration has practical implications for the lowering of homocysteine in women of reproductive age.
Our findings support the method of analysing homocysteine as a continuous variable. The traditional approach of dichotomising homocysteine concentration at a percentile cut-off relative to gestational age at measurement led to some differences in results compared to converting homocysteine to a Z-score or analysing homocysteine as a continuous variable while adjusting for gestational age in the multivariable model. Disadvantages associated with categorising continuous variables for analysis include bias and decreased precision; nevertheless, categorising is often driven by simplicity and clinical interpretability(Reference Bernette and Vickers34– Reference Royston, Altman and Sauerbrei 36 ). With no agreed-upon cut-off for elevated homocysteine during pregnancy, the use of data-derived cut-offs is additionally problematic because different distributions produce different cut-offs, limiting comparability between studies(Reference Bernette and Vickers34).
Interpretation
Our findings of demographic, health, pregnancy-related and metabolic (including genetic) determinants of plasma homocysteine in pregnant women agree with findings from previous studies(Reference Jacques, Bostom and Wilson9,Reference Jacques, Rosenberg and Rogers37,Reference Nygard, Vollset and Refsum38) . The Hordaland Homocysteine study investigated the association of cardiovascular risk factors with non-fasting homocysteine. It is the largest study in the general population on homocysteine determinants; approximately 8000 men and 8000 women aged 40–67 years were recruited in Norway from 1992 to 1993. The strongest determinants of homocysteine were age, cigarette smoking dosage (stronger effects in women) and vitamin intake score(Reference Nygard, Vollset and Refsum38). The Generation R study also investigated factors associated with early-second-trimester homocysteine in a cohort of 5085 participants recruited in the Netherlands from 2002 to 2006(Reference Bergen, Jaddoe and Timmermans4). Smoking, comorbidity, lower education and Caucasian ethnicity were all associated with higher homocysteine. In our adjusted analyses, we found that education level was not associated with homocysteine, which suggests that the effects of adequate folate intake and healthy behaviours can transcend socioeconomic status.
Maternal age and BMI were significant determinants of homocysteine in a U-shaped relation. We also found that nulliparity was associated with higher homocysteine, as did Bergen et al.(Reference Bergen, Jaddoe and Timmermans4), and this may be due to nullipara participants being younger than multipara. A U-shaped relation of homocysteine with age and BMI has been reported in pregnant women(Reference Bergen, Jaddoe and Timmermans4) but not in the general population(Reference Jacques, Bostom and Wilson9,Reference Nygard, Vollset and Refsum38) . A U-shaped association for BMI may be linked to physical activity; in the Hordaland study, BMI modified the effect of physical activity on homocysteine such that participants with lower BMI had an inverse relation between physical activity and homocysteine, whereas participants with a higher BMI had a positive relation between physical activity and homocysteine(Reference Nygard, Vollset and Refsum38).
Homocysteine and folate distributions in the OaK cohort were similar to another Canadian birth cohort that recruited participants in the same time period(Reference Dodds, Fell and Dooley5), but the distributions of homocysteine and folate were lower and higher, respectively, than a European cohort(Reference Bergen, Jaddoe and Timmermans4). Despite the lower distribution of homocysteine, our results were in agreement with other studies, including large meta-analyses, in showing a moderating effect of serum folate with the MTHFR 677C>T polymorphism in determining homocysteine concentration(Reference Holmes, Newcombe and Hubacek13,Reference Clarke, Bennett and Parish14,Reference Jin, Cheng and Chen39) . Our subgroup analyses found that folic acid supplementation was no longer associated with homocysteine concentration; this finding was likely due to the smaller available sample size for the MTHFR subgroup analysis.
Our study demonstrated that continued focus on increasing folate status during pregnancy, particularly via folic acid supplementation >1 mg/d, may no longer result in substantially higher folate and, therefore, lower homocysteine concentration. A Canadian open-label folic acid trial demonstrated that folate concentrations do not reach a steady state during pregnancy(Reference Shere, Nguyen and Tam40), but folic acid supplementation during pregnancy had not demonstrated a substantial benefit to reduce pregnancy outcomes such as preterm birth, stillbirth, low birthweight and neonatal death(Reference Lassi, Salam and Haider41). The multicentre folic acid clinical trial (FACT) found no benefit of high-dose folic acid (i.e., 4 mg daily) taken after the first trimester on the risk of pre-eclampsia in high-risk women(Reference Wen, White and Rybak42). Folate intake has, however, been identified among the major determinants of plasma homocysteine(Reference Jacques, Bostom and Wilson9,Reference Nygård, Refsum and Ueland43) ; in the Hordaland study, a folate intake score was validated against plasma folate in a subsample of study participants. With or without additional changes, preconception folic acid intake has also demonstrated short-term benefits; in a Norwegian population-based cohort study, periconceptional folic acid intake modified the effect of continued smoking during pregnancy on homocysteine concentration in the first trimester(Reference Bakker, Timmermans and Steegers44).
A number of studies conducted in the post-folate fortification era in Canada and the USA demonstrated folate-replete populations(Reference Shere, Kapur and Koren15,Reference Joelson, Fiebig and Wu45– Reference Caudill, Le and Moonie 47 ). Based on current evidence, WHO recommends population-level RBC folate concentrations >906 nmol/l in women of childbearing age to protect against neural tube defects(48). A comparison of mean RBC folate concentrations in women aged 15–44 years in NHANES III (1988–1994) and NHANES 1999 demonstrated a significant increase 3 years after mandatory folic acid fortification of cereal grains was introduced(46). However, the Canadian Health Measures Survey (CHMS) cycle 2007–9 found that although 1 % of the population-based sample was folate-deficient, 22 % of women aged 15–45 years had suboptimal RBC folate status <906 nmol/l(Reference Colapinto, O’Connor and Tremblay49). In a more recent analysis of 1035 physician-ordered tests of RBC folate concentrations in Toronto, Canada, 7 % of women aged 15–45 years had suboptimal folate status(Reference Shere, Kapur and Koren15).
Our study indicated that despite improvements in folate levels through fortification, women with the MTHFR 677C>T polymorphism are one subgroup that is susceptible to low folate and high homocysteine. A population-based folic acid trial in approximately 900 Northern Chinese women of reproductive age, who had no other source of folic acid and were not anaemic or vitamin B12-deficient, found that folic acid dose did not significantly change the effect of the MTHFR variant. Throughout 6 months of supplementation and 3 months of discontinuation, the TT genotype was associated with response (i.e., folate and homocysteine concentrations) to the highest administered folic acid doses of 400 and 4000 µg/d(Reference Crider, Zhu and Hao50).
Women taking hormonal contraceptives are another subgroup of women possibly susceptible to low folate status. A recent systematic review and meta-analysis found a significant RBC and serum folate-lowering effect of oral contraceptive use(Reference Shere, Bapat and Nickel51). However, we found that hormonal contraceptive use prior to conception was not a determinant of homocysteine in the early second trimester of pregnancy. Although homocysteine was measured in the second trimester in OaK participants, introducing a gap in time from ceasing oral contraceptive use to homocysteine measurement, hormonal contraceptive use may be linked to a higher likelihood of planned pregnancy and, therefore, preconception folic acid supplementation. Preconception folic acid supplementation was shown to be associated with lower homocysteine in pregnant women(Reference Bergen, Jaddoe and Timmermans4,Reference Dodds, Fell and Dooley5) . Thus, improved folate intake in the preconception period could offset the folate-lowering effects of hormonal contraceptives.
In the Hordaland study, a 6-year follow-up of participants found reductions in homocysteine concentration associated with increased folate and vitamin B12 concentration, quitting smoking and weight loss(Reference Nurk, Tell and Vollset52). This lowering of homocysteine may additionally explain our finding that history of a placenta-mediated complication was consistently associated with decreased homocysteine concentration, which appears contrary to findings of elevated homocysteine in women with a history of vascular-related pregnancy complications(Reference Vollset, Refsum and Irgens3,Reference Visser, Hermes and Ket53) . It is plausible that women who previously experienced pregnancy complications were more likely to make favourable lifestyle or nutritional changes or have been prescribed multivitamins or folic acid, which may have contributed to a lower homocysteine concentration.
Folic acid trials in women of childbearing age have demonstrated that plasma homocysteine and serum folate tend to return to baseline levels after discontinuing supplementation. In the folic acid trial in Northern Chinese women, homocysteine concentration decreased 17 % with a folic acid dose of 400 µg/d, and approximately 22 % with a dose of 4000 µg/d. However, 3 months after cessation, the effects of intervention were diminished(Reference Crider, Zhu and Hao50). Similarly, in a trial of twenty-seven Dutch women, 500 µg of folic acid was administered for 8 weeks; although homocysteine and folate reached a steady state, RBC folate did not. Moreover, 8 weeks after discontinuation, homocysteine and folate returned to baseline levels(Reference Bakker, de Jong-van den Berg and Fokkema54).
Strengths and limitations
To our knowledge, this is the largest comprehensive study of homocysteine determinants in pregnant women. We used prospective data on second-trimester maternal plasma homocysteine. Our analysis used multiple imputation to deal with missing data and included a wide range of potential determinants. We analysed continuous variables using flexible parametric forms, which allowed for possible non-linear associations with homocysteine.
Although we accounted for a range of potential determinants, we did not have data on physical activity and caffeine consumption, which are among other determinants of homocysteine identified in population-based studies and in studies of pregnant women(Reference Nygård, Refsum and Ueland43,Reference Shiraishi, Haruna and Matsuzaki55) . Additionally, non-fasting blood samples were collected from OaK participants. Although fasting affects folate concentration, fasting for varying lengths of time has demonstrated no measurable effect on homocysteine concentration(Reference Jacques, Rosenberg and Rogers37).
Conclusion
Research is on-going into the role of homocysteine in the development of CVD and vascular-related pregnancy complications. Our findings of the determinants of maternal plasma homocysteine are especially relevant to the lowering of homocysteine in women of childbearing age in the post-folic acid fortification era, which is characterised by folate-replete populations. Clinical trials may point to a benefit of preconception folic acid and/or multivitamin intake on the risk of pregnancy complications. Periconceptional uptake of folic acid is suboptimal worldwide, including in Canada(Reference Ray, Singh and Burrows56– Reference Cawley, Mullaney and Kennedy 63 ). Therefore, promoting timely uptake in the wider population of reproductive-age women is necessary in addition to targeting at-risk groups. A targeted approach based on factors associated with higher homocysteine may prove beneficial. This includes demographic characteristics (younger or older age and considering race or ethnic background), health indicators (lower or higher BMI and chronic conditions such as hypertension) and pregnancy-related factors such as smoking status. Thus, in the post-folic acid fortification era, a focus on periconceptional folic acid supplementation in addition to other modifiable factors – for example, healthy weight and lifestyle choices and other nutritional factors such as vitamin B12 intake – may be an effective approach to lower maternal homocysteine concentration over the long term(Reference Jacques, Bostom and Wilson9,Reference MacFarlane, Greene-finestone and Shi64) .
Acknowledgements
Acknowledgements: Shazia Chaudhry is recipient of a PhD scholarship from the Canadian Institutes of Health Research–Quebec Training Network in Perinatal Research (CIHR-QTNPR). Financial support: This study was supported by grants from the CIHR, grants MOP 53188 and 82802, and FDN 148438. CIHR had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: S.W., M.W., R.R.W., G.S. and M.R. are lead investigators of the OaK Birth Cohort. S.C., S.W. and M.T. designed the study. S.C. analysed patient data and wrote the draft manuscript. S.C., S.W., M.T. and A.M. were involved in data interpretation. L.G., M.W., R.R.W., G.S. and M.R. critically revised the manuscript and contributed to the final version. All authors read and approved the final manuscript. Ethics of human subject participation: The OaK Birth Cohort study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the Ottawa Health Science Network Research Ethics Board (OHSN-REB), formerly the Ottawa Hospital Research Ethics Board (protocol numbers 2002343 and 2007034). Participants’ written informed consent was sought for participation in the cohort study as well as for banking maternal blood, cord blood and contact for long-term follow-up. Ethics approval for secondary analyses of the OaK Birth Cohort was obtained from the OHSN-REB on 31 May 2016 (protocol 20160163-01H).
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980019004002.









