Worldwide, Fe deficiency is the most common and widespread micronutrient malnutrition, and is a public health problem in both industrialized and non-industrialized countries that affects over 24 % of the world’s population, but even more so in developing regions. Fe deficiency can reach all age groups, although young children and women tend to be among those most at risk of developing micronutrient deficiencies( 1 ).
Several studies have shown that the population of countries such as Denmark, Germany, Spain, the Netherlands, the UK, Poland, France, Ireland and Italy has an inadequate intake of Fe, especially among women of reproductive age( Reference Mensink, Fletcher and Gurinovic 2 , Reference Flynn, Hirvonen and Mensink 3 ). Although micronutrient deficiency is highly prevalent in many regions of the world and has a high social impact( 4 ), there are low-cost and highly effective approaches to prevention. Such programmes comprise food diversification to promote the consumption of food sources of Fe, the distribution of supplements and food fortification( 5 ). Food fortification is a safe and cost-effective strategy used in several countries to reduce micronutrient deficiencies( 6 , Reference Bailey, West and Black 7 ). Although many countries combat Fe deficiency with a flour fortification strategy, it seems that only nine of the seventy-eight national fortification programmes can have the desired nutritional impact due to the use of Fe with low bioavailability( Reference Hurrell, Ranum and de Pee 8 ).
In Brazil, there are no past or current figures for Fe deficiency. Since 1999, with the intent being to increase the intake amount of this micronutrient to prevent low stores and Fe-deficiency anemia, the Brazilian Ministry of Health has undertaken some strategies that include the promotion of a healthy diet, the use of supplements in target groups and the fortification of foods. The mandatory fortification of foods with Fe was initiated in 2004; wheat and maize flour was selected as the major vehicle with 4·2 mg of Fe per 100 g of flour( 9 ).
The main objectives of the present study were to assess the Fe intake, calculate the prevalence of inadequate Fe intake and identify the food contributors to Fe intake during 2003 and 2008 in a population-based study, reflecting before and after the mandatory fortification of flour with Fe.
Methods
Study population
For the present analysis, we compared data from two population-based studies: Health Survey–São Paulo (ISA-Capital 2003 and ISA-Capital 2008). The Healthy Survey–São Paulo is a cross-sectional study of health and living conditions among a representative sample of individuals living in São Paulo city, south-eastern Brazil. The ISA-Capital 2003 was conducted during 2003, reflecting the time prior to Fe fortification, and ISA-Capital 2008 was conducted during 2008, reflecting the time after fortification.
The sampling process for ISA-Capital 2003 was carried out in two stages: census tracts and households. For the draw, sectors were gathered into three strata based on the percentage of family heads with university-level education: <5 %, 5–24·9 % and ≥25 %. In total 2386 individuals were interviewed, 183 adolescents (12–13 years), 523 adolescents (14–18 years), 747 adults (19–50 years) and 933 adults (≥51 years), of both sexes.
The sample at ISA-Capital 2008 was defined in eight age domains: <1 year old, 1–11 years old and three more age groups for each sex, namely 12–19 years (adolescents), 20–59 years (adults) and ≥60 years (elderly adults). Two-stage cluster sampling of census tracts and households was performed. In the first stage, by using probability proportional to size, ten census tracts were drawn from each of the strata, making a total of thirty census tracts for each region. In the second stage, households were drawn from each sector. A total of 3271 individuals (197 aged <1 year, 383 aged 1–11 years and 2691 aged ≥12 years) participated in ISA-Capital 2008. For the present study, we invited all individuals older than 12 years from the ISA-Capital 2008 sample to answer one 24 h recall (24HR). Of these, 1662 individuals completed the dietary measurement. One person was excluded owing to supplement use, leaving 1661 individuals: 151 adolescents (12–13 years), 357 adolescents (14–18 years), 529 adults (19–50 years) and 624 adults (≥51 years), of both sexes.
Data collection and processing
In both surveys (ISA-Capital 2003 and ISA-Capital 2008), information on food intake, demographics and socio-economic variables was obtained using structured questionnaires through household interviews. The 24HR was administered in the household by trained interviewers using the multiple-pass method. In this process the respondent is guided through five steps (quick listing, quick listing review, naming meals, detail cycle and general review) using a standardized process that keeps individuals interested and engaged in the interview, which helps them remember all items consumed( Reference Guenther, DeMaio and Ingwersen 10 ). The sampling days for participants covered all the days of the week.
Foods reported in each 24HR were critically reviewed to identify any failures in reporting related to the descriptions of the foods consumed or food preparation techniques, including their apportioning and quantification. Fe intake was analysed using the Nutrition Data System for Research software program version 2007 (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN, USA), which is based on data from tables published by the US Department of Agriculture. The amount of Fe added to fortified products was corrected to account for the quantity of fortification in maize and wheat flour that has been mandatory in Brazil since 2004. There is a difference between the quantities of Fe added to fortified foods in Brazil and the USA. In addition, the Brazilian food composition table was used to verify the adequacy of nutritional values of Fe from food.
Estimating usual iron intake in the pre- and post-fortification periods
Due to day-to-day variation (within-person random error), nutrient intake distributions based on one or a few collection-days of 24HR provide biased estimations of percentiles of intake and consequently biased estimations of the prevalence of inadequacy( Reference Dodd, Guenter and Freedman 11 ). The use of methods to remove within-person variance and estimate usual nutrient intake is widely recommended and has been implemented in several studies worldwide. To do so, at least one replication of the 24HR is needed in a sub-sample of the study population( Reference Nusser, Carriquiry and Dodd 12 ). In the post-fortification period we administered two non-consecutive 24HR, the first was in person and the second a telephone-based interview (in a sample of 50·06 %). Mean time interval between the first and second measurement was about 6 months. Nevertheless, in the pre-fortification period, there was only a single measurement for each participant. According to previous studies( Reference Verly-Jr, Cesar and Fisberg 13 , Reference Jahns, Carriquiry and Arab 14 ), in cases of absence of the repetition of the 24HR, it is advised that the within-person variance component from a study with a similar population should be applied in order to correctly estimate the distribution of usual nutrient intake. Therefore, to correct the distribution of Fe intake in the pre-fortification period, we applied the variance components derived from the post-fortification period. To estimate within-person variance components by each age and sex group and the prevalence of inadequate Fe intake we used the Software for Intake Distribution Estimation (PC-SIDE) that implements the method proposed by Nusser et al. ( Reference Nusser, Carriquiry and Dodd 12 ).
Prevalence of inadequacy
The US Institute of Medicine’s set of intake recommendations for Fe was used as the reference for intake adequacy, specifically the Estimated Average Requirement( 15 ). The prevalence of inadequate Fe intake was calculated as the proportion of individuals whose usual intake fell below the Estimated Average Requirement for a specific age and sex group. To provide valid estimates of prevalence of inadequate intake, the distribution of the intake requirement must be symmetric, which is not the case for women of reproductive age due to Fe loss in the menstrual cycle. In this case, a suitable method to estimate usual intake that accounts for menstrual losses was applied( 16 ). Confidence intervals for the prevalence of inadequate intake were derived from standard errors based on a jackknife replication technique considering the complex sample design. Specifically, for women of reproductive age (14–18 years and 19–50 years) it was not possible to estimate standard errors due to statistical constraints.
The contribution of foods to Fe intake was calculated by the methodology described in Block et al. ( Reference Block, Dresser and Hartman 17 ), considering the study sampling design. This method estimates the major contributors to total Fe intake through a ratio of the daily total Fe provided by the specific food or food group to the daily total intake of Fe from all foods. Subsequently the foods were arranged in decreasing order according to the amount of Fe per food portion, calculated from the median food consumption in grams in the study population.
All analyses were conducted using the appropriate sample weights to account for the complex survey design. For all analyses, the Stata® statistical software package version 12 was used and P<0·05 was considered statistically significant.
Results
Mean Fe intake and the prevalence of inadequate Fe intake in the pre- and post-fortification periods are shown in Table 1. The Fe intake mean increased in all age and sex groups, ranging from 3·91–6·99 mg/d in the pre-fortification period to 7·81–15·20 mg/d in the post-fortification period. There was no identified risk of excessive Fe intake in this population.
Table 1 Prevalence of inadequate intake of iron in the pre- and post-fortification periods according to life stage. São Paulo, Brazil, 2008
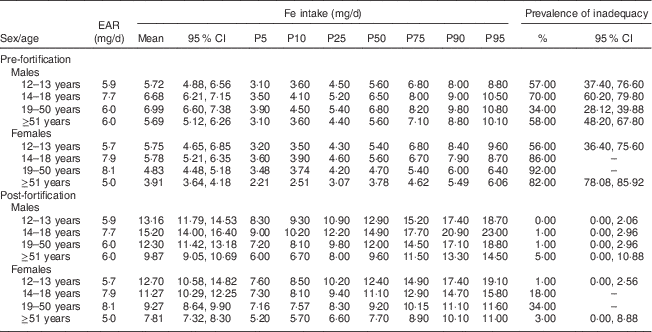
EAR, Estimated Average Requirement; P, percentile.
There was a reduction of over 90 % in the prevalence of inadequacy among men in all age groups analysed. When evaluating women, it was noted that despite the substantial reduction (over 63 %), the prevalence of inadequate Fe intake remained high (34 %) in those aged 19–50 years.
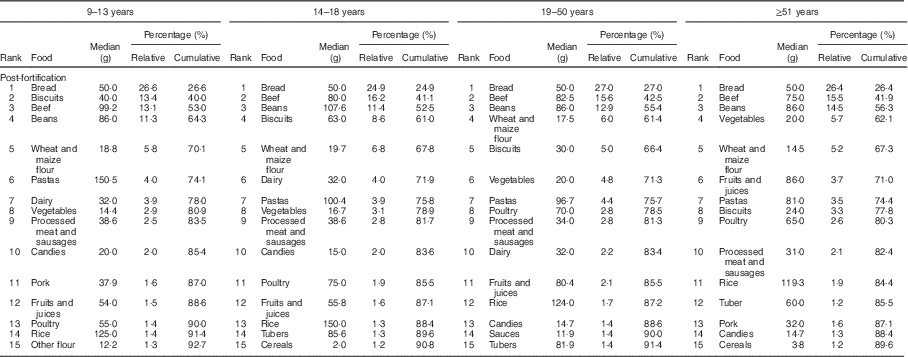
The food groups that contributed most to the intake of Fe before fortification were beans, beef, vegetables and dairy, accounting for more than 58 % of the Fe intake in all age and sex groups studied (Table 2). In the post-fortification period, there was a change in the pattern of contributors: bread, beef, beans and biscuits were main contributors (Table 3).
Table 2 Food contributors to total intake of iron in the pre-fortification period according to life stage. São Paulo, Brazil, 2003
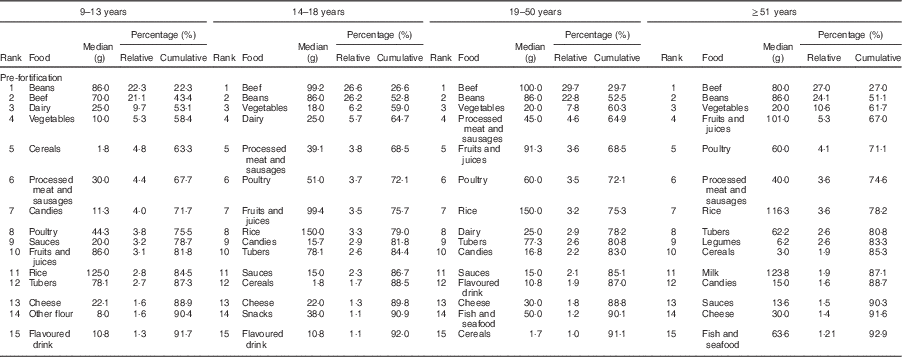
Table 3 Food contributors to total intake of iron in the post-fortification period according to life stage. São Paulo, Brazil, 2008
Discussion
Our results show that fortified foods had an impact in reducing the prevalence of inadequate Fe intake and increasing the mean Fe intake in all life stages, regardless of sex.
The mean Fe intake in the post-fortification period in all age and sex groups was similar to that found by Santos et al. ( Reference Santos, Nilson and Verly-Junior 18 ) in their evaluation of the flour fortification programme in a representative sample of Brazilians. Berner et al. ( Reference Berner, Keast and Bailey 19 ) found that food fortification with Fe contributed to reducing the prevalence of inadequacy of this nutrient in adolescents, similar to the results of the present study. Martorell et al. ( Reference Martorell, Ascencio and Tacsan 20 ) showed that the Fe fortification policy in Costa Rica reduced the prevalence of anaemia from 18·4 to 10·2 % in adult women. In South Africa, the fortification policy implemented in 2003 also led to an increase in Fe intake( Reference Steyn, Wolmarans and Nel 21 ). The average Fe intake in men and adult women was higher than that evidenced in the present study. Fulgoni et al. ( Reference Fulgoni, Keast and Bailey 22 ) assessed the contributions to various micronutrients from the usual diet according to different sources (natural, fortified or enriched and dietary supplement) among individuals over two years according to National Health and Nutrition Examination Survey 2003–2006 data, and found that the percentage of the population with usual intakes below the Estimated Average Requirement decreased from 21·8 to 6·5 %.
The prevalence of inadequate Fe intake in women of reproductive age was over 20 % in the present study, even after the mandatory fortification. This high prevalence in this age group suggests that the policy has limited effectiveness and it is necessary to consume a greater amount of fortified flour( 6 ). However, the incentive to increase consumption of flour should not stimulate the growing prevalence of weight excess( 23 ). Thus, other approaches to increase Fe intake (e.g. supplementation) should be considered in this population group in a region where anaemia is highly prevalent( 24 ).
Bioavailability is the other relevant issue regarding Fe fortification. In Brazil, reduced Fe is the main source used by the industry in the fortification policy( 25 ). However, this type of Fe provides a low bioavailability compared with ferrous sulfate and ferrous fumarate( Reference Germani, Ascheri and Silva 26 ). The extent of its use is due to the low cost and stability when added in flour. The low bioavailability of this source may explain the findings of Assunção and colleagues( Reference Assunção, Santos and Barros 27 ) who showed that Fe fortification in Brazil had no impact on anaemia in children under 6 years old living in the urban area of the city of Pelotas, southern Brazil. Hurrell et al.( Reference Hurrell, Ranum and de Pee 8 ) reviewed the efficacy and effectiveness studies with various Fe-fortified foods and found strong evidence that reduced Fe and other forms of Fe with low bioavailability cannot be efficacious to a have satisfactory impact on Fe status. Thus, it is necessary that the government review the fortification policy in order to increase the effectiveness of the programme by use of an Fe form with better bioavailability.
Despite a significant increase in Fe intake after fortification, the population in the present study showed no intakes near the tolerable upper intake level of this micronutrient. The 95th percentile of intake (11–19 mg Fe/d) in the post-fortification period is lower than that observed in the adult female population of Europe, whose 95th percentile of Fe intake ranged from 13 mg/d (Ireland) to 20 mg/d (Germany). However, the 95th percentile of Fe intake observed in adult men in the current study population is similar to levels found in Denmark, the Netherlands and Spain( Reference Flynn, Hirvonen and Mensink 3 ).
Although there is no evidence of risk of adverse effects related to higher intake of Fe from the fortification( Reference Flynn, Hirvonen and Mensink 3 , Reference Hennessy, Walton and Flynn 28 ), studies are needed to assess the impact of fortification in individuals with low nutritional risk. Abtahi and colleagues( Reference Abtahi, Neyestani and Pouraram 29 ) evaluated the effects of Fe-fortified bread consumption on oxidative stress in healthy individuals in Iran. They showed that there was an increase in the level of superoxide dismutase and a reduction in the value of the total antioxidant capacity in men. These results suggest that consumption of flour fortified with Fe in non-anaemic adults in the long term cannot be without adverse effects. Furthermore, monitoring of Fe addition to flour is essential to assess compliance to the fortified flour policy and to guarantee a safe Fe intake.
The panorama of foods that contributed to the Fe intake also changed after fortification. In 2003, the main contributors were food groups that were natural sources of this nutrient, such as beef and beans. However, there was an important change after fortification, in which bread, biscuits, wheat and maize flour were among the top five contributors. Therefore, flour was a good vehicle for Fe fortification, corroborating results observed in previous studies, in which higher intakes of Fe were associated with high consumption of fortified foods( Reference Flynn, Hirvonen and Mensink 3 , Reference Berner, Keast and Bailey 19 , Reference Fulgoni and Buckley 30 ).
Food fortification is a safe and cost-effective strategy used in several countries to reduce micronutrient deficiencies( 6 , Reference Bailey, West and Black 7 ). Baltussen and co-workers( Reference Baltussen, Knai and Sharan 31 ) estimated the cost-effectiveness of Fe supplementation and Fe fortification programmes, at different coverage levels, in four subregions of the world. The cost-effectiveness of fortification was always lower than the cost-effectiveness of supplementation, regardless of the coverage level. Fiedler and Macdonald( Reference Fiedler and Macdonald 32 ) estimated that in Brazil the fortification of wheat and maize flour has a cost, over 10 years, of about $US 41 per disability-adjusted life year saved. If health interventions with a good cost-effectiveness are those with a cost lower than $US 200 per disability-adjusted life year saved, as suggested by the World Bank, then the fortification of flour with Fe appears to be a public health strategy with good cost-effectiveness( 33 ).
Few studies in Brazil have evaluated the effectiveness of the flour fortification policy in a representative sample and in different age groups. Thus, the present study is the first to provide representative estimates of the prevalence of inadequate Fe intake in the pre- and post-fortification periods in this country. The reduction in inadequacy may be associated with reducing Fe deficiency, but due to the methodological limitations inherent in the evaluation methods of dietary intake and the lack of knowledge of Fe bioavailability, this result may not have a significant impact on body stores of this nutrient. Another limitation of the study is the use of the food composition tables from the US Department of Agriculture. However, the Fe contents of fortified foods available in the Brazilian food composition table were corrected. Moreover, during the pre-fortification period there was no replication of the 24HR, so the variance components derived from the post-fortification period were used to estimate the within-person variance.
Conclusion
In conclusion, the mandatory fortification of wheat and maize flour with Fe significantly furthered the reduction in the prevalence of inadequate intake, except among women of reproductive age, and changed the main contributors to this nutrient in the studied population. Therefore, monitoring of Fe addition in flour is essential to assess compliance to the fortified flour policy and to guarantee a safe Fe intake for all the population.
Acknowledgements
Acknowledgements: The authors would like to thank the participants of the study and researchers of the Dietary Assessment Group (GAC; Grupo de Pesquisa de Avaliação do Consumo Alimentar). Financial support: This study was funded by the São Paulo Research Foundation (FAPESP; grant number 2012/22113-9); the National Council of Technological and Scientific Development (CNPq; grant number 472873/2012-1); and scholarships offered by FAPESP (grant number 2013/06979-9). FAPESP and CNPq had no role in the design, analysis or writing of this article. Conflict of interest: The authors confirm that there is no conflict of interest. Authorship: D.A.S.V. contributed with analysis, interpretation of data and wrote the manuscript. J.S. contributed with interpretation of data and wrote the manuscript. E.V.J. contributed with analysis of the manuscript. R.M.F. conceived the study, designed the study and revised the article critically. D.M.M. contributed with design of the study and revising the article critically. All authors read and approved the submitted version. Ethics of human subject participation: The study protocol in both surveys was reviewed and approved by the Ethics Committee at the School of Public Health, University of São Paulo. All participants enrolled in the study after providing free and written informed consent forms signed by themselves or by their guardians, when younger than 18 years.






