Hypertension has been identified as one of the leading causes of CVD and premature mortality in the world(Reference Murray and Lopez1, Reference Muntner, He and Cutler2). In the past few decades, the prevalence of hypertension has increased dramatically worldwide(Reference Muntner, He and Cutler2–Reference Chobanian, Bakris and Black4). In Brazil, for example, hypertension prevalence has increased substantially to as high as 33 % in Aracaju among adults aged ≥18 years(Reference Ala, Gill and Gurgel5, Reference Ordunez, Silva and Rodriguez6). The rising prevalence of hypertension in Brazil reflects well on the high prevalence of CVD. IHD (25 % of total deaths) and cerebrovascular diseases (34 %) are the leading causes of mortality in Brazil(7).
In children, blood pressure (BP) tracking patterns confirm that persistent BP increase may be related to hypertension in adulthood(Reference Bao, Threefoot and Srinivasan8, Reference Lauer and Clarke9). Increased BP in childhood has also been linked to left ventricular hypertrophy(Reference Daniels, Loggie and Khoury10). As a result, detection and management of high BP at an early age may be an important approach for limiting the disease burden ascribed to high BP(11).
The prevalence of high BP in children in Western countries ranges from 7 % to 19 % on the basis of measurements taken on one visit(Reference Sorof, Lai and Turner12–Reference Genovesi, Giussani and Pieruzzi14). In contrast, few population-based studies have been conducted in children and adolescents in middle- and low-income countries(Reference Jafar, Islam and Poulter15–Reference Costanzi, Halpern and Rech22). Continuous assessment of high BP in children is relevant for health-care policy and prevention strategies.
Furthermore, childhood obesity has increased considerably over the past few decades. Many studies have shown that overweight and obesity are associated with BP in children and adolescents(Reference Muntner, He and Cutler2, Reference Falkner, Gidding and Ramirez-Garnica3, Reference Torrance, McGuire and Lewanczuk23–Reference Weiss, Dziura and Burgert25). In the USA, for example, overweight children have been shown to be two to four times more likely than non-overweight children to have high BP(Reference Falkner, Gidding and Ramirez-Garnica3). However, information on the relationship between body size and high BP in children and adolescents in non-Western countries is limited. In addition, some studies suggest that BP levels in children have recently increased in parallel to the obesity epidemic(Reference Muntner, He and Cutler2, Reference Ford, Mokdad and Ajani26). Other studies, by contrast, have shown that BP levels have decreased over time in spite of upward trends of obesity(Reference Watkins, McCarron and Murray27). These findings suggest that the strength of the relationship between BP and body weight in children and adolescents may differ between populations in different settings.
In Brazil, information on high BP in adolescents is limited(Reference Guimarães, de Almeida and Santos20–Reference Costanzi, Halpern and Rech22, Reference Mendonça da Silva, Romero Rivera and Goretti Barbosa de Souza28). Evidence also suggests that overweight and obesity in children are becoming a major problem in Brazil. The prevalence of overweight and obesity in adolescents has increased dramatically from 4·0 % and 8·2 % in 1975 to 17·9 % and 15·4 % in 2003 in both boys and girls, respectively(29). More recent studies show even higher prevalence rates of overweight and obesity ranging from 24·0 % in São Paulo to 36·9 % in Salvador, Bahia(Reference Guimarães, de Almeida and Santos20, Reference Nobre, Domingues and da Silva30). Despite the increasing prevalence of overweight and obesity, information on the relationship between body size and high BP in adolescents is limited in Brazil. Therefore, the main aim of the present study was to estimate the prevalence of high BP and the association of overweight and obesity with high BP among adolescents in Aracaju, Brazil.
Methods
Study area
The present study was carried out in Aracaju, the capital city of Sergipe State, Brazil. Aracaju is located in the north-eastern part of Brazil, about 350 km north of Salvador. Aracaju has a population of about 544 039 inhabitants, which represents approximately 33 % of the state population. Sugar cane and petroleum extraction are its main economic activities. The majority of the population (about three-quarters) is of African descent and of mixed African and Portuguese descent.
Study design
Data were collected between February and May 2008 from healthy adolescents aged between 12 and 17 years from thirty different schools. The schools were randomly selected from all eighty-one schools (fifty public and thirty-one private schools) in the five districts of Aracaju. According to official statistics, the total number of adolescents (10–18 years of age) in Aracaju is 72 683. For each of the five districts, four public schools and two private schools were randomly selected. The selection of schools in this way allowed us to obtain representative samples of students from different school types and locality. In each school, we randomly selected fifty students among those aged 12–17 years. All the selected students were given an informed consent form to be signed by their parents. Of the 1500 students, 1016 returned the consent form (response rate: 67·7 %). The response rate was relatively similar in both public (68·0 %) and private (64·5 %) schools. The 12-year-olds had a lower response rate (57 %) than 13–17-year-olds, ranging from 60 % to 78 %. Fourteen adolescents were excluded from the study because they did not meet the age criterion. Ethical approval was obtained from the Research Ethics Committee of the Federal University of Sergipe, Brazil.
Physical measurements
All measurements were taken in a private room provided by the schools.
Main outcome measure
The main outcome measure was high BP, which was measured according to the European Society of Hypertension Guidelines(Reference Parati, Stergiou and Asmar31). BP was measured with a validated oscillometric automated digital BP device (Omron M-6; Omron Healthcare Europe BV, Hoofddorp, The Netherlands) by trained medical students from Aracaju (Brazil) and Amsterdam (The Netherlands). Using appropriate cuff sizes, three readings of 2 min intervals were taken on the right arm in a seated position after at least 5 min rest. The mean of the last two readings was used for analysis. Sex-, age- and height-specific percentile levels were defined using US normative BP tables for children and adolescents(11, 32). High BP was defined as systolic BP and/or diastolic BP ≥ 95th percentile(11).
Primary covariate
The main predictor variables were overweight and obesity. Height was measured without shoes with a measuring tape to the nearest 0·01 m. Weight was measured to the nearest 0·1 kg after removal of shoes, jackets, heavy clothing and pocket contents using a digital Seca scale. BMI was calculated as weight in kilograms divided by the square of height in metres (kg/m2). Overweight and obesity were defined using the sex- and age-specific BMI criteria of the International Obesity Task Force(Reference Cole, Bellizzi and Flegal33).
Other covariates
In addition, participants were asked to complete a questionnaire including questions on age, sex and physical activity. Physical activity was based on the number of days per week engaged in leisure-time physical activity outside school. The type of school (private or public school) was used as proxy for socio-economic status. Public schools are free of charge, but private schools charge a tuition fee.
Data analysis
Cross-tabulations were analysed using the χ 2 test. Continuous outcome variables were analysed by independent samples t test. Linear regression analyses were used to assess the relationship between overweight and obesity and mean BP. Prevalence ratios (PR) of high BP and their 95 % CI were estimated by means of Poisson regression with robust variance to assess the relationship between overweight and obesity and high BP(Reference Barros and Hirakata34), adjusting for factors that are known to be associated with high BP in adolescents (age, socio-economic status and physical activity)(Reference Ala, Gill and Gurgel5, Reference Costanzi, Halpern and Rech22). The analyses were stratified by sex, because there was a significant interaction between sex and overweight and obesity. P values and 95 % CI were calculated with two-tailed tests with correction for the cluster design effect. All statistical analyses were performed using the STATA statistical software package version 11·0 (StataCorp., College Station, TX, USA).
Results
Characteristics of the study population
In Table 1, the characteristics of the study population are shown for boys and girls. Girls were shorter, lighter and had a somewhat higher BMI than boys. Boys were more physically active than girls.
Table 1 Characteristics of the study population of Aracaju adolescents by sex
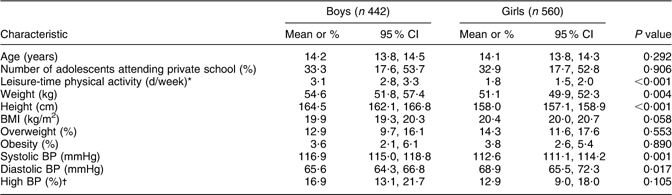
BP, blood pressure.
*Leisure-time physical activity was defined as days per week the participant was physically active outside school.
†High BP is defined as ≥95th percentile.
Blood pressure levels and association of overweight and obesity with high blood pressure among Aracaju adolescents
The mean systolic BP was higher but diastolic BP was lower in boys than in girls. The prevalence of high BP was higher in boys than in girls, although the 95 % CI overlapped slightly.
Overweight and obese boys and girls had higher mean systolic and diastolic BP levels compared with their normal-weight peers (Table 2). The differences hardly changed after adjusting for type of school and leisure-time physical activity in linear regression models. These differences were observed at all ages for systolic BP (Fig. 1a, b) and diastolic BP (Fig. 2a, b) in both boys and girls, with the systolic BP differences being statistically significant for 14- and 17-year-old boys and for all age groups in girls.
Table 2 Mean and regression coefficient of systolic and diastolic BP by body size among Aracaju adolescents by sex
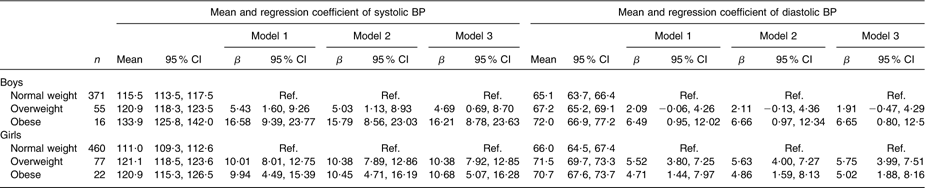
BP, blood pressure; Ref., reference category.
Model 1, adjusted for age; model 2, plus type of school; model 3, plus physical activity.
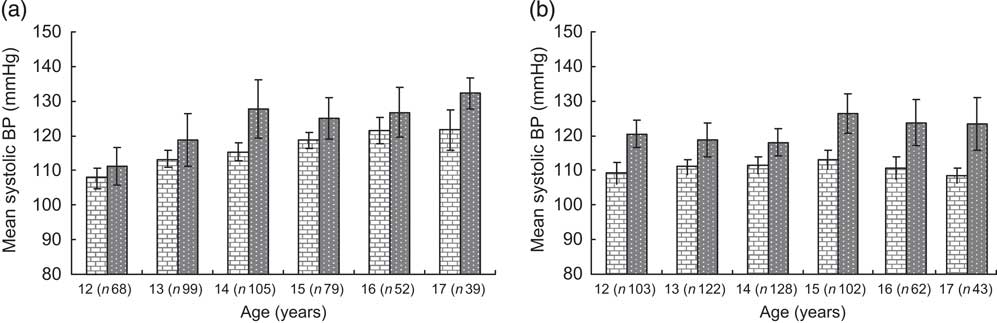
Fig. 1 Mean systolic blood pressure by BMI category and age in boys (a) and girls (b); ![]() , normal weight;
, normal weight; ![]() , overweight/obese
, overweight/obese
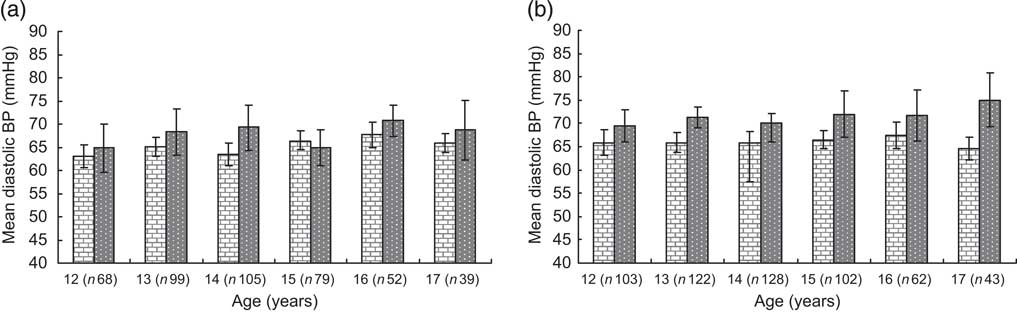
Fig. 2 Mean diastolic blood pressure by BMI category and age in boys (a) and girls (b); ![]() , normal weight;
, normal weight; ![]() , overweight/obese
, overweight/obese
Overweight and obese boys and girls had a higher prevalence of high BP compared with their normal-weight peers (Table 3). The differences persisted after adjustments for age, type of school and physical activity: overweight boys (PR = 1·93, 95 % CI 1·08, 3·48) and obese boys (PR = 4·87, 95 % CI 2·35, 10·11) were more likely than normal-weight boys to have high BP. Overweight girls (PR = 4·34, 95 % CI 2·58, 7·30) and obese girls (PR = 5·18, 95 % CI 2·67, 10·06) also had a higher PR of high BP compared with their normal-weight peers.
Table 3 Adjusted PR of high BP with corresponding 95 % CI by body size and sex among Aracaju adolescents
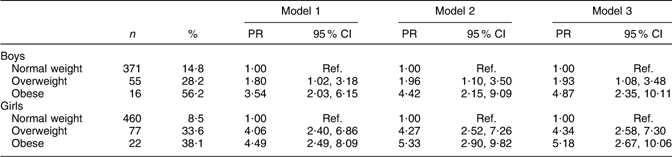
PR, prevalence ratios; BP, blood pressure; Ref., reference category.
Model 1, adjusted for age; model 2, plus type of school; model 3, plus physical activity.
Discussion
Key findings
We found a high prevalence of high BP in both boys and girls. Overweight and obesity were strongly associated with high BP in both boys and girls.
Discussion of the key findings
Studies on high BP and association of overweight and obesity with high BP among adolescents are limited in Brazil(Reference Costanzi, Halpern and Rech22–Reference Aristimuño, Foster and Voors24). In Guimarães et al.'s(Reference Guimarães, de Almeida and Santos20) study, the prevalence rates of high systolic BP and high diastolic BP were 20·4 % and 17·6 %, respectively, in Salvador(Reference Costanzi, Halpern and Rech22). However, high BP was defined as ≥90th percentile and the analyses were based on boys and girls combined. Guimarães et al.'s(Reference Guimarães, de Almeida and Santos20) study was also conducted in a middle-class neighbourhood in Salvador. Evidence suggests that BP increases with socio-economic status in Brazil(7, Reference Aristimuño, Foster and Voors24), which may indicate that the results found by Guimarães et al.(Reference Guimarães, de Almeida and Santos20) may not represent the actual prevalence of high BP in children in urban Brazil. Data were collected in all socio-economic groups in our study and therefore give a better reflection of BP in adolescents in an urban setting in Brazil.
The other studies in Brazil were also based on children or younger adolescents and the results were also reported for boys and girls combined(Reference Rodrigues, Moyses and Bissoli21, Reference Aristimuño, Foster and Voors24). Rodrigues et al. studied 10–14-year-olds in Victoria, Espirito Santo(Reference Rodrigues, Moyses and Bissoli21), and reported a 3·4 % rate of high BP among boys and girls combined. Costanzi et al.(Reference Costanzi, Halpern and Rech22) also studied children aged 7–12 years in Caxias do Sul, State of Rio Grande do Sul, and the rate of high BP (8·4 %) was also based on boys and girls combined. Our findings suggest important difference in BP between boys and girls. The prevalence rates found among adolescents in our study are relatively high compared with the rates found among younger age groups in Brazil. This indicates that high BP increases sharply with age and highlights the need for early intervention among children in Brazil.
The prevalence of high BP in our study is similar to the prevalence found among similar age groups (14–17 years) in Suriname, South America. In the Suriname study, the prevalence of high BP ranged from 9·7 % in Maroon boys to 18·8 % in mixed race boys, and from 3·5 % in Hindustani girls to 12·0 % in mixed race girls(Reference Agyemang, Oudeman and Zijlmans35). The mean systolic BP among Aracaju adolescent boys (116·9 mmHg) was somewhat similar to that of Surinamese adolescent boys, except for Maroon boys (112·5 mmHg) and Hindustani boys (114·2 mmHg). The mean systolic BP was, however, higher in Aracaju girls (112·6 mmHg) than in girls in Suriname in all ethnic groups, ranging from 104·5 mmHg in Hindustani girls to 107·9 mmHg in Creole girls(Reference Agyemang, Oudeman and Zijlmans35).
BP and obesity are strongly related(Reference Muntner, He and Cutler2, Reference Falkner, Gidding and Ramirez-Garnica3, Reference Torrance, McGuire and Lewanczuk23–Reference Weiss, Dziura and Burgert25). Our present findings are in agreement with previous findings in Brazil(Reference Guimarães, de Almeida and Santos20–Reference Costanzi, Halpern and Rech22, Reference Campana, Brandao and Pozzan36). Although the mechanisms by which overweight and obesity may lead to high BP are not well elucidated, it is now generally recognised that overweight and obesity increase the risk of high BP(Reference Sinaiko, Donahue and Jacobs37). Sinaiko et al.'s(Reference Sinaiko, Donahue and Jacobs37) prospective study showed that increases in BMI in early life were significantly related to an increased risk of high BP and other CVD in adulthood. Our findings clearly indicate the need to prevent the increasing prevalence of overweight and obesity early in life to prevent future sequelae of overweight- and obesity-related diseases.
Several studies in children showed that weight loss results in a reduction of BP(Reference Torrance, McGuire and Lewanczuk23). The recommended intervention strategy is a hypoenergetic diet plus physical activity(Reference Ribeiro, Silva and Santos38). Increasing physical activity level in adolescents can be a low-cost intervention to prevent high BP. The physical activity level among adolescents in our study was relatively low. The international guideline for physical activity for children under 18 years of age recommends exercise of moderate intensity for at least 1 h/d(7). The average leisure-time physical activity was 3 d/week for boys and 2 d/week for girls in our study population, which is far less than the international guideline. The physical activity patterns were relatively low in normal-weight, overweight and obese individuals, with only small differences between the groups. This may explain why the result remained the same after adjustment for physical activity in the multivariate analyses. Similar low prevalence of physical activity has also been reported in other parts of Brazil. In a study in Maceio, Alagoas, the prevalence of a sedentary lifestyle, defined as no moderate-to-intense physical activity, was 93·5 % in 7–17-year-olds(Reference Silva, Rivera and Ferraz39). These findings clearly suggest that effective measures are urgently needed to increase physical activity level among adolescents in urban Brazil.
Limitations
Our study has limitations. As in many epidemiological studies, our BP level was based on an average of three measurements at a single visit. A more precise estimate of BP level would be obtained by multiple measurements obtained during several visits(11). The physical activity levels were based on only leisure-time exercise, and was self-reported, which may be subjected to response bias. We had no information on other important factors that are known to influence high BP, such as dietary behaviour, which may also affect our study conclusions. Also the non-response rate was high, particularly in the 12-year-olds (43 %). It is possible that the health of the respondents and non-respondents may differ, which may affect our study conclusions. Nevertheless, the non-response rates were similar in both public (32·0 %) and private (33·6 %) schools. In addition, we corrected for the cluster design effect in the analyses. Furthermore, the distribution of boys and girls in our study is consistent with Aracaju's young population (male/female ratio: 47:53)(Reference Abdelgalil, Gurgel and Theobald40). Finally, our sample was based on Aracaju and its surroundings and therefore the findings may not be representative of other parts of Brazil. Despite these limitations, our present findings provide very important information on the relationship between overweight and obesity and BP among adolescents in Brazil.
In conclusion, our results show a high prevalence of high BP among this population in Brazil. Overweight and obesity were strongly associated with high BP in both boys and girls. Prevention strategies are needed in Brazil to prevent the future burden of hypertension and reduce pressure on the public health-care system. BP reductions in adolescents can be achieved by weight loss through reducing excessive energy intake(Reference Gallist, Sudi and Aigner41) and increasing physical activity strategies(Reference Rocchini, Katch and Anderson42). These cost-effective measures may lead to a significant reduction in BP in adolescents, thereby sparing the next generation from high BP-related complications.
Acknowledgements
The present research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. C.A. was supported by a VENI fellowship (Grant no. 916.76.130) awarded by the Board of the Council for Earth and Life Sciences (ALW) of the Netherlands Organisation for Scientific Research (NWO). The authors have no conflict of interest to declare. R.Q.G., J.P., R.R., R.E.d.O.R. and J.A.S.B.-F. were responsible for data collection; J.P., C.A., J.F.W., J.S.d.M. and R.Q.G. were responsible for analysis and interpretation of data; R.R. and C.A. drafted the manuscript and all were involved in critical revision of the manuscript. All authors were responsible for study concept and design.







