Both low and high birth weight have been associated with increased risk of type 2 diabetes and CVD in adulthood in epidemiological studies( Reference Whincup, Kaye and Owen 1 ). Children born small- and large-for-gestational-age have been reported to have higher insulin resistance than children born appropriate-for-gestational-age( Reference Chiavaroli, Giannini and D’Adamo 2 ), suggesting that abnormal prenatal growth increases cardiometabolic risk already in childhood.
One possible mechanism for the relationship between birth weight and later disease risk is programming of metabolism during fetal life( Reference Barker 3 ). It has been suggested that also appetite and taste preferences may be programmed by the intra-uterine environment( Reference Stein, Cowart and Beauchamp 4 , Reference Ayres, Agranonik and Portella 5 ). For example, lower birth weight has been associated with a higher acceptance of salty taste( Reference Stein, Cowart and Beauchamp 4 ) and sweet taste( Reference Ayres, Agranonik and Portella 5 ) in childhood, which may lead to higher intakes of salty and sweet foods. Alternatively, the association of low and high birth weight with later diseases could be mediated by harmful parental lifestyle factors, such as an unhealthy diet, related to the birth weight of the offspring.
Evidence on the associations of birth weight with dietary factors in later life is limited. One previous study reported that undernutrition during fetal life was associated with a higher intake of fat, particularly saturated fat, in adults born in the 1940s( Reference Lussana, Painter and Ocke 6 ). In similar vein, a lower birth weight was associated with a higher intake of fat and a lower intake of carbohydrates in adults born in the 1930s to 1940s( Reference Perälä, Männistö and Kaartinen 7 ). In contrast, severe intra-uterine growth restriction was associated with a higher carbohydrate intake in a study among young Brazilian adults born in the 1970s( Reference Barbieri, Portella and Silveira 8 ). Children born preterm at very low birth weight had a lower consumption of vegetables, fruit, berries and dairy products than children born at term in a study among young Finnish adults born in the 1970s to 1980s( Reference Kaseva, Wehkalampi and Hemiö 9 ).
There are few reports on the associations of birth weight with dietary habits among healthy, full-term children in Western countries in recent decades, when famine is absent and when overnutrition during pregnancy is more common than undernutrition( Reference Stafford and Lucas 10 , Reference Shultis, Leary and Ness 11 ). Only two studies among children born in the early 1990s have reported a relationship between a lower birth weight and a higher intake of fat( Reference Stafford and Lucas 10 ) and a higher intake of saturated fat( Reference Shultis, Leary and Ness 11 ) at pre-school age. However, there are no studies on the associations of birth weight with overall diet quality or eating frequency in healthy, full-term children. We therefore explored the associations of birth weight with overall diet quality, food consumption, energy and nutrient intakes, and the number of main meals and snacks daily at the age of 6–8 years in a population sample of Finnish girls and boys born at term in the early 2000s.
Methods
Study design and study population
The present analyses are based on the baseline data of the Physical Activity and Nutrition in Children (PANIC) study, which is an ongoing physical activity and dietary intervention study in a population sample of primary-school children from the city of Kuopio, Finland (ClinicalTrials.gov registration number NCT01803776). Altogether 736 children born in 1999–2002 were invited to participate in the study by letters delivered to their parents via schools when the children were 6–8 years old. Of 736 invited children, 512 (70 %) participated in the baseline examinations that were conducted in 2007–2009. The participants did not differ in sex distribution, age or BMI standard deviation score (BMI-SDS) from other children of the same age living in the city of Kuopio based on available school health examination data (data not shown). Of the whole PANIC study sample, we excluded fifty-five children who had no valid data on birth weight or gestational age in the national register, who were not singletons and who were born before 37 gestational weeks. Moreover, we excluded seventy-five children who had inaccurate data on food consumption and fifteen children who had severe chronic, diagnosed diseases or conditions that could affect fetal growth or diet (e.g. epilepsy, rheumatic disease, type 1 diabetes, inflammatory bowel disease, Asperger’s syndrome, attention-deficit hyperactivity disorder). The final study sample for these analyses consisted of 367 children (179 girls, 188 boys).
Assessment of gestational age and birth size
We collected data on gestational age, birth weight and birth length retrospectively from the birth register provided by the National Institute for Health and Welfare. We calculated birth weight SDS based on Finnish population birth size reference values( Reference Sankilampi, Hannila and Saari 12 ). These reference values were developed using Finnish birth register data from all Finnish infants born from 1996 to 2008. The values are specific for sex, gestational age and plurality. We also divided birth weight SDS into three categories (<−1 SDS, −1 to 1 SDS, >1 SDS).
Assessment of diet
We assessed food consumption, energy and nutrient intakes, and the number of main meals and snacks daily at the age of 6–8 years by food records administered by the parents on four predefined consecutive days, including either two weekdays and two weekend days or three weekdays and one weekend day. The parents were instructed to record all foods and drinks using household measures (e.g. tablespoons, decilitres, centimetres) and to ask their child about foods eaten outside their home. Schools and afternoon nurseries were asked for the menus and details on the foods served to the children; for example, cooking fat and spread on bread. A clinical nutritionist reviewed and completed the food records at return. For details on portion sizes, a picture booklet of portion sizes was used. We analysed the food records and calculated the intakes of energy and nutrients using the Micro Nutrica® dietary analysis software, version 2.5 (The Social Insurance Institution of Finland), that utilizes Finnish and international data on nutrient composition of foods( Reference Rastas, Seppänen and Knuts 13 ). Food consumption was analysed in grams divided by total energy intake to control for energy intake in a population that varies a lot in size and energy needs. A clinical nutritionist defined main meals and snacks according to the recorded time and type of foods. Breakfast, lunch and dinner were classified as main meals and all eating and drinking occasions between them as snacks.
As an indicator of a healthy diet, we used the Finnish Children Healthy Eating Index (FCHEI) that has been reported to describe well the dietary quality in 1-, 3- and 6-year-old Finnish children( Reference Kyttälä, Erkkola and Lehtinen-Jacks 14 ). In 6-year-old children, higher FCHEI has been strongly correlated with lower intakes of saturated fat (correlation coefficient r=−0·27) and sugars (r=−0·40), lower energy density (r=−0·24) and higher intakes of vitamin E (r=0·24) and vitamin D (r=0·37), indicating that a higher FCHEI reflects a healthier diet( Reference Kyttälä, Erkkola and Lehtinen-Jacks 14 ). We computed the FCHEI as described previously( Reference Kyttälä, Erkkola and Lehtinen-Jacks 14 ). In brief, the consumption of five food groups, including vegetables, fruit and berries, oils and vegetable oil-based margarines (fat ≥60 %), foods containing high amounts of sugar (sugar-sweetened beverages, fruit juice, added sugar, chocolate, sweets, pastries, biscuits, ice cream and puddings), fish and skimmed milk, were divided by energy intake and categorized to deciles according to their variation. The lowest decile achieved the minimum score of 1 and the other deciles were scored ascendingly. Reverse scoring was applied for foods containing high amounts of sugar. The resulting component scores were summed to create the overall FCHEI (range 5–40). A higher score indicates a higher diet quality.
Other assessments
We measured body height of children at the age of 6–8 years successively three times using a wall-mounted stadiometer in the Frankfurt position. The mean of the nearest two values was used in the analyses. Body weight was measured successively twice using the InBody® 720 device (Biospace, Seoul, Korea) after overnight fasting, empty-bladdered and standing in light underwear. The mean of the two values was used in the analyses. BMI-SDS was calculated based on Finnish references( Reference Saari, Sankilampi and Hannila 15 ). Chronic diseases and conditions and parental education and household income were asked by a questionnaire administered by the parents. Parental education was defined as the highest completed or ongoing degree of the parents (vocational school or less, polytechnic, university). Annual household income was reported to accuracy of 10000€ and was categorized as ≤30000€/year, 30001–60000€/year or >60000€/year.
We collected data on maternal age at child’s birth, possible multiple pregnancy, number of previous births (0 or ≥1) and smoking during pregnancy (no smoking, smoked but quit during the first trimester, smoked after the first trimester) retrospectively from the birth register of the National Institute for Health and Welfare. We also collected data on maternal body weight and height before pregnancy and gestational diabetes mellitus from the birth register of Kuopio University Hospital. Maternal BMI before pregnancy was calculated as weight (in kilograms) divided by the square of height (in metres). The data on BMI were available only for a sub-sample of 294 mothers and the data on gestational diabetes for a sub-sample of 299 mothers, who had delivered in the Kuopio University Hospital.
Statistical methods
We performed all data analyses using the statistical software package IBM SPSS Statistics version 21.0. The level of significance was set at P<0·05. We also used the Bonferroni correction for multiple testing, with the level of significance at P<0·002. The sample size of the present study was based on the original power calculation of the PANIC study( Reference Viitasalo, Eloranta and Lintu 16 ). A post hoc power calculation indicates that the minimally detectable effect size is 0·15 with a power of 80 %, a two-sided level of significance P<0·05 and the sample size of 367 children.
We compared the characteristics between girls and boys using Student’s t test and Pearson’s χ 2 test. The associations of birth weight SDS with dietary factors were investigated using multivariate linear regression analyses adjusted for sex, gestational age, age and BMI-SDS at the time of dietary data collection, maternal age at child’s birth, number of previous births, smoking during pregnancy, BMI before pregnancy and gestational diabetes, and parental education and household income at the time of dietary data collection. These covariates were chosen based on prior evidence( Reference Kyttälä, Erkkola and Lehtinen-Jacks 14 , Reference Kramer, Olivier and McLean 17 ). The linear regression analyses included only those participants with complete data (n 278). We used general linear models to test the interaction of sex and birth weight on dietary factors. If there was a statistically significant interaction, linear regression analyses on the association of birth weight with dietary factors were additionally performed for girls and boys separately. We present the results of the multivariate linear regression analyses as standardized regression coefficients (β) with 95 % CI that are standardized so that the variances of dependent and independent variables are 1. The standardized coefficients refer to how many standard deviations a dependent variable will change per one standard deviation increase in the predictor variable.
Because the association of birth weight with the risk of type 2 diabetes and CVD has been found to be U-shaped( Reference Whincup, Kaye and Owen 1 ), also the association of birth weight with diet is potentially non-linear. We therefore analysed the differences in dietary factors across three categories of birth weight SDS (<−1 SDS, −1 to 1 SDS, >1 SDS) using general linear models adjusted for same covariates as in the primary analyses. We did pairwise comparisons among all categories as post hoc tests and report the mean intakes and 95 % CI of those two categories that differed statistically significantly from each other. The presented P values are the P value for trend across the three categories.
Results
Characteristics
Boys were heavier and longer at birth and taller at the age of 6–8 years than girls (Table 1). Boys were also more likely to have a parent with a university degree and less likely to have a parent with a polytechnic degree than girls. Moreover, boys had lower vegetable and fruit and berry consumption, higher sausage consumption and higher energy intake than girls (Table 2).
Table 1 Characteristics of children and their parents, the Physical Activity and Nutrition in Children (PANIC) study, Kuopio, Finland, 2007–2009
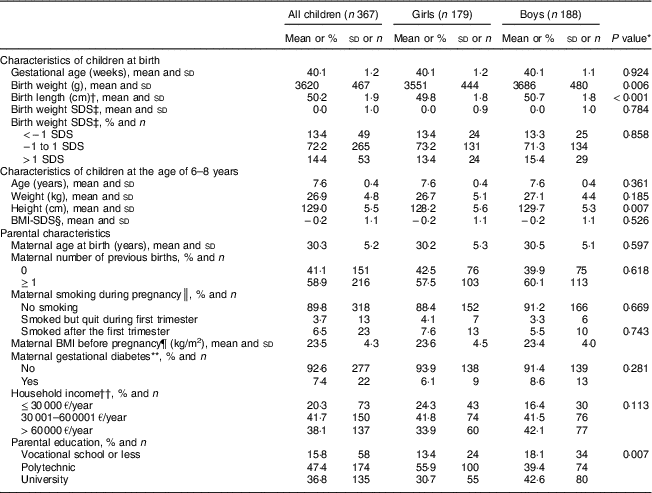
SDS, standard deviation score.
* Differences between girls and boys were assessed using Student’s t-test and Pearson’s χ 2 test.
† n 177 in girls, n 185 in boys.
‡ Calculated based on Finnish references( Reference Sankilampi, Hannila and Saari 12 ).
§ Calculated based on Finnish references( Reference Saari, Sankilampi and Hannila 15 ).
║ n 172 in girls, n 182 in boys.
¶ n 145 in girls, n 149 in boys.
** n 147 in girls, n 152 in boys.
†† n 177 in girls, n 183 in boys.
Table 2 Dietary factors of children at the age of 6–8 years, the Physical Activity and Nutrition in Children (PANIC) study, Kuopio, Finland, 2007–2009
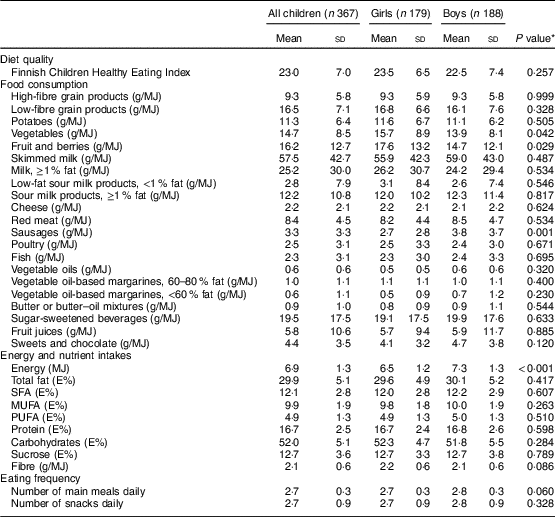
E%, percentage of energy intake.
* Differences between girls and boys were assessed using Student’s t test.
The association of birth weight with dietary factors
A higher birth weight SDS was associated with a lower FCHEI in all children adjusted for child’s sex, gestational age, age and BMI-SDS at dietary data collection, maternal age at child’s birth, number of previous births, smoking during pregnancy, BMI before pregnancy and gestational diabetes, and parental education and household income (Table 3). This association was observed in boys (β=−0·27, 95 % CI −0·45, −0·09, P=0·003) but not in girls (β=−0·01, 95 % CI −0·21, 0·18, P=0·911; P=0·044 for interaction). A higher birth weight SDS was also associated with lower fruit and berry consumption, higher total energy intake, higher sucrose intake and lower fibre intake in all children after these adjustments (Table 3). None of these associations remained statistically significant after Bonferroni correction for multiple testing.
Table 3 The associations of birth weight standard deviation score (SDS) with diet quality, food consumption, energy and nutrient intakes, and eating frequency at the age of 6–8 years (n 278), the Physical Activity and Nutrition in Children (PANIC) study, Kuopio, Finland, 2007–2009

E%, percentage of energy intake.
Data are standardized regression coefficients (β), 95 % CI and P values from linear regression models adjusted for gestational age, age and BMI-SDS at dietary data collection, and sex when appropriate, maternal age at birth, number of previous births, smoking during pregnancy, BMI before pregnancy and gestational diabetes, and parental education and household income. The threshold of statistical significance with Bonferroni correction is 0·002.
* Calculated based on Finnish references( Reference Sankilampi, Hannila and Saari 12 ).
The association of birth weight categories with dietary factors
Children who had a birth weight of >1 SDS had higher sucrose intake as a percentage of energy intake (mean intake 14·3 E%, 95 % CI 12·6, 16·0 E%) than children with a birth weight of −1 to 1 SDS (mean intake 12·8 E%, 95 % CI 11·6, 14·0 E%) or <−1 SDS (mean intake 12·4 E%, 95 % CI 10·8, 13·9 E%) adjusted for child’s sex, gestational age, age and BMI-SDS at dietary data collection, maternal age at child’s birth, number of previous births, smoking during pregnancy, BMI before pregnancy and gestational diabetes, and parental education and household income (P=0·036 for trend across the categories). Other differences in dietary factors across three categories of birth weight were not observed (data not shown).
Discussion
The results of the current study showed that higher birth weight was associated with poorer overall diet quality at the age of 6–8 years independent of child’s sex, gestational age, age and BMI-SDS at dietary data collection, maternal age at child’s birth, number of previous births, smoking during pregnancy, BMI before pregnancy and gestational diabetes, and parental socio-economic status in a population sample of full-term, healthy children and particularly in boys. Moreover, higher birth weight was related to lower fruit and berries consumption, higher energy and sucrose intakes and lower fibre intake. However, none of these associations remained statistically significant after correction for multiple testing.
We found that higher birth weight was associated with poorer diet quality assessed using a diet quality index. This association was independent of many potential confounding factors but weakened after correction for multiple testing. This finding increases our understanding on eating habits related to birth weight in a more holistic approach. Instead of a preference for single nutrients or foods, as previously reported( Reference Lussana, Painter and Ocke 6 – Reference Shultis, Leary and Ness 11 ), a higher birth weight may be related to an overall unhealthier diet that can be slightly different at different periods of time. Moreover, we found that birth weight was associated with diet quality in boys but not in girls, when sexes were studied separately. Some previous studies have also reported that the associations of birth weight with dietary factors were stronger in boys than in girls in young children( Reference Stafford and Lucas 10 , Reference Shultis, Leary and Ness 11 ). However, another study reported no interactions between the effects of sex and birth weight on diet in adults( Reference Perälä, Männistö and Kaartinen 7 ). Because these associations were not statistically significant after the correction for multiple testing, these findings need to be verified in other large samples of children.
Only few previous studies have investigated the association of birth weight with food consumption. In Finnish studies, lower birth weight has been associated with lower consumption of fruit and berries( Reference Perälä, Männistö and Kaartinen 7 , Reference Kaseva, Wehkalampi and Hemiö 9 ), vegetables( Reference Kaseva, Wehkalampi and Hemiö 9 ) and dairy products( Reference Kaseva, Wehkalampi and Hemiö 9 ) in adults. In contrast, we found that higher birth weight was associated with lower consumption of fruit and berries in children. One possible explanation for these inconsistent findings is that these previous studies investigated diet in adults, whereas we investigated diet in primary-school children. Diet in children reflects probably more the diet of their mothers, which has also affected the birth weight of the child, than diet in adults. Because we found an association between higher birth weight and lower quality of diet, such as lower consumption of fruit and berries, this may explain the associations of birth weight with diet found in the present study. On the other hand, diet in children may directly reflect intra-uterinely programmed food preferences, whereas the effects of these food preferences on diet may be weakened in adults by other factors, such as diseases related to intra-uterine growth. For example, lower birth weight was found to be associated with a higher consumption of fruit and berries in a sample of 56–70-year-olds( Reference Perälä, Männistö and Kaartinen 7 ). At that age, the occurrence of type 2 diabetes or CVD may have induced diet changes in a healthier direction. Moreover, one previous study investigated very-low-birth-weight preterm children( Reference Perälä, Männistö and Kaartinen 7 ). Such children have more hypersensitivity and oral motor problems than children born at term( Reference Samara, Johnson and Lamberts 18 ). These problems may affect the dietary choices of these individuals, such as avoiding bitter-tasting, hard-structured vegetables, fruit and berries. Therefore, it may be that both low and high birth weight are related to similar dietary preferences and deficiencies, although the likely mechanisms are different.
A previous Finnish study reported that higher birth weight among term-born children was associated with higher intake of sucrose in adults( Reference Perälä, Männistö and Kaartinen 7 ). In similar vein, we found that this association was pronounced already in children. Instead, we did not find an association of birth weight with fat intake in contrast to previous studies that have reported a consistent association between lower birth weight and higher fat intake( Reference Lussana, Painter and Ocke 6 , Reference Perälä, Männistö and Kaartinen 7 , Reference Stafford and Lucas 10 , Reference Shultis, Leary and Ness 11 ). One explanation for the inconsistent findings of these studies may be that the preference for a high fat intake appears with more serious intra-uterine growth restriction but not in full-term born children with appropriate birth weight. Only 13 % of children in our population sample of children born in the early 2000s had a birth weight less than 1 SDS. Therefore, it is possible that our general population did not include enough variance at the lower end of birth weight to show the association of low birth weight with the preference for a fatty diet. Instead, it may be that the preference for a diet high in sucrose appears already in children with birth weight at the higher end of appropriate levels. Moreover, the mean intake of fat was less than 30 E%, which is lower than in a previous study that has reported an association of lower birth weight with higher fat intake( Reference Perälä, Männistö and Kaartinen 7 ). In that study, an association of lower birth weight with higher fat intake was observed for an average fat intake of 33 E%( Reference Perälä, Männistö and Kaartinen 7 ). It may be that the average diet in the 2000s includes less high-fat products than earlier.
Previous studies have reported that high birth weight is associated with an increased cardiometabolic risk in adolescence( Reference Stansfield, Fain and Bhatia 19 ) and with an increased risk of type 2 diabetes in adulthood( Reference Whincup, Kaye and Owen 1 ). Our results suggest that these associations may be partly mediated by poor diet quality. For example, a lower consumption of fruit and berries has been linked to a higher risk of CVD( Reference Mirmiran, Noori and Zavareh 20 ). On the other hand, poor diet may also be associated with a higher adiposity, which then may lead to a higher risk of CVD. Moreover, poor diet quality may increase the risk of other chronic diseases in children with a high birth weight. For example, a higher sucrose intake may lead to an increased risk for poor dental health( Reference Anderson, Curzon and Van Loveren 21 ).
The relationship of birth weight with diet is likely to be explained by a complex aetiological network of both biological and social mechanisms. For example, previous studies have suggested that one potential mechanism for the association between birth weight and diet in adulthood is the biological early programming of appetite and taste preferences during fetal life( Reference Portella, Kajantie and Hovi 22 ). On the other hand, the associations of birth weight with dietary factors have also been suggested to be mediated by factors related to the health or socio-economic status of the mother or the whole family. Surprisingly, we found the association of higher birth weight with lower dietary quality after adjustment for several maternal characteristics, including age, previous births, smoking during pregnancy, BMI before pregnancy and gestational diabetes, and parental socio-economic status. Another potential social explanation for these associations is that poor quality of diet of the mother during pregnancy is related to higher birth weight of the child who then adopts the poor diet of the family. However, we had no data on maternal diet and thus were unable to test the confounding effect of maternal diet on the association of birth weight with diet in childhood. Nevertheless, women at reproductive age and their families may be a target for dietary interventions to prevent future generations from dietary shortcomings and chronic diseases. The reason for observing the association of higher birth weight with poor diet in boys but not in girls is unknown. One explanation for this finding could be that parents feed girls and boys with a higher birth weight differently, because a large boy may be more desired than a large girl. Moreover, in the present study, boys had a slightly larger standard deviation in birth weight and a higher consumption of foods, which may have affected the statistical power due to a higher frequency of extreme values in boys than in girls. However, we only found potential signals on possible differences between girls and boys, since the results were not statistically significant after correction for multiple testing. Possible sex differences need to be replicated in other samples of children.
The strengths of the present study are that gestational and birth data were obtained from reliable national records instead of self-reports and that dietary intake was assessed using 4 d food records that were individually instructed, reviewed and completed. The food record method has previously been validated against the observation method in primary-school children( Reference Klesges, Hanson and Eck 23 , Reference Crawford, Obarzanek and Morrison 24 ). We were also able to adjust the associations for several possible confounding factors. One of the main strengths was the relatively large representative population sample of children. Because of detailed background data, we were able to exclude twins and preterm-born children and children who had severe diseases that could have affected or mediated the studied associations. Due to our population sample, we had a low number of children with birth weight in the very extremes and were not able to divide the sample according to generally accepted cut-offs for small-for-gestational-age (<−2 SDS), appropriate-for-gestational-age (−2 to 2 SDS) and large-for-gestational-age (>2 SDS). Instead, we used cut-offs at −1 SDS and 1 SDS. A limitation of the study is the lack of data on maternal diet and other lifestyle factors during pregnancy, which could have confounded the observed associations. Moreover, we had data on maternal BMI before pregnancy and gestational diabetes only in a sub-sample of mothers, which may have limited the statistical power related to these variables. Another limitation is the large number of analyses, which raises the concern that the associations may have been found by chance. Moreover, our findings are specific to healthy, full-term born Finnish children aged 6–8 years and the generalizability of the findings in other populations needs to be investigated.
Conclusion
In conclusion, the findings of the current study suggest that children with a high birth weight may have a higher risk of having an overall unhealthy diet, particularly lower fruit and berries consumption, higher energy and sucrose intakes and lower fibre intake at the age of 6–8 years. However, this was an exploratory analysis and because the associations did not remain statistically significant after correction for multiple testing, the findings present only potential signals that need to be replicated in other samples of children. Then, dietary counselling targeted to children with a high birth weight could potentially decrease the risk of chronic diseases among these individuals.
Acknowledgements
Acknowledgements: The authors thank all voluntary subjects and their families participating in the PANIC study. They are also grateful to the members of the PANIC research team for their skilful contribution in performing the study. Financial support: This work was financially supported by grants from Ministry of Social Affairs and Health of Finland, Ministry of Education and Culture of Finland, Finnish Innovation Fund Sitra, Social Insurance Institution of Finland, Finnish Cultural Foundation, Juho Vainio Foundation, Foundation for Paediatric Research, Doctoral Programs in Public Health, Paavo Nurmi Foundation, Paulo Foundation, Yrjö Jahnsson Foundation, Diabetes Research Foundation, Finnish Foundation for Cardiovascular Research, Research Committee of the Kuopio University Hospital Catchment Area (State Research Funding), Kuopio University Hospital (EVO funding number 5031343) and the city of Kuopio. The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: A.-M.E. conducted the statistical analyses, interpreted the findings and wrote the draft of the manuscript. J.J., U.S. and V.L. contributed to the design of the study, the interpretation of the findings and the critical revision of the manuscript. T.V., H.J., S.K., and A.M. contributed to the critical revision of the manuscript. T.A.L. was the principal investigator of the PANIC study, contributed to the design of the study, the interpretation of the findings and the critical revision of the manuscript. All authors read and approved the final version of the manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures were approved by the Research Ethics Committee of the Hospital District of Northern Savo. Written informed consent was obtained from all participating children and their parents.





