Owing to advances in cancer treatment and screening, there are now over 10 million cancer survivors in the USA(Reference Rowland, Mariotto and Aziz1). Given the ageing of the US population and estimates of 5-year survival as high as 64 %(Reference Rowland, Mariotto and Aziz1), this number is expected to continue to increase. Cancer survivors are at increased risk for recurrent disease or second primary malignancies, as well as for higher mortality from other chronic diseases including CVD, diabetes and osteoporosis(Reference Demark-Wahnefried, Aziz and Rowland2); they also have higher mortality rates from non-cancer causes than does the general population(Reference Brown, Brauner and Minnotte3).
As the number of cancer survivors continues to grow, tertiary prevention strategies aimed at reducing disease burden in this population are becoming increasingly important. Because of limited evidence on health behaviours and cancer survival, recommendations for cancer survivors are based primarily on cancer prevention guidelines for the general population(4). Poor diet and physical inactivity operating independently or through obesity-mediated pathways, as well as alcohol consumption and smoking, form the foundation for cancer prevention recommendations with respect to modifiable health behaviours(4–Reference Kushi, Byers and Doyle6). On the basis of estimates that approximately two-thirds of all cancer deaths may be attributable to tobacco use, poor diet, physical inactivity and excess weight(7), improvements in these behaviours after a diagnosis of cancer may have a significant impact on the overall health and life expectancy of cancer survivors.
Better information regarding the extent to which cancer survivors are altering their health behaviours following the diagnosis of cancer can provide a basis for targeted programmes to reduce their long-term morbidity and mortality. Previous studies have found that survivors report improvements in modifiable health behaviours(Reference Blanchard, Denniston and Baker8–Reference Maunsell, Drolet and Brisson12) (for a complete review, see Demark-Wahnefried et al.(Reference Demark-Wahnefried, Aziz and Rowland2)); although prevalence studies cannot directly assess behavioural changes among cancer survivors, data from national health surveys have failed to show differences in health behaviours between cancer survivors and the general population(Reference Coups and Ostroff13–Reference Mayer, Terrin and Menon16). Therefore, the purpose of the present study was to examine whether modifiable health behaviours differed between cancer survivors and participants with no history of cancer at baseline in the Multiethnic Cohort Study (MEC) and whether associations remained consistent across cancer sites or among ethnic groups. Specifically, differences in dietary intake, physical activity, BMI, smoking and alcohol consumption were examined. Recommendations by the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) 2007(4) for reducing the risk of cancer associated with nutrition and physical activity were compared for behaviours addressed in the report. On the basis of the findings of previous reports, we hypothesized that there would be few differences between cancer survivors and participants reporting no history of cancer with respect to the health behaviours examined. A lack of previous data for several ethnic groups examined in the present study precluded any a priori hypotheses regarding ethnic differences in health behaviours of cancer survivors.
Methods
Study population
The MEC is a longitudinal study designed to investigate the role of lifestyle and dietary factors in the aetiology of cancer in a multiethnic population of US adults and has been described previously in detail(Reference Kolonel, Henderson and Hankin17). Briefly, from 1993 to 1996, 215 831 men and women between 45 and 75 years of age at recruitment and residing in Hawaii or California consented to participate in the study by completing a baseline questionnaire. Participants were comprised primarily of five main self-reported ethnic groups – African Americans, Japanese Americans, Latinos, Native Hawaiians and Whites – and were recruited through drivers’ licence records, voter registration lists and Health Care Financing Administration files to obtain adequate and representative samples for each ethnic group. Participants reporting mixed ethnicity were classified using the following hierarchy: African American, Native Hawaiian, Latino, Japanese American, White and others. More than 95 % of all non-Hawaiians reported only one ethnic group. At cohort entry, participants completed a twenty-six-page questionnaire with questions pertaining to demographic characteristics, anthropometrics, medical history, family history, reproductive history, cancer screening practices, occupational history, physical activity and diet. The study was approved by the Institutional Review Boards of the University of Hawaii and the University of Southern California. For the present analysis, participants who were not classified into one of the five main ethnic groups (n 13 994), who reported implausible dietary values of 3 sd from a truncated normal distribution using the middle 80 % of logged energy values and 3·5 sd for logged fats, proteins and carbohydrates within each sex–ethnic group (n 8265) or had missing covariate data (n 16 569) were excluded. Therefore, data on 177 003 participants were available for analysis.
Measures
Identification of cancer survivors
Cancer survivors for the present study were identified either through the baseline questionnaire or through the tumour registry linkage. In the baseline questionnaire, participants were asked whether they had ever been told by a doctor that they had cancer of the stomach, breast, prostate, cervix, uterus, colon or rectum, melanoma, other skin or other cancers not listed. Participants were considered to be cancer survivors if they answered ‘yes’ to having cancer at any site other than non-melanoma skin cancer in the baseline questionnaire or if they were identified as a case before cohort entry through linkage to the Surveillance, Epidemiology, and End Results (SEER) cancer registries for Hawaii (Hawaii Tumor Registry) and California (the Cancer Surveillance Program for Los Angeles County, California State Cancer Registry). Of participants included in the present analysis, 8362 reported a history of cancer and were registry confirmed; 2600 additional cases were found in the registries, whereas 5384 self-reported cases could not be linked to the two statewide registries. In addition to possible false-positive reports, the latter number could reflect cases diagnosed outside Hawaii and California. Only 33 % of registry cases not self-reported were among the specific sites listed on the survey, despite the high prevalence of these cancers. Because no differences by source of cancer information were observed in preliminary analyses, all results included non-duplicative cases identified either by questionnaire or by tumour registry.
Dietary assessment
Dietary intake information was obtained for the MEC using a Quantitative FFQ (QFFQ) designed for use in this multiethnic population. Food items included in the questionnaire were those identified from 3 d measured food records as the minimum set that could explain ≥85 % of nutrient intake for nutrients of interest in each ethnic group(Reference Kolonel, Henderson and Hankin17). Foods traditionally consumed by ethnic populations targeted in the study were added to the questionnaire regardless of their contribution to nutrient intake. A calibration study of the QFFQ was conducted using three 24 h recalls from a random subsample of participants selected within sex–ethnic groups and revealed a high correlation between the QFFQ and 24 h recalls for energy-adjusted nutrients(Reference Stram, Hankin and Wilkens18). Dietary values were computed from the QFFQ using the food composition database designed and maintained by the Cancer Research Center of Hawaii(Reference Kolonel, Henderson and Hankin17).
Demographic characteristics and health behaviours
Demographic information on age, sex, ethnicity and educational attainment was collected through the baseline questionnaire. Self-reported height and weight were used to calculate BMI (kg/m2). Table 1 shows how the recommendations were operationalized for the present study. Smoking status was assessed by asking participants if they had ever smoked more than twenty packs of cigarettes in their lifetime. Those reporting ‘no’ were considered to be never smokers; those reporting ‘yes, but I quit smoking’ were former smokers; and those reporting ‘yes, and I currently smoke’ were classified as current smokers. A BMI within the normal range (18·5–24·9 kg/m2) was considered in agreement with the recommendation, although the WCRF/AICR advice is to ‘be as lean as possible within the normal range of body weight’(4). Women who reported consuming one or less and men who reported consuming two or less alcoholic beverages per day were classified as meeting the WCRF/AICR recommendation for alcohol consumption. Physical activity was estimated from baseline questions on work and leisure-time physical activity and sedentary activities; engaging in at least an average of 30 min of moderate or vigorous physical activity per day was considered adequate. Values for dietary variables were obtained from the QFFQ and dichotomized to reflect adherence to the WCRF/AICR recommendations whenever possible or to be in agreement with the 2005 Dietary Guidelines for Americans(19). Participants reporting intakes of ≥400 g of fruit and vegetables per day, <500 g of red meat per week, <2·4 g of Na per day, ≥25 g of dietary fibre per day, <30 % of total energy from fat and <10 % of total energy from saturated fat were considered as meeting recommendations.
Table 1 Recommendations for health behaviours examined in the present study
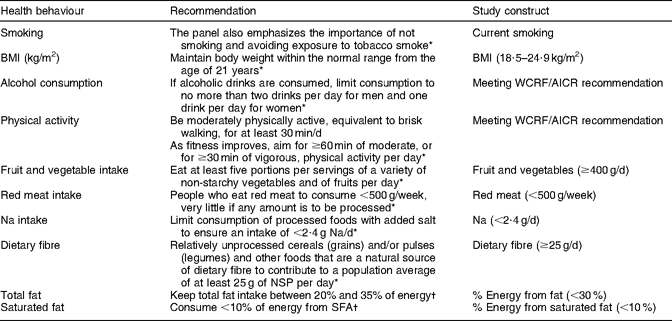
Two index scores were created from questionnaire variables. For adherence to the WCRF/AICR recommendations specified in the 2007 report(4), we assigned one point for meeting recommendations that could be operationalized (BMI, physical activity, alcohol consumption, fruit and vegetables, red meat and Na). We then added the values for each participant, allowing for a maximum possible score of 6. To assess whether a history of cancer had a unique impact on dietary behaviours, an index score was created to examine adherence to dietary recommendations for several food items (fruit and vegetables, red meat, Na, fibre, total fat) specifically addressed in the WCRF/AICR 2007 report or listed as having a ‘probable’ or ‘suggestive’ role in the aetiology of cancer(4). Saturated fat intake was also included in the dietary index score, given its consistent association with CVD. One point was assigned for meeting each dietary recommendation and the values were then added, allowing for a maximum possible score of 6.
Statistical analysis
Descriptive statistics were calculated for demographic characteristics, BMI, smoking status, alcohol consumption, physical activity and diet for cancer survivors and participants with no history of cancer. Age, sex, ethnicity and educational level were found to differ by cancer status and were therefore included as covariates in regression models. BMI was also found to differ and was included only in models of specific behaviours, as it was a component of the WCRF/AICR score and did not affect diet score results. Unconditional multivariable logistic regression was used to obtain OR and 95 % CI for cancer survivors as compared with participants with no history of cancer. Because regression models indicated that behaviours did not differ by time from diagnosis, cancer status source (questionnaire or registry) or by age in decades, results stratified on these subgroups are not presented. The heterogeneity of effect of health behaviours across ethnic groups was tested by a Wald test of the cross-product terms between ethnicity and behaviour, with a P value of ≤0·05 being considered as significant. All data analyses were performed using the SAS statistical software package version 9·2 (SAS Institute Inc., Cary, NC, USA).
Results
Through the questionnaire and tumour registry linkage, 16 346 unique prevalent cases of cancer were identified at baseline (Table 2). Unadjusted results showed that cancer survivors reported meeting a greater number of WCRF/AICR and dietary recommendations than did participants with no history of cancer (Table 3). Survivors were older, more often female and of African-American or white ethnicity, and were less often reported to be current smokers or to meet physical activity recommendations. The proportion of participants meeting dietary recommendations was similar for cancer survivors and for participants with no history of cancer (Table 3).
Table 2 Site of diagnosis for cancer survivorsFootnote *
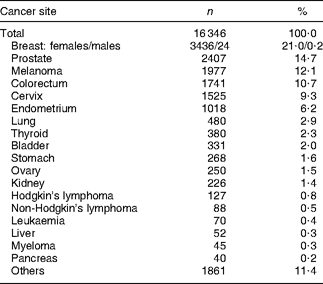
* Case status obtained from the baseline questionnaire or tumour registry linkage.
Table 3 Baseline demographic characteristics and health behaviours of participants in the Multiethnic Cohort Study by history of cancer status
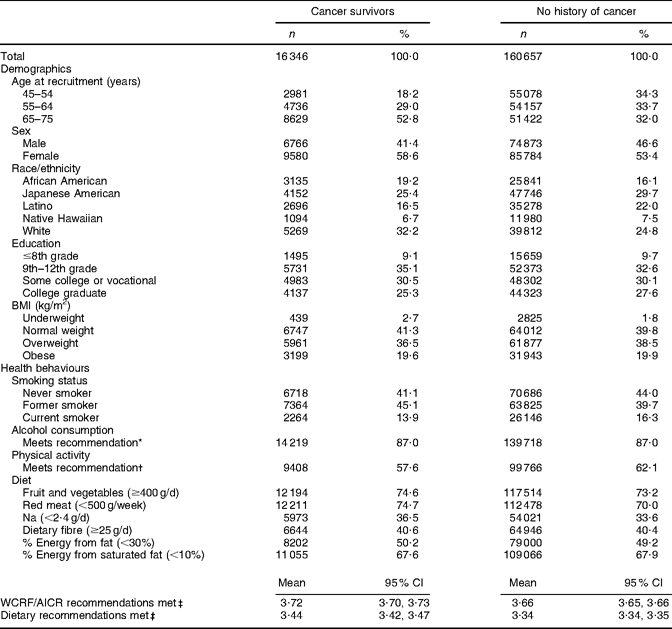
WCRF/AICR, World Cancer Research Fund/American Institute for Cancer Research.
*WCRF/AICR recommendation for those who choose to drink alcohol is ≤1 drink/d for women and ≤2 drinks/d for men.
†WCRF/AICR physical activity recommendation is to engage in at least 30 min of moderate or vigorous physical activity per day.
‡Max score is 6.
Adjusted for age, sex, ethnicity and educational attainment, survivors of cancer at any site were less likely to meet WCRF/AICR recommendations, although not to differ on meeting dietary recommendations alone, than participants with no history of cancer (Table 4). Findings for survivors of colorectal cancer were similar to those of survivors of cancer at any site for adherence to both index scores; however, breast and prostate cancer survivors reported diets that were more likely to meet recommendations.
Table 4 OR and 95 % CI for selected health behaviours in cancer survivors v. persons without cancer at baseline in the Multiethnic Cohort Study

WCRF/AICR, World Cancer Research Fund/American Institute for Cancer Research; Ref., reference category.
*Analysis included only females.
†Adjusted for age, ethnicity, sex and education.
‡Adjusted for age, ethnicity, sex, education and BMI.
§WCRF/AICR recommendation for those who choose to drink alcohol is ≤1 drink/d for women and ≤2 drinks/d for men.
∥WCRF/AICR physical activity recommendation is to engage in at least 30 min of moderate or vigorous physical activity per day.
With regard to specific behaviours, survivors of cancer at any site were more likely to be underweight or former smokers, and less likely to meet the physical activity or saturated fat recommendations. Survivors of colorectal cancer were more likely to report being obese or former smokers and less likely to report meeting the physical activity recommendation. Breast cancer survivors were less likely to meet the recommendation regarding physical activity or to be a current smoker, but were more likely to meet recommendations regarding red meat, Na, total fat and saturated fat intake. Interestingly, survivors of prostate cancer were less likely to be former or current smokers or to meet the physical activity recommendation, but more likely to meet the recommendations regarding alcohol and red meat consumption.
African-American, Japanese-American, Native Hawaiian and white survivors of cancer at any site did not differ from participants with no history of cancer with regard to meeting WCRF/AICR or dietary recommendations (Table 5). However, Latino cancer survivors were less likely than their counterparts with no history of cancer to meet WCRF/AICR recommendations, although not the dietary recommendations alone. Tests of heterogeneity for specific behaviours showed differences among ethnic groups on smoking status, alcohol consumption, physical activity, dietary fibre and energy from fat (P < 0·05). Japanese-American cancer survivors were the only group with a lower likelihood of current smoking and increased likelihood of consuming ≥25 g of dietary fibre per day. White cancer survivors were the only ethnic group less likely to meet the recommendation regarding alcohol consumption, and African-American survivors were the only ethnic group less likely to consume <30 % of energy from fat.
Table 5 OR and 95 % CI by ethnicity for selected health behaviours in cancer survivors (any site) v. persons without cancer at baseline in the Multiethnic Cohort Study
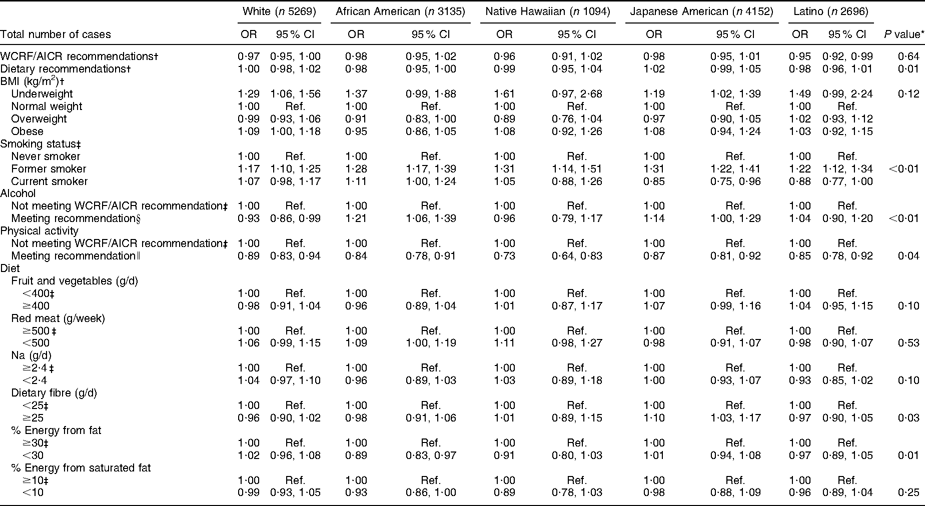
WCRF/AICR, World Cancer Research Fund/American Institute for Cancer Research; Ref., reference category.
*P value refers to test of heterogeneity for the effect of health behaviours across ethnic groups.
†Adjusted for age, sex and education.
‡Adjusted for age, sex, education and BMI.
§WCRF/AICR recommendation for those who choose to drink alcohol is ≤1 drink/d for women and ≤2 drinks/d for men.
∥WCRF/AICR physical activity recommendation is to engage in at least 30 min of moderate or vigorous physical activity per day.
Discussion
In a cross-sectional analysis utilizing baseline data from the MEC, few differences were found between cancer survivors and participants with no history of cancer with respect to meeting recommendations regarding modifiable health behaviours that may reduce the risk of cancer and other chronic diseases. Survivors of cancer at any site and those of colorectal cancer were less likely to meet WCRF/AICR recommendations, whereas breast and prostate cancer survivors were more likely to meet dietary recommendations. However, the magnitude of these differences suggests that a diagnosis of cancer is not associated with meaningful differences in health behaviours in a large, multiethnic sample of older adults. It should be noted that the MEC was based on general population sampling and that participants were shown to be reasonably representative, on the basis of comparisons with US Census data(Reference Kolonel, Henderson and Hankin17). Similarities between cancer survivors and participants with no history of cancer were found to be consistent across ethnic groups.
Although Latino survivors had the lowest likelihood of meeting WCRF/AICR recommendations compared with their controls, the differences by ethnic group were not significant. Differences between ethnic groups for meeting dietary recommendations were detected; although for no ethnic group did associations differ from unity.
Our findings that the prevalence of overweight and obesity, current smoking, alcohol consumption and diet was similar for cancer survivors and older adults with no history of cancer are generally consistent with those of previous studies(Reference Coups and Ostroff13–Reference Mayer, Terrin and Menon16). Coups and Ostroff(Reference Coups and Ostroff13) reported similar findings for diet, physical activity, alcohol consumption, current smoking and BMI in the 2000 National Health Interview Survey (NHIS). Bellizzi et al.(Reference Bellizzi, Rowland and Jeffery14) also found no difference in current smoking or alcohol use between cancer survivors and non-cancer controls; however, in contrast to the present study and previous findings(Reference Coups and Ostroff13), reported survivors were more likely (9 %) to engage in physical activity when combining data from the 1998–2001 NHIS. Mayer et al.(Reference Mayer, Terrin and Menon16) found no differences in BMI, physical activity, current smoking or fruit and vegetable consumption between cancer survivors and the general population in the National Cancer Institute's 2003 Health Information National Trends Survey. Rogers et al.(Reference Rogers, Courneya and Paragi-Gururaja15) examining prostate cancer survivors surveyed in the 2002 Behavioral Risk Factor Surveillance System reported results similar to ours for BMI, but in contrast found that survivors were more likely to consume fruit and vegetables but not to differ in current smoking or alcohol consumption. Our finding that cancer survivors were less likely to engage in physical activity has been observed in only one other study and was restricted to participants 40–64 years of age(Reference Coups and Ostroff13). Discrepancies in the findings among studies may reflect such factors as differences in the distributions of cancers, differences in analytical approaches and differences in the age distributions of study samples.
The fact that a diagnosis of cancer was not associated with meaningful differences in the behavioural risk factors for cancer is of concern, given the higher risk of cancer and other chronic diseases for cancer survivors. Although evidence directly linking health behaviours in cancer survivors to reductions in disease is limited, improvements in these behaviours are likely to be of benefit in this population. However, as previously discussed(Reference Coups and Ostroff13), improvements in modifiable health behaviours may be of less relevance among those with advanced disease or poorer prognosis, with changes in certain behaviours more or less beneficial for specific cancers. Further studies examining behaviour change within individuals after a diagnosis of cancer(Reference Satia, Campbell and Galanko20–Reference Tangney, Young and Murtaugh22) with sufficient follow-up time are needed to examine the impact of specific behaviours on subsequent disease risk more comprehensively.
Strengths of the present study include: (i) the representativeness of the study population; (ii) the large number of cases; (iii) stratification by cancer site and ethnicity; (iv) the ethnic diversity of the sample and novel findings among Native Hawaiian, Japanese-Americans and Latino cancer survivors; (v) registry confirmation of cases and analyses demonstrating similar results for cases identified by questionnaire or registry; and (vi) detailed dietary information. There were some limitations as well. First, all data on health behaviours were obtained from self-report collected after a diagnosis of cancer and were thus subject to potential recall bias. In addition, the QFFQ has been found to rank individuals well on dietary factors, but has not been validated for identification of individuals meeting dietary recommendations. However, the accuracy would be expected to be similar for cancer survivors and others. Second, the cross-sectional nature of the present study prevents any ability to assess changes in behaviours over time. Because the health behaviours examined are thought to be risk factors for disease, small differences in these behaviours may be indiscernible if they were engaged in less frequently by survivors before their diagnosis of disease. Third, data utilized in the present study examined patterns of behaviour between 1993 and 1996 and, therefore, may not reflect current behaviours. Fourth, for some comparisons, the large sample size could possibly detect as significant those findings that are unimportant clinically.
In conclusion, cancer survivors do not appear to differ from the general population with respect to meeting recommendations regarding diet, physical activity, smoking, alcohol consumption or BMI, despite their increased risk of disease. Treatment practices for cancer survivors, with a more focused approach on behaviour modification during the post-treatment period in order to capitalize on the ‘teachable moment’, as well as follow-up programmes aimed at behavioural maintenance, may assist cancer survivors to achieve improved health-related behaviours. Additional longitudinal studies are needed to determine the extent to which cancer survivors are engaging in long-term behaviour modification, and whether subsequent changes translate into a reduced risk of disease.
Acknowledgements
The Multiethnic Cohort Study has been supported by Grant no. R37 CA 54281 from the National Cancer Institute. The tumour registries are supported by NCI contracts N01-PC-35137 and N01-PC-35139. N.J.O. was supported by a post-doctoral fellowship on Grant no. R25 CA 90956. The authors have no conflict of interest to declare. N.J.O. designed and carried out the statistical analysis, interpreted results and wrote the manuscript; G.M. originally conceived of the analysis and helped with the analytic strategy and interpretation of results; L.R.W. provided expertise pertaining to analytic strategy, statistical analysis and interpretation of results; L.N.K. provided insight into the analytic strategy and interpretation of results. All authors provided feedback on the initial draft of the manuscript and approved the final version. The authors thank all participants in the Multiethnic Cohort Study.







