In recent years, governments around the world have focused on the rising burden of chronic disease and its growing impact on health system costs. Nutrition and diet-related activities are recognized elements of proposed national and international risk reduction strategies for chronic disease. In the past decade, several major studies have drawn the link between infant feeding and later-life chronic disease(1, 2).
The higher risk of infectious illnesses and the immunological vulnerability of non-breast-fed infants is well known(Reference Gartner, Morton and Lawrence3). Evidence has recently been accumulating on associated increases in both maternal and infant risk for a number of chronic diseases in later life. These include obesity(Reference Arenz, Ruckerl and Koletzko4, Reference Owen, Martin and Whincup5), diabetes(Reference Owen, Martin and Whincup6), CVD risk including high blood pressure(Reference Martin, Gunnell and Davey Smith7), as well as some childhood cancers(Reference Martin, Gunnell and Owen8), breast cancer in the mother(9) and a range of chronic digestive (ulcerative colitis, Crohn’s disease and coeliac disease)(Reference Akobeng, Ramanan and Buchan10, Reference Klement and Reif11) and allergic diseases, including asthma(Reference Gdalevich, Mimouni and David12–Reference Oddy14). In 2006, following a review of potential prevention strategies(15), the European Council of Ministers endorsed breast-feeding as a key action area for addressing obesity(16). The World Cancer Research Fund Expert Report has recommended that mothers breast-feed and that children be breast-fed to reduce their risk of cancer(17). In Australia, a key implementation action area in the National Chronic Diseases Strategy(18) is to increase the rates of full breast-feeding at 6 months. The strategy regards the need to start early to ensure success in preventative measures and concludes:
Full breastfeeding for at least the first six months of life offers considerable health benefits to infants, and potential benefits over the entire lifespan of the individual. Breast fed infants are less likely to develop high blood pressure, some infectious diseases, and some diet related chronic diseases later in life(18).
Few populations around the world achieve the WHO recommendation for infant feeding, i.e. for exclusive breast-feedingFootnote † to 6 months with continued breast-feeding along with appropriate complementary foods to 2 years and beyond(19). For example, in Australia, only about half the infants are still breast-feeding at 6 months(20). In situations where a large proportion of the population is exposed, public health implications of even small risks can be striking(Reference Egger, Schneider and Davey Smith21).
Infant feeding practices are determined by a wide range of cultural, social and economic factors, not just potential health impacts. Opinions differ on whether breast-feeding saves time and work for mothers, but there is evidence that breast-fed infants spend more time interacting with their mothers, including on feeding, and that employment is associated with reduced breast-feeding(Reference Baxter and Smith22–Reference Ryan, Zhou and Arensberg26). Indeed, the health promotion model of breast-feeding has been criticized because of dilemmas for maternal employment(Reference Hausman27–Reference Hausman30). Media attention focuses intensely on any studies purporting to challenge the importance of breast-feeding(Reference Smith31–33) and questions have been raised about promoting breast-feeding as environmental pollutants can be measured in breast milk(Reference Eggesbø, Stigum and Longnecker34–Reference Stein, Savitz and Dougan36).
The aim of the present study is to provide a summary assessment of the public health significance of premature weaning of infants from breast milk and breast-feeding for the prevalence of chronic illness in the Australian population.
Method
The objective of the present study is to estimate the population-attributable proportion of chronic disease associated with artificial feeding in infancy in a developed country such as Australia in order to evaluate the public health significance of breast-feeding for chronic disease prevention.
First, we review evidence from recent meta-analyses on links between lack of breast-feeding in infancy and later-life chronic disease. We identify those chronic diseases with evidence supporting possible or probable links between artificial feeding and later-life chronic disease and illness: obesity, type 1 and type 2 diabetes, CVD, asthma, coeliac disease, inflammatory bowel disease and childhood cancer. We conducted a Medline search (1966–2007) to identify relevant meta-analyses and hand-searched cited articles for other studies. We reviewed these meta-analyses to obtain the most reliable and recent estimates of OR and relative risk. In most cases, there were only one or two meta-analyses. We prioritized results from higher-quality studies if ambiguity existed between meta-analyses and, where possible, used results that reflected adjustment for important potential confounding sociodemographic, economic and anthropometric variables.
Second, we reviewed evidence on risk exposure levels, using long-term historical data on breast-feeding rates that were collected at child health clinics in Victoria, Australia, since the 1920s. As ethical considerations limited experimental studies in this field, we also provided a commentary on methodological issues currently encountered in breast-feeding research and plausible causal mechanisms.
It is not well established that the degree of exposure to artificial feeding is associated with heightened later chronic illness risk, because existing research uses a variety of definitions of exposure to premature weaning to compare chronic disease prevalence in breast-fed with non-breast-fed groups. We therefore estimate the attributable proportion of chronic disease incidence in Australia for six scenarios regarding the extent of early-life exposure to artificial feeding. These estimates correspond to the potential health burden for three distinct historical cohorts and for feeding status at either 6 months or at hospital discharge. This approach allows us to assess the extent of chronic disease that is potentially avoided by increased breast-feeding for the population as a whole in a developed country like Australia in a manner that allows for uncertainty arising from existing limitations in breast-feeding research.
Results
Relative risk estimates
Our search identified fourteen meta-analyses, which provided estimates of pooled adjusted relative risks or OR for eight chronic disease conditions. Table 1 presents a summary of results of these meta-analyses. Our preferred relative risk estimates and the studies they were drawn from are highlighted in the table. Below we discuss the various meta-analyses and the strengths of those that are selected as the basis of our preferred relative risk estimates.
Table 1 Results from meta-analyses of epidemiological studies on infant feeding and later disease riskFootnote *

RR, relative risk; N/A, not applicable.
* Shading represents our preferred estimates for use in later calculations.
† Assuming RR approximates the inverse of the OR, where OR represents the protective effect of breast-feeding and RR represents the risk of artificial feeding.
‡ Confidence limits are based on 95 % CI of OR.
Obesity
There have been five recent meta-analyses in which the outcome was the risk of obesity or overweight(Reference Arenz, Ruckerl and Koletzko4, Reference Owen, Martin and Whincup5, Reference Harder, Bergmann and Kallischnigg37–Reference van Rossum, Buchner and Hoekstra39). The relative risk for the artificially fed group was found to be 11–28 % higher than for breast-fed infants. Our preferred estimate is that of Horta et al.(Reference Horta, Bahl and Martines38), which included the largest number of studies, including the most recent one. The authors reported that controlling for confounding by socio-economic status and parental anthropometry did not modify the effect of breast-feeding. A dose-dependent relationship was evident in two of the meta-analyses(Reference Owen, Martin and Whincup5, Reference Harder, Bergmann and Kallischnigg37). For example, Harder et al.’s(Reference Harder, Bergmann and Kallischnigg37) meta-analysis of seventeen different studies measuring the duration of breast-feeding (121 000 participants) found that the probability of overweight/obesity in later life was increased by 4 % for each month of not breast-feeding.
Diabetes
The risk of type 1 diabetes among different infant feeding groups has been examined by two meta-analyses(Reference Gerstein40, Reference Norris and Scott41). Norris and Scott(Reference Norris and Scott41) were concerned with the confounding issue of recall bias in their meta-analysis of seventeen case–control studies, whereas Gerstein(Reference Gerstein40) assessed four case–control studies which met the high-quality methodological criteria. Commonly considered confounders in these studies were family history of type 1 diabetes, neonatal illness, maternal age at birth, birth order, maternal/parental education and type of delivery. The present study combines both adjusted and unadjusted OR to estimate a relative risk of 1·43 for those artificially fed younger than 3 months of age compared to those who experienced any breast-feeding beyond 3 months(Reference Gerstein40).
Two meta-analyses of studies on the risk of type 2 diabetes were identified. Owen et al.(Reference Owen, Martin and Whincup6) and Horta et al.(Reference Horta, Bahl and Martines38) covered the same studies and obtained similar estimates. However, as the earlier review included a larger number of studies, this meta-analysis is preferred. This review of seven studies included 76 744 infants. A dose-dependent effect of exposure to artificial feeding was found by both the research groups(Reference Owen, Martin and Whincup6, Reference Horta, Bahl and Martines38). Owen et al. noted that maternal social class, maternal weight and low birth weight could influence both the likelihood of breast-feeding and the risk of later diabetes. However, adjustment for such confounders had little effect on the association between breast-feeding and risk of diabetes.
Heart, stroke and vascular disease
Two studies have examined the relationship between infant feeding practices and blood pressure as an indicator of later-life cardiovascular health(Reference Martin, Gunnell and Davey Smith7, Reference Owen, Whincup and Gilg42). The meta-analysis by Martin et al.(Reference Martin, Gunnell and Davey Smith7) includes data on extra infants from additional studies. It reports that reductions in population mean blood pressure levels of the magnitude found, if causal, ‘could reduce the prevalence of hypertension by up to 17 percent, the number of coronary heart disease events by 6 percent, and strokes and transient ischemic attacks by 15 percent’ (p. 24). This was not influenced by adjustment for accelerated postnatal weight gain, although residual confounding for socio-economic factors was possible. We have estimated the relative risks for hypertension, CHD and strokes/ischaemic attacks based upon these percentages.
Asthma
Two meta-analyses have examined the effects of infant feeding on childhood risk of asthma and have concluded that lack of breast-feeding is associated with an increased risk of developing asthma(Reference Gdalevich, Mimouni and Mimouni13, Reference Ip, Chung and Raman43). This association is stronger for those infants with a family history of asthma. Data presented in Table 1 show the risk of developing asthma for artificially fed infants without a family history of asthma.
Ip et al.(Reference Ip, Chung and Raman43) included two additional studies to the five examined by Gdalevich et al.(Reference Gdalevich, Mimouni and Mimouni13) and found a similar increased risk of approximately 37 % for asthma in children that were artificially fed as infants without a family history of asthma. Potential confounders, including age, socio-economic status, family history of atopy and parental smoking, were controlled for. No significant relationship has yet been determined between the timing of weaning from breast-feeding and the prevalence of asthma(Reference Ip, Chung and Raman43).
Coeliac disease
Only one meta-analysis has examined coeliac disease and its association with infant feeding. Akobeng et al.’s(Reference Akobeng, Ramanan and Buchan10) analysis of six studies found that among infants who were not breast-feeding at the time when gluten was introduced into the infant’s diet, the risk of coeliac disease in later life was doubled. Studies controlled for age, sex and area of residence, although socio-economic status may not be fully controlled for.
Inflammatory bowel disease
Klement et al.(Reference Klement, Cohen and Boxman44) examined four studies (1359 infants) on Crohn’s disease and ulcerative colitis, concluding that infants who were not breast-fed had an increased risk of both conditions, ranging from 30 % to 49 %. There was little difference between crude and adjusted OR for various confounders (diarrhoeal disease during infancy, sex, age, race, birthplace, sibship size, birth order, maternal age, smoking and the use of oral contraceptives), suggesting that confounding did not significantly bias results.
Childhood cancer
Martin et al.(Reference Martin, Gunnell and Owen8) reviewed twenty-six studies that provided OR for at least one childhood cancer outcome. For artificially fed infants, an increased risk of all childhood cancers was determined from seven studies as 28 %, and of all childhood leukaemias from twelve studies as 12 %. The majority of childhood leukaemia is diagnosed as acute lymphocytic leukaemia and the most recent meta-analysis by Ip et al.(Reference Ip, Chung and Raman43) found an increased risk of this chronic disease of 25 % for infants breast-fed for <6 months. A dose-dependent relationship was evident(Reference Ip, Chung and Raman43, Reference Kwan, Buffler and Abrams45). The result accounts for known confounding variables, including socio-economic status, although biological mechanisms, including via infectious exposures, remain unclear.
Population risk exposures
Official national data on historical trends and patterns in infant feeding are lacking in Australia, with only a small number of national surveys and difficulties in comparability over time(46). However, a variety of nationwide(47–49) and state surveys(Reference Gabriel, Pollard and Suleman50, Reference Hector, Webb and Lymer51) suggest that only 5 % of Australian infants are exclusively breast-fed at 6 months, and only 10 % receive any breast milk at 12 months. Approximately 60 % of Australian infants are fully weaned from breast milk by 6 months of age(Reference Hector, Webb and Lymer52). Nationally, individuals in the lowest two socio-economic deciles are twice as likely as those in the top two deciles to have never been breast-fed(53). Up to a third of indigenous Australians are never breast-fed(Reference Hector, Webb and Lymer52).
Long-term trends in infant feeding in Australia can be compiled from historical data sets on breast-feeding(Reference Mein-Smith54–Reference Siskind, Del-Mar and Schofield56). Victorian infant health clinic data for the period from 1927 to the present provide an important source of information on long-term trends in the rates of ‘full’ breast-feeding.Footnote * This is supplemented by data from Queensland available for the period 1939–1976.
On the basis of available data, the approximate exposure rates of the current Australian population of infants and children, young or middle-aged and elderly adults can be represented as in Table 2. In the present study, we have chosen to discuss three population age cohorts with distinct exposures categorized as follows: ‘low exposure’ (aged adults who were born before 1940); ‘moderate exposure’ (current infants, children, adolescents and young adults who have been born since 1980) and ‘high exposure’ (adults born during 1965–1975). As shown in Fig. 1(Reference Smith57) and Table 2, an exposure of 30 % in infancy was experienced by current adults (aged 35–45 years) born during 1965–1975.
Table 2 Approximate risk exposures to artificial baby milk

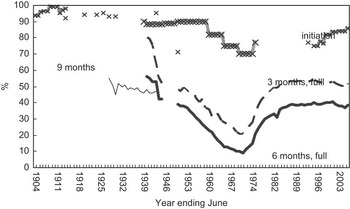
Fig. 1 Infant feeding practices in Australia during 1945–2005 (reproduced from Smith(Reference Smith57))
Public health significance
Table 3 presents estimates of population-attributable proportion of chronic disease risk in Australia, assuming that 30 % of the population is exposed to higher chronic disease risk due to artificial feeding in infancy.
Table 3 Attributable proportion of chronic disease risk assuming 30 % exposure

RR, relative risk.
*Population-attributable proportion is calculated as P e(RR − 1)/[1 + P e(RR − 1)], where P e is the prevalence of exposure to artificial feeding and RR is the relative risk calculated as the ratio of the incidence of morbidity in artificially fed infants to the incidence in breast-fed infants.
†The RR ratio of 2·08 for coeliac disease is that associated with being weaned from breast-feeding before solids are introduced. We have calculated the population-attributable proportion here by assuming that 30% of infants are not being breast-fed at the time of introduction of solid food (P e).
‡A RR ratio of 1·40 is calculated from an average of the OR for Crohn’s disease and ulcerative colitis.
The attributable proportion of chronic disease incidence in the current child and adult population will depend on the particular age cohort under consideration, as well as the range of possibilities regarding the critical level of exposure to artificial infant feeding. For example, if approximately 60 % of infants are exposed to premature weaning from breast-feeding (a definition corresponding approximately to the proportion of Australian infants born since 1980, who were weaned from exclusive breast-feeding before 6 months of age), a range of 11–36 % of chronic disease incidence might be avoided through more optimal breast-feeding rates (Table 4). If breast-feeding rates were improved to Australia’s National Health Target levels of around 80 %, i.e. an exposure reduced to 20 % of the population, this intervention would reduce the attributable proportion of chronic disease in the population to a range of 4–18 %.
Table 4 Attributable proportion of chronic disease risk for different scenarios or cohorts of exposure to lack of breast-feeding

IBD, inflammatory bowel disease; RR, relative risk.
*The RR ratio of 2·08 for coeliac disease is that associated with being weaned from breast-feeding before solids are introduced. We have calculated this figure for coeliac disease by assuming that the exposure (P e) is not being breast-fed at the time of introduction of solid food (P e).
Discussion
The effect estimates from the meta-analyses above may appear small in size (e.g. the relative risk for CHD is 1·06), but they have the potential to translate into the prevention of a substantial prevalence of chronic disease in cohorts or population subgroups in which the risk exposure is high(Reference Egger, Schneider and Davey Smith21).
Our analysis uses meta-analyses of risk of chronic disease to identify robust relative risk estimates from the literature. The advantage of meta-analyses is that they make available in summary form large quantities of information, which helps to establish generality and consistency of effects(Reference Mulrow58). Well-conducted meta-analyses can thus contribute to a more objective appraisal of epidemiological evidence and provide a more precise estimate of treatment effects(Reference Egger and Smith59). Despite their advantages as a scientific tool, however, meta-analysis of observational studies can produce spurious results if these studies are distorted by confounding or selection bias(Reference Egger, Schneider and Davey Smith21). Methodological standards for assessing quality in breast-feeding research established by Bauchner et al.(Reference Bauchner, Leventhal and Shapiro60) and Kramer(Reference Kramer61) are control for important confounding variables, adequate statistical power, clear definition of ‘breast-feeding’(Reference Labbok and Krasovec62, Reference Heinig63), avoidance of detection bias and clearly defined outcome events, including information on the severity of outcome.
The meta-analyses discussed in the present study illustrate some important methodological issues in infant feeding research, which result in continuing uncertainty about the critical level of exposure and about the magnitude of the effects on chronic disease risk of artificial feeding in infancy. First, there are very few randomized control trials that compare the effects of breast-feeding v. artificial feeding. This is because it would be unethical to deliberately deprive infants of breast-feeding since breast-feeding is known to be important and desirable in nearly all cases. Therefore, most studies included in the meta-analyses to date are observational. It is difficult for such studies to show causation and the results can also be confounded by unobserved differences in the groups’ characteristics, other than the infant feeding method, which also affect the risk of chronic disease. This difficulty is especially so if the studies are comparing later-life outcomes, when many environmental or behavioural factors could affect the outcome.
It is also important to consider whether the study is large enough to show an effect. Many studies on infant feeding are small in scale and have too few infants who are exclusively breast-fed or exclusively artificially fed for the study to have adequate statistical power to confirm differences between the infant feeding methods. When there are also problems in how the feeding groups are defined, small studies showing no difference between feeding groups are even more unreliable.
The inconsistent or inappropriate definitions of breast-feeding introduce a significant lack of precision that is a major problem in existing breast-feeding research(Reference Labbok and Krasovec62). For example, in the meta-analyses reviewed in the present study, the infant feeding categories were highly diverse. Artificially fed groups included infants fed cow’s milk, standard formula, preterm formula, fatty acid supplemented formula or breast-fed for x number of days/weeks/months only. Similarly, ‘breast-fed’ was variously defined as ever breast-fed, breast-fed for more than x number of days/weeks/months or exclusively breast-fed for more than x number of days/weeks/months. Artificially fed infants were more often misclassified as breast-fed. The most common classification in the studies included in the meta-analyses was ‘ever’ v. ‘never’ breast-fed. For example, a study of childhood cancer states that ‘in 92 % of reviewed studies, measurement of exposure was limited to whether the child had ever or never been breastfed’(Reference Martin, Gunnell and Owen8). Therefore, there is a likely understatement of the effect of infant feeding due to non-random misclassification. This will affect both statistical significance and effect sizes in such studies. Indeed, most studies wrongly conceptualize breast-fed infants as the exposed or intervention group, rather than characterizing artificial feeding as the exposure(Reference Smith, Dunstone and Elliott-Rudder64).
Risk exposure is admittedly difficult to define in the area of infant feeding. Definitions that are suitable for monitoring and surveillance of breast-feeding trends may not be optimal for clinical or epidemiological research(Reference Labbok and Krasovec62, Reference Webb, Marks and Lund-Adams65). For some conditions, a single exposure to artificial feeding in early infancy may be expected to trigger increased risk of chronic disease(Reference Host66), but for other conditions with a different aetiology, different health outcomes are expected from varying durations of exposure over several weeks or months. The greater effect size we observed in studies with more precise measurement of duration or intensity of exposure to artificial feeding suggests that pooled estimates from meta-analyses, which include studies with ambiguous measures of exposure and inadequate specification of the different feeding groups, may understate the magnitude of the effects of early infant feeding on chronic disease in later life.
It also noteworthy that the findings of consistent reductions in risk for breast-fed infants across many long-term health outcomes are based on data for populations exposed to a variety of environmental contaminants during their infancy and adult life. The present study includes a broad range of outcomes and is important in providing a balanced perspective on this issue. The common practice of accurately measuring exposures to environmental pollution via the convenient, non-invasive testing of collected breast milk may confuse and distort the perspectives on risks and infant feeding by wrongly inferring that breast milk (the ‘messenger’) rather than environmental pollution is the health risk, or that formula feeding involves no health risks(Reference Van Esterik67). Neurotoxic effects have rarely been linked to exposure during infancy or childhood(Reference Jacobson and Jacobson68) and in virtually all studies, beneficial effects of breast-feeding outweigh any potential adverse effect of milk organochlorine contaminants(Reference Korrick and Sagiv69). Lower weight gain of breast-fed infants is presented in some studies(Reference Grandjean, Budtz-Jorgensen and Steuerwald35) as evidence of harm from postnatal exposure, but such an interpretation is at odds with concerns about adverse early growth implications for later risk of obesity in non-breast-fed infants(Reference Gunnarsdottir, Schack-Nielsen and Michaelsen70). Such an interpretation also contrasts with the paradigm underlying new WHO growth charts that establish breast-fed, not formula-fed, infant growth patterns as the biological norm(Reference De Onis, Onyango and Borghi71).
Mechanisms
Our analysis uses relative risk estimates from the meta-analyses of studies that are mainly observational rather than experimental. As noted earlier, it is difficult for observational studies to show ‘causation’. Experimental studies of animals may also contribute to an understanding of these mechanisms. Findings are more conclusive if there is a dose-dependent relationship, i.e. if earlier or more complete artificial feeding shows larger differences or effects than when artificial baby milk is introduced later, or when artificial baby milk is combined with breast-feeding. A number of studies have found a dose response of various chronic diseases to artificial feeding in early life as noted above in our review of the meta-analyses.
Findings are also more persuasive if there are biologically plausible ways in which an ‘exposure’ (such as artificial feeding) could result in a higher incidence of a subsequent condition or disease. The aetiology of many of the chronic diseases examined here is multifactorial. Both genetic susceptibility and environmental factors play a significant role in the development of asthma(Reference Warner72), childhood cancer(Reference Ortega-Garcia, Ferris-Tortajada and Torres-Cantero73), type 1 diabetes(Reference Peng and Hagopian74) and inflammatory bowel disease(Reference Klement, Cohen and Boxman44). It is still unclear how artificial feeding exerts its effect as an environmental factor. The compositional differences between breast milk and artificial baby milk are likely to be important as are the complex metabolic programming effects of human milk(Reference Dietz75–Reference Dewey77). There is growing evidence that diet in infancy has short- and long-term effects on how the body metabolizes food, as well as influencing food intake levels and composition(Reference Dietz75, Reference Plagemann and Harder78–Reference Singhal and Lucas81). In some cases, early feeding practices that affect later feeding or eating behaviours may also play a role(Reference Ip, Chung and Raman43, Reference Gillman, Rifas-Shiman and Camargo76).
Figure 2 details a conceptual pathway for chronic risk of disease in artificially fed infants. Different exposures to nutrients and bioactive factors, either present in breast milk or absent in artificial baby milk, may lead to a number of different responses or the abnormal development of regulatory processes. These systems and responses, in turn, produce undesirable physiological outcomes and eventual development of chronic disease.

Fig. 2 Conceptual pathway for underlying chronic disease risk in artificially fed infants
The components in breast milk (including a complex and dynamic mix of nutrients, hormones, growth factors and cytokines) play a key role in developing body systems to appropriately regulate food intake, process fats and sugars, and influence body weight and infant growth(Reference Weaver82–Reference Singhal91). A higher energy intake by artificially fed infants leads to a higher body weight gain during the critical neonatal period compared with breast-fed infants(Reference Heinig, Nommsen and Peerson79, Reference Baker, Michaelsen and Rasmussen92). Such growth acceleration in early infancy has been linked to ‘malprogramming’ and a lasting increased risk of obesity, diabetes and CVD(Reference Gillman, Rifas-Shiman and Camargo76, Reference Plagemann and Harder78, Reference Singhal and Lucas81, Reference Weaver82, Reference Singhal91, Reference Stettler, Zemel and Kumanyika93). Lower levels of sodium in breast milk are thought to provide a plausible explanation for reduced blood pressure in later life(Reference Martin, Gunnell and Owen8).
Various complex and interrelated components in human breast milk are not replicated in artificial baby milk. For example, the long-chain PUFA found in breast milk may play a crucial role in energy metabolism, as they are a source of energy; in blood pressure control, since they are important structural components of tissue membrane systems, including the vascular endothelium; and in molecular signalling, with the synthesis of prostaglandins and leucotrienes(Reference Warner72, Reference Martin, Ness and Gunnell94). These fatty acids are also inversely correlated to fasting glucose levels and disruption of these levels may lead to changes in the skeletal muscle membrane, increased fasting glucose levels and too much insulin in the blood, eventual β-cell failure and diabetes(Reference Horta, Bahl and Martines38). Another hormone found in breast milk, leptin, has been found to exert a protective effect against excessive infant weight gain(Reference Miralles, Sanchez and Palou87), via appetite suppression and regulation of energy intake(Reference Singhal, Farooqi and O’Rahilly86). Artificially fed infants, however, are thought to develop resistance to leptin, leaving them at risk of excess weight gain(Reference Singhal, Farooqi and O’Rahilly86).
In addition to lowering blood pressure and reducing the risk of CVD(Reference Lawlor, Riddoch and Page95), hormones contained within breast milk also promote the functional maturity of the intestinal mucosal tissues. Such mucosal defences are important in protection from infection and may limit the development of diabetes(Reference Peng and Hagopian74), asthma(Reference van Rossum, Buchner and Hoekstra96), inflammatory bowel disease(Reference Whorwell, Holdstock and Whorwell97) and coeliac disease(Reference Akobeng, Ramanan and Buchan10) in susceptible individuals. The mode of feeding can also influence the levels of the hormone insulin. Artificial feeding has been linked to increased plasma insulin levels, possibly as a result of higher protein intakes, and the later development of obesity and diabetes(Reference Dewey77) due to greater fat deposition and development of adipocytes(Reference von Kries, Koletzko and Sauerwald98). Such altered levels of insulin may also lead to insulin resistance, which is thought to be associated with increased blood pressure(Reference Martin, Ness and Gunnell94).
Breast milk has anti-microbial, anti-inflammatory and immunomodulatory properties due, in part, to the cytokines, hormones and growth factors present. The immunomodulatory properties of human breast milk (via passive immunity, a T-cell-specific suppressive effect or diminished immune responses) may confer protection against postnatal antigenic exposures and the subsequent development of type 1 diabetes(Reference Gerstein40), childhood cancer(Reference Martin, Gunnell and Owen8, Reference Greaves99), asthma(Reference van Rossum, Buchner and Hoekstra39), coeliac disease(Reference Akobeng, Ramanan and Buchan10) and inflammatory bowel disease(Reference Klement, Cohen and Boxman44). Indeed, the protection afforded by breast milk may also be due to delayed exposure to harmful bacteria or foreign food antigens that may be otherwise present in artificial milk(Reference Virtanen and Knip100).
Finally, different feeding behaviours may play a role in chronic disease development, e.g. suckling patterns, and the infant’s degree of control over meal sizes and feeding intervals are altered in artificially fed as compared with breast-fed infants(Reference Agras, Kraemer and Berkowitz101–Reference Ventura, Savage and May103). Intake of artificial baby milk is determined by the mother, whereas breast-feeding facilitates development of an infant’s self-control(Reference Gillman, Rifas-Shiman and Camargo76, Reference Ventura, Savage and May103). Later food preferences may then tend towards healthy eating and better nutrition, since components of human milk and the suckling experience affect feeding behaviours and preferences of the mother and/or the child(Reference Martin, Ness and Gunnell94, Reference Birch and Fisher102–Reference Sullivan and Birch105).
Conclusion
There are many uncertainties about the links between nutrition in infancy and risk of chronic disease in later life due to the methodological flaws in existing research. These flaws would most likely work in the direction of understating the effect on the risk of chronic diseases from artificial feeding in the context of how breast-feeding is defined. Conversely, the risks may be overstated if residual confounding is not adequately accounted for. Overall, we suggest that poor measurement of breast-feeding lowers the measured effects in these studies at least as much as inadequate control for relevant confounding does. Confidence in many studies is also weakened by their small sample sizes. High-quality studies (such as those with large sample sizes, clear comparisons of substantially breast-fed and substantially artificially fed infants, and appropriate adjustment for confounding variables) are more likely to find an association between lack of breast-feeding in infancy and increased incidence of chronic diseases in later life.
While conclusive evidence is still lacking because of flawed research design and ethical barriers to randomized control trials, a wide range of biological, animal and epidemiological studies and some randomized control trials point to a small but consistent effect of exclusive artificial feeding in infancy in increasing the risk of chronic disease in later life. The relationship appears to be dose-dependent, with larger positive effects on the risk of disease associated with more exclusive or longer duration of breast-feeding.
While the clinical effect sizes are relatively small (with relative risks of chronic disease ranging from 1·2 to 2·1), the risk of exposure in a developed country population such as Australia is substantial. Approximately 90 % of current 35–45-year-olds were weaned from breast milk before they reached 6 months of age. Around 20–60 % of current Australian infants, especially those in low socio-economic groups, are still exposed to heightened levels of risk of chronic disease in later life due to weaning from breast-feeding either as newborns or before 6 months. These large population-level exposures to artificial baby milk suggest that infant feeding practices may not only contribute importantly to explaining current levels of chronic disease in current middle-aged adults, but also provide a potential avenue for reducing future chronic disease burden and health system costs.
Despite the uncertainties, we suggest that there is enough evidence to show that breast-feeding affects chronic disease incidence at the population level and is, therefore, of significance to public health policy. Although the average effects are modest, widespread population exposure to premature weaning means that relatively small effects from improving breast-feeding rates have a potentially large impact on population health.
Our findings have potentially significant economic implications. Health system cost impacts of premature weaning on common childhood and infectious illnesses are reasonably well documented. Several studies have identified substantial short-term health system, hospital or health fund cost savings from reducing premature weaning and associated infections, such as gastrointestinal illness or respiratory illness(Reference Weimer106–Reference Smith, Thompson and Ellwood108).
Many interventions currently proposed to reduce obesity and related chronic illnesses are expensive, but are not sustained and are, therefore, not cost-effective. Breast-feeding can be considered to be a one-off ‘intervention’ that continues to reduce risk of chronic disease throughout the life cycle. Unlike other interventions, such as exercise programmes or dietary changes, it does not have to be continued throughout the life cycle in order to maintain this protection and, therefore, has no ongoing costs. There are few other preventive health interventions that have proven to be permanently effective in reducing risk factors for chronic disease or chronic disease in a variety of settings.
With these lasting effects over the long term(Reference Plagemann and Harder78), breast-feeding is likely to be cost effective as a disease prevention measure. It is crucial, nevertheless, that public health advocacy addresses significant workplace and cultural barriers to breast-feeding to avoid breast-feeding promotion imposing economic and other costs on women(Reference Smith and Ellwood23, Reference Smith109). Further research is needed to quantify these costs and cost savings, but meanwhile, it would seem that implementing cost-effective interventions to support breast-feeding could be an important element of strategies to restrain future escalation of health costs of chronic disease burdens.
Acknowledgements
Funding for the project and fellowship to J.P.S. came from the Australian Research Council. The authors have no conflict of interest to declare. Each author contributed to implementation of the study, data collection and analysis and writing of the manuscript. J.P.S. was responsible for study design and wrote the first draft of the manuscript. Each author has seen and approved the contents of the manuscript. The present manuscript is an original work and has not been published previously. It is not currently being considered by any other journal and, if accepted by Public Health Nutrition, it will not be published elsewhere in the same form, in English or in any other language, without the written consent of the Nutrition Society.








