Vitamin A deficiency (VAD) remains an ongoing public health problem in many areas of the world. Children and women of childbearing age are most affected, with an estimated 190 million children of pre-school age and 19 million pregnant women having VAD( 1 ). Based on night blindness (XN; the ocular symptom of VAD) and xerophthalmia (the first stage of ocular manifestations due to VAD), it is estimated that 5·2 million pre-school children and 9·8 million pregnant women are impacted, corresponding to 0·9 % and 7·8 % of these populations worldwide( 1 , 2 ).
A national survey conducted in Brazil examined VAD among children of pre-school age and women of reproductive age( 3 ). Analyses of serum retinol, which is the biochemical indicator of VAD, revealed that women from the Southeast region evinced the highest prevalence of VAD in the country (14·0 %), surpassing those in the Northeast region (12·1 %), which is considered at higher risk for this condition( 3 ). These findings are supported by data from the WHO showing that the prevalence of VAD among pregnant/postpartum women living in Rio de Janeiro, RJ (Southeast region of Brazil) is higher than in those living in the Northeast region, based on gestational XN( 4 ).
The WHO( 5 ) considers VAD one of the aggravating factors for maternal mortality because it may be associated with worsening of hypertensive disorders of pregnancy due to the antioxidant role of vitamin A. In addition, VAD contributes to the worsening of puerperal infections because of the effects of vitamin A on the immune system and it is often associated with anaemia in pregnant women( Reference Campos, Saunders and Ramalho 6 – Reference Thorne-Lyman and Fawzi 11 ).
Hypertension, haemorrhage and puerperal infection are among the main direct causes of maternal death among Brazilian women( 12 ). The maternal mortality ratio is considered high in Brazil. The Brazilian Ministry of Health estimates sixty-eight maternal deaths per 100 000 live births( 12 ).
Thus, the diagnosis of gestational XN is currently a marker of high-risk pregnancy and may be useful during prenatal care to identify women requiring special attention( 13 , Reference Christian 14 ). In addition, the prevalence of XN can also indicate nutritional deficiency due to, for example, acute changes in living conditions, economic crises or reduction in employment rates, which affect the prevalence of XN in pregnant women and pre-school children( Reference West and Mehra 15 ).
Inadequate dietary intake of vitamin A is the most recognized risk factor for VAD( 2 , Reference Christian, West and Khatry 8 , Reference Akhtar, Ahmed and Randhawa 16 – Reference Pandey, Lin and Collier-Tenison 18 ). However, other factors need to be further studied, such as lack of education, poor sanitation, food insecurity, low maternal socio-economic status, poorly diversified and plant-based diets and low consumption of vitamin A-rich foods, whose association with VAD has been well described in studies conducted in Asia and Africa( Reference Akhtar, Ahmed and Randhawa 16 , Reference Gebreselassie, Gase and Deressa 19 , Reference Abebe, Abebe and Loha 20 ). In Brazil, such studies remain inconclusive. The majority of the available studies were conducted in the Northeast region, a region classically recognized as having a higher prevalence of VAD among children( 4 ). The determinants of gestational XN in the country are still not clear, stalling the adoption of effective measures to prevent and control this condition.
The present study aimed to describe the prevalence of gestational XN, and identify the factors that determine this ocular symptom, using a hierarchical analysis in adult pregnant women who received care in a hospital in Rio de Janeiro, Brazil.
Methods
Study population and inclusion criteria
The present cross-sectional study is part of the project entitled ‘Health and Nutrition Profile of Postpartum Women and Newborn Infants in the Maternity Hospital of the Federal University of Rio de Janeiro during 1999–2008’ (Perfil de Saúde e Nutrição de Puérperas e Recém-nascidos da Maternidade Escola da UFRJ no período de 1999–2008; Principal Investigator C Saunders; Sigma/Universidade Federal do Rio de Janeiro (UFRJ) registration number 19022, March 2010) and was conducted with a representative sample of pregnant/postpartum women receiving care in this hospital from 1999–2001 (n 225) and 2005–2008 (n 602), totalling 827 women.
The maternity hospital studied is associated with the Brazilian Public Healthcare System and has prenatal clinics for both low-risk and high-risk pregnancies. A multidisciplinary team provides care to pregnant/postpartum women and newborns. On average, approximately 2100 births are performed annually. Patients receiving care in this hospital are similar to patients (pregnant/postpartum women) receiving care in other health-care units of the city( Reference Saunders, Leal and Gomes 21 ). They are similar to Brazilian women of reproductive age evaluated in a national survey( 3 ), based on marital status, skin colour and the prevalence of both elevated BMI and obesity. In addition, postpartum women evaluated in a national hospital-based study, 2011–2012( Reference Domingues, Dias and Nakamura-Pereira 22 ), were similar to the present sample based on marital status and skin colour.
Study groups
Taking into consideration the original study, we selected 606 women who met the inclusion criteria: adult (aged ≥20 years) with a singleton pregnancy, without chronic diseases, attending prenatal care and who delivered in a public maternity hospital in Rio de Janeiro. Of the total (n 606), 225 (37·1 %) women were followed in prenatal care and had their births in the period 1999–2001 (group I; GI) and 381 (62·9 %) were followed in prenatal care and had their births in the period 2005–2008 (group II; GII).
Selection of GI occurred at the time of admission to the maternity ward for delivery and/or immediately postpartum (up to 6 h after birth). Women who met the inclusion criteria and agreed to participate in the study were interviewed. In addition, the medical records of the pregnant woman and her child were reviewed. Selection of GII was done via the original study, in which women were enrolled in the waiting room for prenatal consultations in the studied maternity ward. The same data collection techniques were used for GII as for GI. Data were collected by a team of investigators with expertise in pregnancy and postpartum care, and who were properly trained and supervised.
Nutrition assistance during prenatal care
During the years 1999–2001, prenatal nutritional assistance offered in the hospital was limited, individualized care with a nutritionist during prenatal care was rare, and any assistance was often initiated late in pregnancy for pregnant women exhibiting weight deviations or pregnancy complications( Reference Chagas, Ramalho and Padilha 23 , Reference Neves, Saunders and Padilha 24 ). There was no minimum number of appointments throughout the pregnancy and no definition of the ideal gestational age for referral to the nutritionist( Reference Chagas, Ramalho and Padilha 23 , Reference Neves, Saunders and Padilha 24 ).
Studies conducted in this hospital led to changes in the prenatal routine beginning in 2005. It was found that at least four visits with a nutritionist during pregnancy, starting concomitantly with prenatal care, improved perinatal outcomes. Prenatal care was restructured with the expansion of nutritionist coverage to all pregnant women. Low-risk pregnant women attend at least one group visit with the nutritionist during pregnancy. Risk is monitored throughout pregnancy based on BMI and pregnancy complications (e.g. anaemia, gestational diabetes, hypertension, digestive symptoms), with a minimum of four individual consultations and a maximum interval of 30 d between them( Reference Chagas, Ramalho and Padilha 23 , Reference Neves, Saunders and Padilha 24 ).
During the consultations, a nutritionist provides teaching and encouragement to consume vitamin A from natural sources and vitamin A-fortified foods. Multivitamin supplementation containing vitamin A, Fe, folate and vitamin C is a routine procedure of the maternity hospital and focuses mainly on the prevention or treatment of gestational anaemia. The amounts supplemented conform to the safe total levels of vitamin A for the women’s reproductive age( 25 ).
Variables analysed
The gestational XN outcome was assessed using an interview that was standardized by the WHO( 26 ) and confirmed by biochemical assay (serum retinol, as reported by Saunders et al.)( Reference Saunders, Ramalho and Lima 27 ). The interview included the following questions: (i) ‘Do you have difficulty seeing during the day?’ (ii) ‘Do you have difficulty seeing in low light or at night?’ (iii) ‘Do you have night blindness?’ Gestational XN was considered when the woman answered ‘no’ to the first question and ‘yes’ to either the second or third question, or both. If the pregnant woman presented with any vision problem, her vision capacity was evaluated with glasses or contact lenses to correct the vision problem. The interview was conducted using simple language and examples of places with low light( 26 ).
Socio-economic and environmental factors, prenatal care data and biological, clinical and obstetric data were the independent variables tested. The theoretical hierarchical model proposed is shown in Fig. 1. The choice of possible predictive risk factors of gestational XN was based on data from the literature( Reference Christian, West and Khatry 8 , Reference Saunders, Ramalho and Lima 27 – Reference Katz, Khatry and West 30 ).
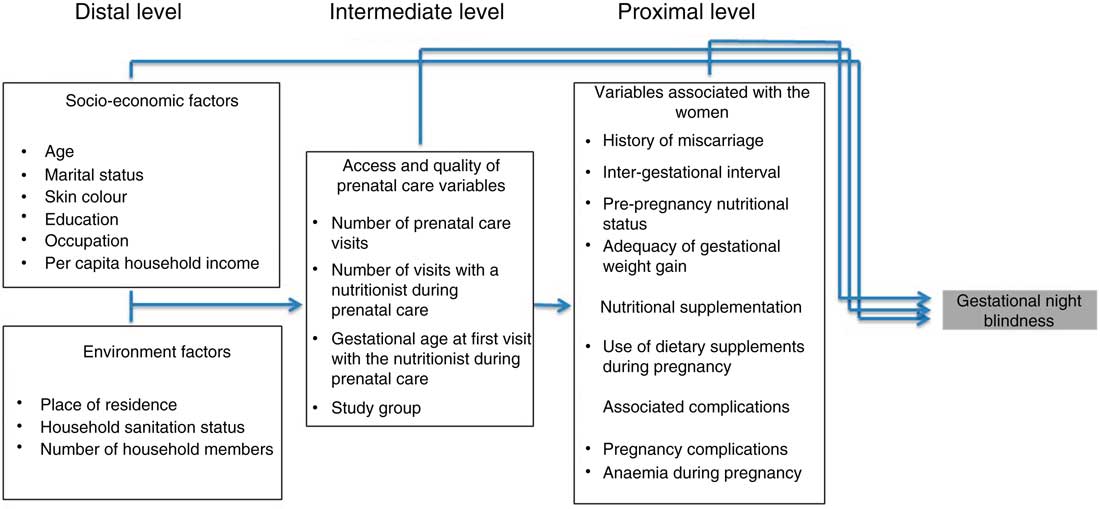
Fig. 1 Conceptual hierarchical model of the process of determining gestational night blindness in adult women seen in a public maternity hospital in Rio de Janeiro, RJ, Brazil
To characterize socio-economic status during prenatal care as well as the biological, obstetric and clinical data of the pregnant women, variables that are part of the prenatal care protocol of the maternity hospital were selected and obtained by searching the medical records.
Anthropometric measurements and pregnancy complication assessments
The following anthropometric assessments were performed: weight before pregnancy reported or measured before the 13th week of gestation; and height and weight before delivery or those recorded in the last prenatal care visit before delivery. The pre-pregnancy BMI was calculated and evaluated according to the cut-offs proposed by the Institute of Medicine( 31 ). The total pregnancy weight gain was estimated by subtracting the pre-pregnancy weight from the weight before delivery or that recorded in the last prenatal care visit occurring no later than 7 d before delivery. The adequacy of pregnancy weight gain was classified as adequate weight gain (as recommended) and inadequate weight gain (below or above the recommended)( 31 ).
The following pregnancy complications were considered: gestational diabetes, gestational hypertension, urinary infection and others, according to the patients’ medical records and in agreement with the recommendations of the Brazilian Ministry of Health( 32 ). Anaemia was diagnosed when the woman’s Hb level was below 110 g/l( 32 ).
Hierarchical model
The variables selected as described below were grouped into three levels (blocks). The ‘distal level’ comprised the following socio-economic variables: maternal age (20–34 years or ≥35 years), marital status (living with a partner or living without a partner), skin colour (white or non-white), education (incomplete primary education or complete primary education), occupation (homemaker or working outside the home) and per capita household income in minimum wages (MW; <1 MW, 1 to <2 MW or ≥2 MW); and the following environmental variables: residence (South Zone of Rio de Janeiro or others) and number of household members (≥5 members, 3 or 4 members or ≤2 members).
The ‘intermediate level’ comprised the following variables of access and quality of prenatal care: number of prenatal visits (<6 or ≥6), number of visits with a nutritionist during prenatal care (<4 or ≥4), gestational age at first visit with the nutritionist during prenatal care (<14th week of gestation or ≥14th week of gestation) and study group (GI or GII).
The ‘proximal level’ comprised the following variables associated with the women: obstetric characteristics, i.e. history of all-cause abortion (yes or no) and inter-gestational interval (<24 months or ≥24 months); biological characteristics, i.e. pre-pregnancy nutritional status (underweight/normal weight or overweight/obesity) and adequacy of gestational weight gain (below or adequate and above); and clinical characteristics, i.e. dietary supplementation during pregnancy (yes or no), pregnancy complications (yes or no) and anaemia in the first or second trimester of pregnancy (yes or no).
Statistical analysis
Measures of central tendency, as well as the means and standard deviations of continuous variables, were calculated to describe the sample. In the data analysis, associations between possible determinants of XN during pregnancy were initially tested through bivariate analysis with all variables of each hierarchical level (distal, intermediate and proximal levels). Crude odds ratios were estimated with 95 % confidence intervals using simple logistic regression.
To construct the final model, the variables were added into the model step by step, considering the hierarchical levels: distal, intermediate and proximal. A P value of <0·25 obtained in the bivariate analysis was adopted as the criterion to include variables in the model. To fit the model into the hierarchical levels, variables with P<0·05 remained in the model at each level of analysis.
In the final model, the adjusted odds ratios were estimated with their respective 95 % confidence intervals using a hierarchical logistic regression, and the results were expressed as the crude and adjusted odds ratio according to each hierarchical level. The statistical software package IBM SPSS Statistics version 20 was used to perform the analysis.
Sample size
Because the number of women with available information for the present analysis was smaller than the total sample of the original study, post hoc calculations were performed. Assuming a 10 % prevalence of gestational XN and a significance level of 5 % and 80 % power, the sample size of the current study (606 women) was able to detect differences of at least 6 % in the prevalence of gestational XN between the groups.
Ethical issues
The research projects that generated the databases were in agreement with the guidelines of Resolution 196/96 of the Brazilian National Health Council (signatory of the Declaration of Helsinki) and were approved by the Ethics Committee of the Maternity Hospital, Federal University of Rio de Janeiro (Opinion no. 35/04, dated 25/02/2002) and the National School of Public Health, Oswaldo Cruz Foundation (Opinion no. 75/02, dated 04/09/2002). All participants went through an informed consent process and signed an informed consent form.
Results
The prevalence of gestational XN observed in the study was 9·9 %. Only twenty-three women were able to accurately report at which gestational age the ocular symptom started; for these women, it began on average during week 18 (sd 8·09) of pregnancy, with minimum and maximum gestational ages of 8 and 35 weeks, respectively. The mean maternal age was 27·6 (sd 5·2) years. Among the pregnant women analysed in the study, 59·2 % lived in the South Zone of Rio de Janeiro; 78·4 % were living with a partner; 63·2 % were non-white (black or mulatto/mixed); 70·3 % had completed primary education (Table 1); and 88·4 % attended six or more prenatal visits (Table 2).
Table 1 Socio-economic determinants (distal) of gestational night blindness (XN) in adult women seen in a public maternity hospital in Rio de Janeiro, RJ, Brazil (1999–2008)

MW, minimum wage; Ref., reference category.
Table 2 Prenatal care-related determinants (intermediate) of gestational night blindness (XN) in adult women seen in a public maternity hospital in Rio de Janeiro, RJ, Brazil (1999–2008)
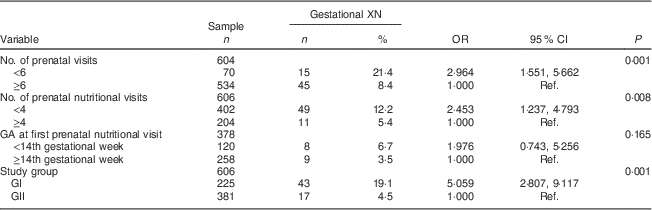
GA, gestational age; GI, group I, pregnant women seen in the hospital from 1999 to 2001; GII, group II, pregnant women seen in the hospital from 2005 to 2008; Ref., reference category.
The bivariate analysis revealed possible determinants of gestational XN within the three hierarchical levels (Tables 1–3). With respect to socio-economic factors (distal level), place of residence (P=0·009), skin colour (P=0·219), per capita household income (P<0·05) and number of household members (P<0·05) were associated with gestational XN.
Table 3 Maternal-related determinants (biological and obstetric characteristics, proximal) of gestational night blindness (XN) in adult women seen in a public maternity hospital in Rio de Janeiro, RJ, Brazil (1999–2008)
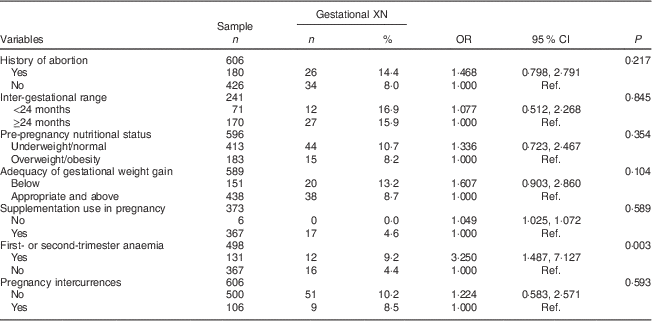
Ref., reference category.
A greater chance of gestational XN was observed among women who lived outside the South Zone of Rio de Janeiro (OR=2·044; 95 % CI 1·193, 3·504) or in a home with a large number of members (≥5 members: OR=2·751; 95 % CI 1·284, 5·894; Table 1). Pregnant women living in the South Zone of Rio de Janeiro had better living conditions (higher per capita household income and fewer household members). Higher per capita household income represented a protective factor against gestational XN (1 to <2 MW: OR=0·483; 95 % CI 0·248, 0·940; ≥2 MW: OR=0·427; 95 % CI 0·202, 0·802; Table 1).
All variables included in the intermediate level met the criteria for entry into the multivariate analysis, and the prevalence of XN decreased fivefold in the group of pregnant women who had access to adequate nutritional assistance (GII; Table 2).
Considering that information about gestational age at the first visit with the nutritionist during prenatal care was obtained only from 378 pregnant women, it was assumed that the variable study group would be a proxy for the quality of nutritional assistance offered to the pregnant women (Table 3).
The proximal-level variables selected for the multivariate analysis were: history of abortion (P=0·217), adequacy of gestational weight gain (P=0·104) and anaemia in the first or second trimester of pregnancy (P=0·003). The crude and adjusted odds ratios for the proximal-level variables and their corresponding 95 % confidence intervals obtained in the hierarchical model are shown in Tables 3 and 4.
The final model revealed that not living in the South Zone of Rio de Janeiro (distal level: adjusted OR=1·846; 95 % CI 1·002, 3·401), belonging to GI (intermediate level: adjusted OR=2·183; 95 % CI 1·066, 4·471), history of abortion (proximal level: adjusted OR=2·840; 95 % CI 1·134, 7·115) and an anaemia diagnosis in the first or second trimester of pregnancy (proximal level: adjusted OR=3·776; 95 % CI 1·579, 9·029) were the determinants of gestational XN (Table 4).
Table 4 Final hierarchical model with crude and adjusted odds ratios, and 95 % confidence intervals, to estimate the determinants of gestational night blindness in adult women seen in a public maternity hospital in Rio de Janeiro, RJ, Brazil (1999–2008)
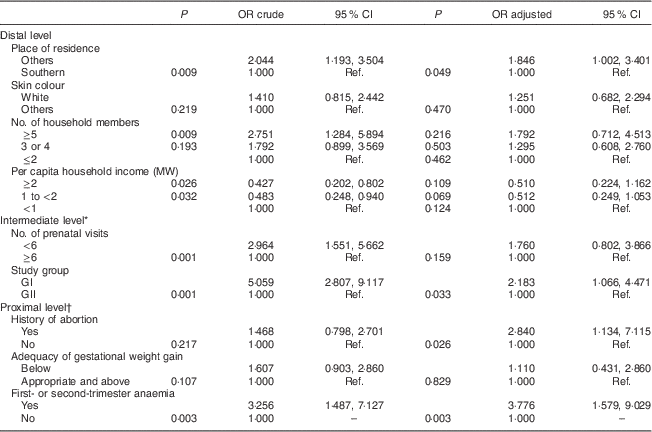
MW, minimum wage; GI, group I, pregnant women seen in the hospital from 1999 to 2001; GII, group II, pregnant women seen in the hospital from 2005 to 2008; Ref., reference category.
* Adjusted for living place.
† Adjusted for study group.
Discussion
The present study identifies determinants of gestational XN in adult pregnant women residing in the city of Rio de Janeiro. These determinants were zone of residence, access to adequate nutritional assistance, history of all-cause abortion and gestational anaemia, which is another common nutritional deficiency among pregnant women. The environmental conditions represented by place of residence (distal level) as well as the nutritional monitoring represented by the variable study group (intermediate level) were associated with the chance of developing gestational XN. A history of abortion and a diagnosis of anaemia (proximal level) were associated with gestational XN regardless of socio-economic conditions, prenatal care conditions and maternal anthropometric characteristics.
The hierarchical multivariate model was useful in the present study because it allowed for the identification of the determinants of gestational XN for the first time in the national literature. The current analysis elucidates associations between variables still poorly investigated in studies about XN in Brazilian pregnant women, in addition to identifying the periods during which they have greater impact( Reference Lima, Carvalho and Vasconcelos 33 ).
The measured prevalence of gestational XN was similar to values found in other national studies but higher than the value reported by Santos et al.( Reference Santos, Velarde and Ferreira 34 ), who studied pregnant women receiving care in health-care units in the city of Diamantina, Minas Gerais state, a region considered at higher risk for VAD, where a prevalence of 8·7 % was observed( Reference Santos, Velarde and Ferreira 34 ). Another Brazilian study conducted with adult pregnant women in the same hospital analysed in the present study revealed that 18·0 % had XN during pregnancy over the period 1999–2001( Reference Saunders, Ramalho and Lima 27 ). After the implementation of a nutritional intervention into the prenatal care programme in this hospital (an increase in the number of visits with a nutritionist that started concomitantly with prenatal care and the adoption of practices recommended to prevent and treat VAD), the prevalence of gestational XN among adult pregnant women decreased from 19·1 % to 4·5 %( Reference Chagas, Ramalho and Padilha 23 ).
These findings corroborate the results of the present study, as the final model demonstrated that the changes adopted over the years in the routine prenatal nutritional assistance programme of the studied maternity hospital were beneficial in reducing gestational XN. The systematic nutritional assistance practised in this hospital included an earlier initiation of monitoring by a nutritionist, guidance regarding a healthy and adequate diet to meet the increased nutritional needs during pregnancy associated with the classic strategies recommended for combating VAD, such as encouragement to consume foods rich in vitamin A and fortified foods, and adherence to the supplementation scheme prescribed during pregnancy( 35 , 36 ). The consumption of beef liver once weekly (lunch or dinner) and of other animal and plant sources of vitamin A was promoted during the visits. This is similar to recommendations described in studies of adult( Reference Rouse 37 ) and adolescent( Reference Wrieden and Symon 38 ) pregnant women, as well as other strategies aimed at reducing high-impact deficiencies like anaemia( Reference Chagas, Ramalho and Padilha 23 ).
The nutritional assistance practised in this maternity hospital can be adapted to other levels of prenatal care. In addition, other health-care professionals can be made aware of and trained to assess for gestational XN with the application of the standardized interview, and to implement the aforementioned strategies for combating VAD. This nutritional intervention can contribute to the reduction of gestational XN and improve maternal/child health, in addition to helping to reduce maternal mortality( 5 ).
The importance of prenatal care to detect and treat nutritional deficiencies in pregnant women has been emphasized by the WHO since 1999( 5 ). In a 2011 committee statement, it was noted that many pregnant women present with nutritional deficiencies, especially anaemia, VAD and iodine deficiency, during early pregnancy. The committee recognized that these deficiencies can affect birth weight and chances of survival, and that inadequate intake of vitamin A increases the risk of gestational XN( 36 ).
The high prevalence of gestational XN (9·9 %) found in the present study reflects the vulnerability of women of reproductive age in the city of Rio de Janeiro to VAD. This finding aligns with the results from the last Children and Women National Demographic and Health Survey (Pesquisa Nacional de Demografia e Saúde da Criança e da Mulher (PNDS), 2006)( 3 ), which, based on serum retinol concentrations, demonstrated that 15·5 % of women from the Southeast region of Brazil presented with VAD, the highest value observed in the country( 3 ). These data suggest that VAD must be monitored in women of reproductive age and during pregnancy because it is known that women who begin a pregnancy with low stores of vitamin A and maintain a diet poor in this nutrient during pregnancy have a greater chance of developing VAD and gestational XN, particularly during the third trimester( 25 , 36 ). Consequently, both mother and child will be exposed to the adverse effects of this deficiency.
Recent studies suggest that vitamin A supplementation in women of reproductive age may contribute to reduced mortality only in populations that present a high prevalence of VAD (>10 % gestational XN) and high maternal mortality rates (>500 deaths per 100 000 live births)( Reference West, Christian and Katz 39 ). These data reinforce the notion that other strategies, such as those used in the present study, must be designed and implemented in order to improve the nutritional status of pregnant women and women of reproductive age. Because supplementation with vitamin A has a short-term impact, after this period other interventions must be implemented to maintain adequate levels of this nutrient, including, for example, nutritional assistance, food fortification with vitamin A and dietary diversification( 1 , 36 ).
Due to higher physiological demand for vitamin A during pregnancy, even pregnant women presenting with subclinical VAD can develop gestational XN, which is known to be associated with other biochemical indicators of VAD( 26 ). Among the negative consequences to the health of pregnant women are higher incidence of preterm birth, complications such as gestational hypertension, increased risk of maternal mortality caused by respiratory infection and other infections( Reference Christian, West and Khatry 7 ), diarrhaea( Reference Semba, de Pee and Panagides 40 ) and increased risk of postpartum maternal mortality( Reference Christian, West and Khatry 7 , Reference Christian, Katz and Wu 41 ). The presence of gestational XN also increases the risk of low birth weight and detracts from the health of the newborn in early childhood, as it is associated with an increased risk of diarrhoea, dysentery, acute respiratory infections and poor growth measurements at 6 months of life( Reference Tielsch, Rahmathullah and Katz 42 ).
The presence of gestational XN also increases the risk of neonatal death in the first 6 months of life( Reference Christian, West and Khatry 8 , Reference Christian, West and Khatry 9 ). The negative impact of maternal VAD on the health of the child during childhood and even adolescence was described in a study conducted in Nepal. In the latter study, the authors demonstrated in a population with chronic VAD that supplementation with vitamin A before, during and after pregnancy resulted in improved lung function in children at 9–13 years of age( Reference Checkley, West and Wise 43 ).
The present study demonstrates that place of residence is one of the socio-economic determinants of gestational XN. A higher percentage of women with better living conditions (higher per capita household income and fewer household members) live in the South Zone of Rio de Janeiro. This association suggests that this group of women have better access to a healthy diet and consequently to foods rich in vitamin A. These women are also less exposed to infections and parasites, which can be one of the factors associated with VAD( Reference Pandey, Lin and Collier-Tenison 18 ).
A history of all-cause abortion is another determinant of gestational XN. Adjusted odds ratios were higher in the final model because the sample size used in the final analysis was smaller (n 340), which revealed a greater difference between women with and women without a history of abortion. The association between history of abortion and gestational VAD was previously described by Simsek et al.( Reference Simsek, Naziroglu and Simsek 44 ), who showed lower serum levels of vitamin A among women with a history of abortion. In another study, Neela and Raman( Reference Neela and Raman 45 ) reported increased serum retinol levels among women with a history of abortion. An association between history of abortion and a short inter-gestational interval, which can contribute to the depletion of maternal reserves of vitamin A and is considered a trigger for gestational XN, has also been described( Reference Saunders, Leal and Gomes 21 ).
Maconochie et al.( Reference Maconochie, Doyle and Prior 46 ) suggest that abortion is associated with nutritional status and diet. The authors offer that a low pre-pregnancy BMI and the low consumption of micronutrient-rich foods (fruits and vegetables) are some factors associated with this outcome. This information is of particular importance for women from developing countries, where the main dietary sources of vitamin A and other micronutrients are vegetables( Reference Penniston and Tanumihardjo 47 ). Therefore, adequate dietary intake, as well as the correct intake of vitamin supplements, may have some impact in reducing abortion( Reference Maconochie, Doyle and Prior 46 ).
Anaemia is another important nutritional deficiency in Brazil( 3 ) and the present study demonstrates that it is a risk factor for gestational XN. The Brazilian Ministry of Health estimates that the prevalence of gestational anaemia is 50 %( 12 ) and it is associated with 20 % of total maternal deaths( 17 ). Some studies report that in communities where anaemia is prevalent among pregnant women, VAD should also be assessed( 3 , Reference Lopes, Ramos and Bressani 10 , Reference Thorne-Lyman and Fawzi 11 ).
A study performed in Recife, Brazil, revealed anaemia and VAD in 65·3 % and 25·0 % of postpartum adult and adolescent women, respectively( Reference Lopes, Ramos and Bressani 10 ). A population-based case–control study conducted in Nepal also demonstrated that pregnant women who had gestational XN were more likely to be anaemic, malnourished and to consume a diet low in vitamin A( Reference Christian, West and Khatry 8 ).
The association between these two nutritional deficiencies remains inconclusive. It is believed that VAD can impair the absorption, transport and storage of Fe( Reference Thorne-Lyman and Fawzi 11 , Reference McLaren and Frigg 48 ). Several hypotheses have been proposed, including the potential action of vitamin A in the mobilization of hepatic Fe stores, increased erythropoiesis, decreased ‘anaemia of inflammation’ through increased circulating Fe via reduction of infection and increased absorption of Fe( Reference Thorne-Lyman and Fawzi 11 ).
Brazil has guidelines in place for Fe supplementation during pregnancy( 49 ), as well as fortification of flour with Fe and folic acid to prevent and treat anaemia. The benefit of flour fortification in reducing gestational anaemia in pregnant women has been described in Rio de Janeiro( Reference Silva, Saunders and Szarfarc 50 ). Similar to the case with anaemia, VAD among pregnant women should be a focus of nationwide health programmes as a measure to promote maternal and child health. As observed in the present study, prenatal nutritional monitoring associated with routine prenatal care, including encouragement to use vitamin A supplementation, was one of the protective factors against gestational XN. The WHO( 36 ) recommends weekly or daily vitamin A supplementation for pregnant women living in areas where VAD is a severe public health problem (population prevalence of XN ≥5 % in pregnant women or in children aged 24–59 months) to prevent ocular symptoms( 36 ), in addition to other strategies such as dietary diversification, education and encouragement regarding healthy eating habits, and the use of fortified foods.
The fact that among the sixty women diagnosed with gestational XN, only twenty-three were able to accurately report the onset of ocular symptoms during pregnancy was a limitation of the present study. In addition, lack of data about adherence to vitamin A supplementation and the dose of vitamin A consumed via both supplementation and regular diet were also limiting factors. Such information would be instructive and allow for comparison of the present results with those from other studies.
Conclusion
The present study reveals a significant prevalence (9·9 %) of gestational XN among adult pregnant women in Rio de Janeiro. The determinants were socio-economic status (specifically, place of residence); study group (a proxy for the presence and quality of prenatal nutritional assistance); and history of abortion and presence of anaemia in the first and second trimesters of pregnancy.
These results indicate that VAD should be assessed for during routine prenatal care, and that gestational XN should be assessed for in all pregnant women, especially those living in deprived areas of the city of Rio de Janeiro and those with a history of abortion or who have been diagnosed with gestational anaemia. In addition, nutritional monitoring during pregnancy may have a positive impact on the prevention and control of gestational XN. Therefore, nutritional monitoring should be initiated concurrently with the beginning of prenatal care and continued throughout the entire gestational period.
These data also suggest that in the city of Rio de Janeiro, despite it not being a region classically considered as at higher risk, VAD among women of reproductive age and during the pregnancy/postpartum period may be an underestimated problem. Further investigation at other maternity hospitals and health-care units is warranted, with adequate prevention and control of this nutritional deficiency as needed, in order to head off serious consequences for the health of both mother and child.
Acknowledgements
Financial support: This study was financially supported by the National Counsel for Technological and Scientific Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)) (notice 51/2005, which funded the project entitled ‘Assessment of the impact of prenatal nutritional assistance on obstetric outcome’ (login sigma/UFRJ 12127)) and by CNPq Research Productivity (RP) Fellowships to C.S. and M.d.C.L. Conflict of interest: None. Authorship: C.S. and M.d.C.L. designed and planned the study; C.S. and P.d.C.P. collected the data; C.S., M.d.C.L. and A.O.C.S. analysed the data; C.S., M.d.C.L., P.A.R.N., P.d.C.P. and L.B.d.G. participated in the writing and revision of the final version of the manuscript. Ethics of human subject participation: The study was designed according to Resolution 196/96 of the Brazilian National Health Council/Ministry of Health, which is a signatory of the Declaration of Helsinki.








