Iodine is a trace element essential for the synthesis of the thyroid hormones triiodothyronine and thyroxine, and plays an important role in physical growth and mental development( Reference Zimmermann 1 ). Iodine deficiency can impair mental function and physical development, particularly when it occurs during pregnancy or early infancy( Reference Eastman and Zimmermann 2 ). For exclusively breast-fed infants, breast milk provides the sole source of iodine. Breast-milk iodine concentration (BMIC) has been shown to vary between populations, such that women from areas of iodine sufficiency have higher BMIC than women from areas of iodine deficiency( Reference Semba and Delange 3 ). A BMIC of at least 100 µg/l is considered sufficient to provide adequate iodine to meet the needs of breast-fed term infants( Reference Semba and Delange 3 ).
Despite the well-established negative effect of severe iodine deficiency in the perinatal period on infant and child development( Reference Zimmermann 4 ), data regarding the BMIC of Australian lactating women are scarce. Only one small study (n 49), which was conducted over a decade ago, reported a median BMIC of 84 µg/l at ~4 d postpartum( Reference Chan, Hams and Wiley 5 ), suggesting that the iodine content of breast milk was inadequate to meet infant requirements. The Australian National Iodine Nutrition Study of school-aged children in 2006 also suggested the re-emergence of iodine deficiency in Australia( Reference Li, Eastman and Waite 6 ).
In response to concerns about the re-emergence of iodine deficiency in Australia, mandatory use of iodised salt in bread making was introduced in 2009( 7 ). The mandatory iodine fortification was closely followed by recommendations from the National Health and Medical Research Council in 2010 for all pregnant and lactating women to take an iodine supplement of 150 µg/d( 8 ). A small study (n 60) suggested that the iodine status of breast-feeding women in Australia, as assessed by urinary iodine concentration, has improved since the introduction of iodine fortification( Reference Axford, Charlton and Yeatman 9 ). However, there are no studies to date which have assessed BMIC in Australian women after the introduction of mandatory iodine fortification and the National Health and Medical Research Council’s recommendation for iodine supplements.
Therefore, the primary aim of the present study was to compare BMIC from lactating women living in the same region of Australia pre and post mandatory iodine fortification. An additional aim of the study was to evaluate the effect of iodine supplementation on BMIC in the context of mandatory iodine fortification.
Methods
Study setting and participants
The study utilised breast milk samples collected from South Australian women participating in two separate pregnancy nutrition studies: the DOMInO (DHA to Optimise Mother Infant Outcome)( Reference Makrides, Gibson and McPhee 10 ) and the PINK (Pregnancy Iodine and Neurodevelopment in Kids) studies( Reference Condo, Skeaff and Ryan 11 ). Breast milk samples were collected between 2006 and 2007 in the DOMInO study (pre-fortification) and between 2012 and 2013 in the PINK study (post-fortification).
The inclusion criteria for the DOMInO study were women with a singleton pregnancy less than 20 weeks’ gestation who were not taking fish oil or prenatal supplements containing DHA. The inclusion criteria for the PINK study were any women less than 20 weeks’ gestation without a history of thyroid disease( Reference Condo, Skeaff and Ryan 11 ). Both studies excluded women with known major fetal abnormalities or a history of drug or alcohol abuse. Women were also excluded in both studies if English was not the main language spoken at home. All women were recruited at the Women’s and Children’s Hospital, Flinders Medical Centre or Flinders Private Hospital in Adelaide, South Australia.
Assessment of breast-milk iodine concentration
The breast milk samples were collected at either the hospital or the participant’s home within 7 d of delivery using sterile 70 ml specimen collection containers (Techno Plas, Australia). In the DOMInO study breast milk samples were stored at −20°C after collection until analysis, whereas in PINK study samples were stored at −20°C for an average of 9 d before being delivered to the central laboratory after which they were stored at −80°C until analysis.
BMIC was determined using a validated method of inductively coupled plasma–mass spectrometry( Reference Huynh, Zhou and Gibson 12 ). Using this method the results obtained for the external standard, NIST 1549 milk powder (National Institute of Standards and Technology (NIST), Gaithersburg, MD, USA), was 3·38 (sd 0·02) mg/kg compared with the certified value of 3·38 (sd 0·02) mg/kg. The method’s quantification limit was 1·6 µg/l. The intra-assay and inter-assay CV was <1 % and 3·5 %, respectively.
Sociodemographic information
Information on sociodemographic characteristics of the women, including age, ethnicity, education, employment status, parity, alcohol consumption and smoking status, was collected at the time of enrolment in both the DOMInO and PINK studies based on self-reporting. Ethnicity was classified as Caucasian, Aboriginal/Torres Strait Islander, Pacific Islander, Maori, Asian, Indian, African Black and other. Only a small number of participants reported their ethnicity as not being Caucasian (6 % pre-fortification and 16 % post-fortification). Therefore, participants were classified as being of either ‘Caucasian’ or ‘non-Caucasian’ ethnicity for the purpose of the present study. Height and weight of women in both cohorts were also obtained at study entry and used to calculate maternal BMI (kg/m2).
No specific information regarding intake of supplements containing iodine was collected in the DOMInO study. However, a surveyed conducted in July 2009 (two years after the commencement of the DOMInO study) reported that a number of dietary supplements targeted at pregnant women contained at least some iodine( Reference Gallego, Goodall and Eastman 13 ). Thus, in order to assess the effect of iodine fortification alone on BMIC, women in the pre-fortification (DOMInO) cohort who reported taking dietary supplements that potentially contained iodine were grouped as the ‘iodine supplement’ group. The remaining women were grouped as the ‘non-iodine supplement’ group. The PINK study collected more detailed information on the specific brand/name of supplements taken by women; on the basis of this information, women were classified into either the ‘iodine supplement’ group if they were consuming any supplements containing iodine or the ‘non-iodine supplement’ group if they did not take any dietary supplements or if the supplements they were taking did not contain iodine.
Data analysis
The statistical software package SPSS Statistics Version 17.0 was used for all statistical analyses. Differences in sociodemographic, maternal health and lifestyle baseline characteristics between the pre- and post-fortification samples were determined by the Mann–Whitney test for non-normally distributed continuous variables or the χ 2 test for categorical variables. Evidence from the sole iodine balance study to date indicated that a positive iodine balance in full-term infants is achieved only when iodine intake is 15 µg/kg per d, which is equivalent to a BMIC of approximately 100 µg/l( Reference Semba and Delange 3 ). Therefore, our study used the cut-off of 100 µg/l based on a review of the literature. The median BMIC was determined and the proportion of women with BMIC below 100 µg/l, the suggested cut-off for providing an adequate iodine supply for breast-fed infants( Reference Semba and Delange 3 ), was also determined. A linear regression model was fitted to estimate the effect of mandatory iodine fortification on BMIC. The log of breast-milk iodine was used for analyses due to the skewed distribution of BMIC. The estimates (differences in means on the log scale) were subsequently back-transformed to the original scale and are reported as ratios of geometric means. An interaction term was fitted to test for effect modification of iodine fortification by supplement use and estimates of the effect of fortification were derived separately for the iodine supplement and non-iodine supplement groups. Due to differences in some key sociodemographic characteristics between women in the pre- and post-fortification samples, the analyses were adjusted for maternal BMI at study entry, maternal smoking during pregnancy, maternal alcohol consumption during pregnancy, parity (0 v. ≥1) and completion of secondary education. Additional linear models, using data from both the pre- and post-fortification cohorts and BMIC as a continuous variable, were also used to identify significant predictors of BMIC. A P value of <0·05 was considered statistically significant.
Results
A total of 1660 pregnant women were enrolled in the DOMInO study in Adelaide sites between 2006 and 2007, and 1129 (68 %) of these women reported having ever breast-fed their infant. Breast milk samples were collected from 571 (51 %) of these women for the analysis of breast-milk fatty acid profile as part of the DOMInO study. Of the breast milk samples collected, surplus breast milk samples from 291 women were available for the assessment of BMIC in the current study. There were no differences in sociodemographic (ethnicity, education, currently in paid employment, parity and age) and lifestyle (smoking and alcohol consumption at study entry) characteristics between breast-feeding women whose samples were included in the analysis of BMIC and those who either did not provide a sample or for whom there was no surplus sample available for the analysis.
Of the 783 pregnant women who were recruited into the PINK study, 708 (90 %) were breast-feeding at birth and breast milk samples were collected at birth from 653 (92 %) of these women.
The baseline demographic information of the women whose BMIC was assessed in the present study is shown in Table 1. Compared with women in the pre-fortification group, women in the post-fortification sample were older, more educated, and a lower proportion reported smoking or consuming alcohol at the time of enrolment (P<0·01).
Table 1 Demographic characteristics at enrolment (≤20 weeks’ gestation) of South Australian women participating in two separate pregnancy nutrition studies, before (2006–2007) and after (2012–2013) the mandatory iodine fortification of bread in Australia in 2009
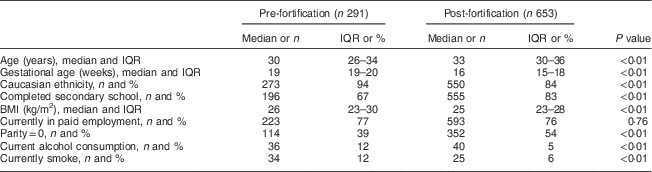
IQR, interquartile range.
Breast-milk iodine concentration pre and post mandatory iodine fortification
BMIC pre and post mandatory iodine fortification are reported in Table 2. Median (interquartile range) BMIC was significantly higher in the post-fortification samples compared with the pre-fortification samples (187 (130–276) v. 103 (73–156) µg/l; P<0·001).
Table 2 Breast-milk iodine concentration (µg/l) of South Australian women participating in two separate pregnancy nutrition studies, before (2006–2007) and after (2012–2013) the mandatory iodine fortification of bread in Australia in 2009
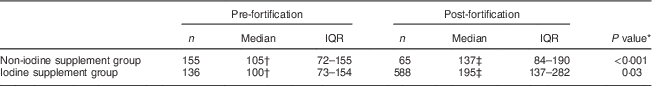
IQR, interquartile range.
* Adjusted for cohort (the effects of pre and post fortification), iodine supplement use, maternal BMI, smoking, alcohol consumption, parity, ethnicity and secondary education.
† There was no difference between the non-iodine supplement and the iodine supplement group in the pre-fortification cohort, P=0·93.
‡ There was a significant difference between the non-iodine supplement and the iodine supplement group in the post-fortification cohort, P<0·001.
There was a statistically significant interaction between cohort (pre- v. post-fortification) and supplement use (P<0·001). Therefore, regression analysis to assess the effect of iodine fortification on BMIC was performed separately in the iodine supplement and the non-iodine supplement groups. After adjustment for the effect of smoking, maternal BMI, alcohol consumption, ethnicity, parity and secondary school completion, the estimated geometric mean BMIC in the post-fortification samples was 1·8 times (95 % CI 1·6, 2·0) that of the pre-fortification samples in the iodine supplement group and 1·2 times (95 % CI 1·0, 1·4) in the non-iodine supplement group.
The proportion of women with BMIC <100 µg/l was lower in the post-fortification compared with the pre-fortification cohort (13 v. 49 %; P<0·001). In the post-fortification cohort, where detailed information on intake of iodine supplements was available, the proportion of women with BMIC <100 µg/l was lower in women who took iodine supplements in pregnancy compared with women who did not take any supplements containing iodine (12 v. 29 %; P<0·01).
Predictors of breast-milk iodine concentration of lactating women
Maternal smoking status, alcohol consumption, ethnicity and parity were all identified as predictors of BMIC, whereas maternal BMI and completion of secondary education were not (Table 3). BMIC was negatively associated with smoking, but positively associated with alcohol consumption. Non-Caucasian mothers had higher BMIC than Caucasian mothers and mothers with parity ≥1 had higher BMIC than nulliparous mothers (Table 3).
Table 3 Predictors of breast-milk iodine concentration* of South Australian women
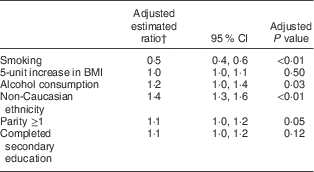
* Breast-milk iodine concentration as a continuous variable.
† Adjusted for cohort (the effects of pre and post fortification), iodine supplement use, maternal BMI, smoking, alcohol consumption, parity, ethnicity and secondary education.
Discussion
The present study is the first to assess the impact of mandatory iodine fortification on BMIC in lactating women in Australia and demonstrates that mandatory iodine fortification has resulted in an increase in the median BMIC in this population.
The median BMIC before the introduction of mandatory iodine fortification could be interpreted as indicating borderline iodine deficiency because almost half of the pre-fortification samples had BMIC below 100 µg/l (the level considered adequate( Reference Semba and Delange 3 )). Although breast milk samples were available for analysis from only a relatively small percentage of the breast-feeding women in the original DOMInO cohort, key sociodemographic and lifestyle characteristics of the women whose samples were analysed in the current study were not different from those of breast-feeding women whose samples were not included. This gives us confidence that the BMIC in these women is representative of the breast-feeding women in the DOMInO cohort as a whole.
The introduction of iodine fortification has resulted in an improvement in the median BMIC as well as a reduction in the percentage of women having BMIC below the cut-off level. However, it is also clear that the proportion of women in the post-fortification cohort with BMIC below the cut-off was notably higher in women who did not take iodine supplements compared with those who did. We interpret these results to suggest that, although the mandatory iodine fortification has resulted in an overall increase in BMIC in Australian women, iodine supplementation may be required in some women to reach the BMIC level that is considered adequate (≥100 µg/l)( Reference Semba and Delange 3 ) to meet the iodine requirement of full-term infants. There is limited information regarding changes in BMIC across lactation and further research investigating whether iodine supplementation is needed throughout lactation to maintain BMIC at or above 100 μg/l is required. Currently there are no data on the relationship between BMIC and growth and development of breast-fed infants. Further research to determine the BMIC that is associated with optimal growth and development of infants is warranted.
Our finding that BMIC was lower in smokers than in non-smokers across the whole population is consistent with previous studies. For example, Laurberg and colleagues found that smoking, as reflected by nicotine level measured at birth, was associated with an approximately 50 % reduction in BMIC( Reference Laurberg, Nohr and Pedersen 14 ). The reduction in BMIC in smokers is thought to be related to the thiocyanate in cigarettes which acts to inhibit iodine uptake from the maternal circulation into breast milk( Reference Laurberg, Nohr and Pedersen 14 – Reference Vinnakota, Peetha and Perrizo 16 ). Although unexpected, the positive association between BMIC and reported alcohol consumption at study entry is consistent with the results of a national survey of pregnant women in Belgium, which reported that women who were consuming alcohol in the first trimester of pregnancy had a lower risk of iodine deficiency in the first and third trimesters of pregnancy than women who did not drink( Reference Vandevijvere, Amsalkhir and Mourri 17 ). Knudsen and colleagues suggested that this may be related to the inhibiting effect of alcohol on thyroid hormone metabolism, which reduces the iodine requirements for thyroid hormone synthesis and increases the iodine available for transfer into breast milk( Reference Knudsen, Bulow and Laurberg 18 ). It is also important to note that both the effect of alcohol consumption on BMIC in our study and the number of women who reported drinking alcohol were small. Therefore, these results need to be interpreted with caution and further studies are required to confirm this finding.
Significant associations between urinary iodine concentration and sociodemographic factors including ethnicity and education level have been reported in several previous studies in adult populations( Reference Luo, Xu and He 19 , Reference Pfeiffer, Sternberg and Caldwell 20 ). Our study is the first to report that non-Caucasian mothers had significantly higher BMIC compared with Caucasian mothers. This finding is in line with the results of a study conducted in Melbourne (Australia) which reported that urinary iodine concentration of Caucasian pregnant women was significantly lower than that of non-Caucasian pregnant women( Reference Hamrosi, Wallace and Riley 21 ). These differences may be due to differences in dietary behaviours between these groups.
Few studies have investigated the association between parity and BMIC, and the results have been inconsistent. A previous study in Portugal reported that BMIC at 3 d and 3 months postpartum were not different between nulliparous and multiparous women( Reference Costeira, Oliveira and Ares 22 ), which is in contrast to our finding of higher BMIC at 3 months postpartum in multiparous women. It should be noted, however, that the previous study included a smaller sample size (n 165) compared with our study (n 944). Furthermore, the iodine status of Portuguese lactating women was deficient while the population of lactating women in the current study was largely iodine sufficient. Therefore, further studies are required to evaluate the relationship between parity and BMIC.
One of the limitations of our study is that the sample storage conditions and duration were different between the pre- and post-fortification samples. However, a pilot study indicated that there were no differences in BMIC when the same sample was stored under two different conditions (at −20°C or −80°C) over a period of up to 18 months (D Huynh, B Muhlhausler, SJ Zhou et al., unpublished results), although we cannot exclude the possibility of differences in BMIC between the two storage conditions beyond 18 months’ storage. However, our finding of a borderline sufficient iodine status in BMIC pre-fortification is consistent with the finding of borderline sufficient iodine status in a survey of schoolchildren in South Australia conducted in the same period( Reference Li, Eastman and Waite 6 ). Furthermore, we did not provide specific instructions to women regarding the collection of fore or hind milk. While no studies to date have compared BMIC in fore and hind milk, the concentrations of a number of other micronutrients are known to differ between these fractions.
Another limitation is that we did not assess dietary intake of the women in the current study. It is possible that dietary habits of lactating women may have changed between the pre- and post-fortification periods which may contribute to the higher BMIC in the post-fortification cohort. However, data from the two latest National Nutrition Surveys (conducted in 1995 and 2011) indicated that in women of childbearing age, intake of the main sources of dietary iodine in Australia including bread and dairy products was lower in the period post-iodine fortification compared with pre-fortification( Reference McLennan and Podger 23 , Reference Palmer 24 ), although the data might not be directly compatible. In addition, it has been shown that there was no difference in the percentage of pregnant and lactating women who used iodised salt before and after the introduction of iodine fortification( Reference Charlton, Yeatman and Lucas 25 ). We also found no difference in iodine status (indicated by urinary iodine concentration) between pregnant women who used iodised salt and those who used non-iodised salt in the post-fortification cohort( Reference Condo, Makrides and Skeaff 26 ). Therefore, it appears unlikely that increased intake of bread or dairy products or use of iodised salt could account for the higher BMIC in the post-fortification cohort.
Although the pre- and post-fortification cohorts were both recruited from the same region of South Australia, the shift in the demography of the pregnant population in South Australia between these periods contributed to differences that may have introduced bias. The differences in the grouping of the participants into ‘iodine supplement’ and ‘non-iodine supplement’ groups between the two cohorts may also lead to other potential biases in the comparison of BMIC.
Conclusion
The current study is the first to report BMIC in lactating women in Australia after the implementation of mandatory iodine fortification of bread and suggests that BMIC in the majority of these women is adequate to meet the iodine requirement of their breast-fed infants. However, iodine supplementation may be required in some women to achieve a BMIC that is considered adequate to meet the iodine requirement of full-term infants. This comparison of BMIC in the same region of Australia supports the suggestion that the mandatory iodine fortification programme has resulted in an increase in the iodine content of breast milk in lactating women. Further research evaluating the BMIC level associated with optimal development in children is warranted.
Acknowledgements
Acknowledgements: The authors are grateful to Waite Analytical Service, School of Agriculture, Food and Wine, University of Adelaide for assistance with method development and validation. They thank Jenni Louise for statistical assistance. B.M. is supported by a Career Development Fellowship from the National Health and Medical Research Council of Australia (NHMRC). R.G. and M.M. are supported by NHMRC Senior Research Fellowships. D.H. is sponsored by the Vietnamese Ministry of Education and Training (project 322), the University of Adelaide and FOODplus Research Centre. D.C. is supported by the University of Adelaide, Women’s and Children’s Health Research Institute and Women’s and Children’s Hospital Foundation. Financial support: This work was supported by the NHMRC (grant number 626800). This funding body had no role in the design, analysis or writing of this article. Conflict of interest: R.G. and M.M. received honoraria for scientific advisory board contributions to Fonterra (Auckland, New Zealand). M.M. also received honoraria for scientific advisory board contributions to the Nestlé Nutrition Institute (Vevey, Switzerland) and Nutricia. All honoraria are paid to their institutes to support continuing education activities for students and postgraduates. The other authors have no conflict of interest. Authorship: R.G., M.M., B.M. and S.J.Z. designed the study; D.C collected samples; D.H. performed breast milk analyses and drafted the manuscript with contributions from all authors. All authors reviewed and approved the manuscript submitted. Ethics of human subject participation: This study were conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving the participants were approved by the Women’s & Children’s Health Network Human Research Ethics Committee (reference numbers REC 1657/2/11 and REC 2230/12/15) and the Southern Adelaide Clinical Human Research Ethics Committee (reference number 199.045). Written informed consents were obtained from all participants.






