During the last decades levels of dental caries seem to be increasing worldwide( Reference Bagramian, Garcia-Godoy and Volpe 1 ) reflecting the well-known development of childhood obesity. This has led to recent interest in the relationship between caries and obesity as they may have common aetiologies that lend themselves to common solutions( Reference Hayden, Bowler and Chambers 2 , Reference Costacurta, DiRenzo and Sicuro 3 ). For instance, intake of sugar-sweetened beverages has been positively associated with BMI in children( Reference Te Morenga, Mallard and Mann 4 ). Sugar, especially in large quantity and when consumed frequently, is also known to contribute to the development of caries( Reference Kawashita, Kitamura and Saito 5 – Reference Touger-Decker and van Loveren 7 ). Further, shorter sleep duration and nocturnal eating have been associated with overweight and obesity( Reference Hense, Pohlabeln and De Henauw 8 – Reference Gallant, Lundgren and Drapeau 10 ) as well as with dental disease( Reference Lundgren, Smith and Spresser 11 , Reference Pieper, Dressler and Heinzel-Gutenbrunner 12 ).
The cariogenic micro-organism mutans streptococci (MS), an important factor in the development of caries, ferments carbohydrates from the diet and contributes to caries by lowering the pH in dental plaque, resulting in tooth demineralization( Reference Kawashita, Kitamura and Saito 5 , Reference Harris, Nicoll and Adair 13 ). High counts of MS in saliva can be used as a biomarker for intake of fermentable carbohydrates( Reference Bradshaw and Lynch 14 ), and positive associations between MS and BMI have been identified in adults( Reference Barkeling, Linné and Lindroos 15 – Reference Vågstrand, Lindroos and Birkhed 17 ) but not in children( Reference Tong, Rudolf and Muyombwe 18 ) or adolescents( Reference Vågstrand, Lindroos and Birkhed 17 ). Given the obesity epidemic, the link between overweight and risk of dental caries is of interest. The objective of present study was to investigate the potential relationship between counts of MS and weight status in children while considering important covariates.
Methods
Participants
The present study included 294 children, aged 4–11 years, from the Swedish cohort of the IDEFICS study (Identification and prevention of Dietary- and lifestyle-induced health Effects In Children and infantS). IDEFICS is a prospective cohort study on child health with an embedded community-based intervention, including eight centres in Europe. The diet part of the intervention aimed to improve dietary habits by increasing daily consumption of water, fruits and vegetables( Reference De Henauw, Verbestel and Marild 19 ) and thereby decreasing intake of added sugars. Ethics approval was obtained from the central ethical review board in Gothenburg. Parents provided written informed consent, and children gave oral consent for examinations and sample collections. Further information about the IDEFICS study can be obtained from previous reports( Reference De Henauw, Verbestel and Marild 19 , Reference Ahrens, Bammann and Siani 20 ).
Data on anthropometrics, diet and sleep were collected during 2009 to 2010 when 1511 (84 %) children from the Swedish cohort returned for a follow-up. Saliva was collected in connection with a dietary sub-study, preceded by a strategic sampling to represent both the control (Alingsås and Mölndal) and intervention (Partille) areas in Sweden, including 728 children, of whom 40 % provided a saliva sample. Reasons for not providing saliva were lack of time, child refused or parents did not want their child to chew the paraffin. After excluding twenty-four children because of inconclusive data on saliva (n 3), missing diet information (n 18) and incomplete anthropometric data (n 3), 271 children are included in the present study.
Cariogenic bacteria in saliva
Paraffin-stimulated saliva was collected during a fasting morning examination and sent to the Department of Cariology at the Dental School in Gothenburg, where it was processed within 24 h. The saliva was shaken on a Whirlimixer, diluted in tenfold steps in 0·05-m phosphate buffer and plated on Mitis Salivarius-Bacitracin agar. The agar plates were incubated anaerobically at 37°C for 2 d. Colony-forming units (CFU) of MS were counted and identified by their colony morphology( Reference Emilson 21 ) and divided into two groups for categorical analysis: medium–high counts (>105 CFU/ml), also referred to as ‘higher’, and low counts (≤105 CFU/ml). These thresholds are commonly used in dental research and known to predict high or low risk of caries( Reference Klock and Krasse 22 ).
Meal frequency, sugar propensity and sleep
Meal frequency and sleep duration were calculated from SACINA (Self-Administered Children and Infant Nutrition Assessment), a 24 h diet recall (24-HDR) program( Reference Vereecken, Covents and Sichert-Hellert 23 ). The parents or other caregivers, assisted by a registered dietitian, reported what the child had been eating and drinking the previous 24 h, as well as wake-up time and bed time.
Due to the high day-to-day variation in diet( Reference Willett 24 , Reference Svensson, Larsson and Eiben 25 ), usual sugar intake was estimated using the sugar propensity ratio derived from a reproducible( Reference Lanfer, Hebestreit and Ahrens 26 ) and validated( Reference Bel-Serrat, Mouratidou and Pala 27 ) FFQ. The sugar propensity ratio is defined as the sum of sugar-rich foods and beverages divided by the sum of all foods reported. A more detailed description of the sugar propensity ratio is presented elsewhere( Reference Lanfer, Knof and Barba 28 , Reference Lissner, Lanfer and Gwozdz 29 ).
Anthropometrics
Weight of the children was measured to the nearest 0·1 kg by a Tanita BC 420 SMA scale and height was measured to the nearest 0·1 cm by a SECA 225 stadiometer. Measurements were done in the morning, with the children fasting and wearing only underwear. Waist circumference was measured according to a standard protocol( Reference Marfell-Jones, Olds and Stewart 30 ). Age- and gender-specific BMI and BMI Z-scores for children and adolescents developed by the International Obesity Task Force( Reference Cole, Bellizzi and Flegal 31 ) were calculated.
Parental education
Data on education level was based on the International Standard Classification of Education (ISCED) for cross-country comparability( 32 ) and used to determine the maximum highest level of the parents’ education, a proxy for socio-economic status. Levels 1–3 represent upper secondary school and are classified as low education level while levels 4–6 represent post-secondary education and are classified as high education level.
Statistics
Descriptive statistics were used to define basic characteristics, i.e. mean and standard deviation for continuous variables, and number and percentage for binary variables. For comparison by MS (medium–high v. low) the Student t test was used for continuous variables (age, sugar propensity ratio, sleep duration, meal frequency, BMI Z-score, waist circumference) and Pearson’s χ 2 test to compare categorical variables (sex, education level, intervention exposure). Age-adjusted univariate logistic regression was used to investigate the association of potential covariates with medium–high counts of MS. The final model was obtained by multiple logistic regression with stepwise forward selection among the whole set of covariates. Area under the receiver-operating characteristic curve was used to estimate how well the final model predicted the outcome( Reference Hosmer and Lemeshow 33 ). To examine the possible effect of dietary under-reporting, and age respectively, sensitivity analyses were performed by excluding participants with only one meal reported (n 4) in the 24-HDR, and by forcing age into the multivariable model. For calculation of the area under the receiver-operating characteristic curve, the statistical software package SAS version 9·3 was used; otherwise data were analysed using IBM SPSS Statistics Version 20. The significance level was set to 0·05.
Results
Descriptive properties and comparison by MS status are presented in Table 1. Medium–high counts of MS were found among 18 % of the children. Low counts were found in 82 % (including children with counts below the detection limit). No differences were found between the low and medium–high counts group regarding the proportion of females (44 v. 52 %, P=0·30), of high parental education level (86 v. 80 %, P=0·40) or of being in the intervention group (59 v. 58 %, P=0·9). Children with higher counts were older (P=0·01), reported greater sugar propensity ratio (P=0·02), less sleep (P=0·00) and more frequent meals (P=0·03). Additionally, they had larger waist circumference (P=0·03) and marginally significantly greater BMI Z-score (P=0·05) compared with those with low counts of MS.
Table 1 Distribution of variables by bacterial status (low v. medium–high counts of mutans streptococci) among children (n 271) aged 4–11 years, the IDEFICS Sweden study
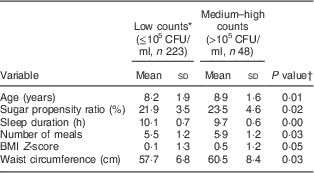
IDEFICS, Identification and prevention of Dietary- and lifestyle-induced health Effects In Children and infantS; CFU, colony-forming units; MS, mutans streptococci.
* No. of participants below detection limit of MS (200 CFU/ml)=183 (67·5 %); median (interquartile range) count of MS among participants with MS above detection limit=1·3 (0·46–13·8)×105 CFU/ml.
† P values from t test for continuous variables.
Our main results are presented in Table 2. Using age-adjusted logistic regression, we found that higher counts of MS were associated with sugar propensity ratio, sleep duration and BMI Z-score. The final multiple logistic regression analysis with stepwise forward selection identified five variables which independently explained the MS categorization of these children. More frequent meals, sugar propensity ratio, BMI Z-score and female sex were all positively associated with medium–high counts, while a negative association was found for longer sleep duration. The area under the receiver-operating characteristic curve was given by 0·78 (95 % CI 0·70, 0·85), indicating good discrimination properties of the final model. The exclusion of children with only one meal reported (n 4) in the 24-HDR did not change the results. A separate sensitivity analysis where age was added to the full model chosen by stepwise procedures confirmed the result of lack of association between MS count and age of the child. However, adding age to the final model attenuated the effect of sex to marginal significance while the other estimates remained unchanged (data not shown). No stratifications were made for age or sex due to the small sample size.
Table 2 Odds ratios for medium–high counts of mutans streptococci among children (n 271) aged 4–11 years, the IDEFICS Sweden studyFootnote *
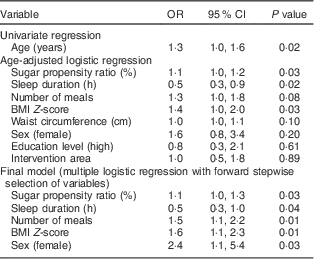
IDEFICS, Identification and prevention of Dietary- and lifestyle-induced health Effects In Children and infants.
* No. of participants included in both analyses=233.
Discussion
Our finding that higher BMI Z-score was positively associated with higher counts of MS in children is novel although in line with earlier findings in adult populations( Reference Barkeling, Linné and Lindroos 15 – Reference Vågstrand, Lindroos and Birkhed 17 ). In the age-adjusted model higher BMI Z-score was positively associated with higher counts of MS and the association was strengthened in the multivariable model. The fact that the association between higher BMI Z-score and counts of MS remained after mutual adjustments suggests that some other common denominator, not accounted for in present study, may be driving the association. Other micro-organisms like e.g. lactobacilli have also been identified as a risk factor for dental caries in children( Reference Kawashita, Kitamura and Saito 5 , Reference Harris, Nicoll and Adair 13 ); however, no associations between salivary counts of lactobacilli and higher BMI Z-score were found in the present sample (data not shown).
The proportion of sugar-rich foods and beverages reported during a typical week was greater in children with higher counts compared with children with low counts, in agreement with other studies( Reference Bradshaw and Lynch 14 , Reference Vågstrand and Birkhed 34 ). Children with higher counts of MS also reported more frequent meals compared with children with low counts. Higher meal frequency implies snacking and less time for oral clearance, which could lead to increased availability of carbohydrates, promoting colonization of MS. Meal frequency( Reference Berteus Forslund, Lindroos and Sjostrom 35 ) and taste preference for sugar-rich foods( Reference Lanfer, Knof and Barba 28 ) have been associated with overweight, and both diet patterns also increase colonization of cariogenic micro-organisms in children( Reference Kawashita, Kitamura and Saito 5 , Reference Touger-Decker and van Loveren 7 , Reference Law, Seow and Townsend 36 ). Therefore, it was surprising that the association between higher BMI Z-score and medium–high counts of MS observed here could not be accounted for by these factors. Reporting errors in parental estimates of usual sugar intake and meal frequency may explain this result.
Children’s food preferences are formed at an early age and are relatively stable during pre-school years( Reference Skinner, Carruth and Bounds 37 ), although there is an increasing soft drink consumption( Reference Lytle, Seifert and Greenstein 38 ) and a higher preference for fruits and foods rich in fat and sugar in school-aged children( Reference Cooke and Wardle 39 ). Therefore it was important to consider the age range by adding age to the final model. However, the estimates remained unchanged suggesting no effect of age on the association between propensity for consuming sugar, meal frequency and counts of MS in the present study. Considering the young age groups included (age span 4–11 years) one can speculate that eating habits are still highly moderated by the parents. Furthermore, in Sweden pre-school and school food environments can be expected to be similar for all age groups, which could explain the lack of an age effect on the associations in the present study.
The recent findings of a negative association between longer sleep duration and the development of overweight( Reference Hense, Pohlabeln and De Henauw 8 , Reference Seegers, Petit and Falissard 9 ) was the reason for investigating the effect of sleep duration on counts of MS. Sleep duration was inversely associated with counts of MS. Night eating has been associated with less sleep, overweight and dental disease in earlier studies( Reference Gallant, Lundgren and Drapeau 10 – Reference Pieper, Dressler and Heinzel-Gutenbrunner 12 ), but since data are not available for the times of sugar consumption, we can only speculate about the possible role of night eating in this context.
Our finding that counts of MS were significantly higher in females has not been reported earlier and could be related to the fact that permanent teeth eruption occurs earlier in girls( Reference Hagg and Taranger 40 , Reference Del Cojo, Lopez and Martinez 41 ), implying a longer period of MS colonization in girls than boys of the same age. This is further supported by the fact that the association between MS and sex was only marginally significant when age was added to the final model.
Despite our unique findings the present study is not without limitations. First, we cannot establish causality from our study. Second, meal frequency and sleep are based on a single 24-HDR which is unlikely to accurately reflect usual habits. However, our finding of meal frequency being positively associated with counts of MS is in line with earlier studies( Reference Kawashita, Kitamura and Saito 5 , Reference Touger-Decker and van Loveren 7 , Reference Law, Seow and Townsend 36 ). Usual sugar intake was assessed by an FFQ, which is considered superior to a single 24-HDR for assessing usual sugar exposure. As with all methods of measuring dietary intake related to obesogenic foods, it is likely that that our study suffers from biased parental reports of usual intake. In contrast to parental reported dietary measures, anthropometric variables and counts of MS were measured objectively and BMI Z-score was analysed as a continuous variable, all of which strengthens the study.
Conclusions
In this sample of 4–11-year-olds BMI Z-score was associated with higher counts of MS. Meal frequency, propensity to consume sugar, sleep duration and female sex were also independently associated with higher counts of MS. Therefore, public health efforts aimed at reducing dental caries and overweight could provide multiple benefits, as these problems might both be resolved in the same fashion. Important targets for joint interventions should include limiting intake frequency of sugar-rich foods and beverages and promoting an adequate amount of sleep.
Acknowledgements
Acknowledgements: The authors thank Ann-Britt Lundberg (Department of Cariology at the Institute of Odontology in Gothenburg) for analysing the saliva samples. Financial support: This study was done as a part of the IDEFICS study (http://www.idefics.eu), which was funded by the European Community within the Sixth RTD Framework Programme Contract No. 016181 (FOOD) with additional financial support from Stiftelsen Fru Mary von Sydows, född Wijk, donations fund and Epilife Teens. These funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorships: L.A. analysed the data and drafted the manuscript; D.B. and S.M. assisted in conceptualizing the study, data interpretation and supervision; L.L. and M.H. assisted in data interpretation and supervision; K.M. assisted in the data analyses; A.L. developed the sugar propensity score; and G.E. assisted in data interpretation and supervision. Ethics of human subject participation: Ethics approval was obtained from the central ethical review board in Gothenburg. Parents provided written informed consent, and children gave oral consent for examinations and sample collections.





