Folate is an essential nutrient for nucleotide synthesis and DNA repair and methylation, and functions as a cofactor for enzymes involved in one-carbon metabolism. Folate plays a key role in cell division and thus is needed during pregnancy and infancy. During pregnancy, folate requirements increase to meet the demands of fetal, placental and maternal tissues( Reference Greenberg, Bell and Guan 1 ). Folate deficiency has been associated with fetal congenital anomalies, including neural tube defects (NTD).
NTD are the most common congenital malformations of the central nervous system, with an incidence of 0·2–10 per 1000 live births( Reference Copp, Stanier and Greene 2 , Reference Yi, Lindemann and Colligs 3 ). NTD are responsible for infant mortality and serious disability. The most common forms of NTD are anencephaly and spina bifida. Although the rate of fatality from spina bifida is lower than that from anecephaly (i.e. the rate of fatality is 7 % in the case of spina bifida, compared with 100 % fatality rate in anecephaly), it can result in severe lifelong morbidity( Reference Atta, Fiest and Frolkis 4 ). In 1991, the Medical Research Council Vitamin Study Research Group reported that the periconceptional use of folic acid prevents the recurrence of NTD and reduces the incidence of NTD by 72 % in offspring( 5 ). A systematic review confirmed that daily supplementation with folic acid can prevent both the incidence and recurrence of NTD( Reference De-Regil, Pena-Rosas and Fernandez-Gaxiola 6 ). Regarding the dose, Wald et al. suggested that supplementation with folic acid at 400 µg/d would reduce the risk of NTD by 36 %, at 1 mg/d would reduce the risk by 57 %, and at 5 mg/d would reduce the risk by 85 %( Reference Wald, Law and Morris 7 ). This preventive effect was confirmed in various ethnic groups in Hungary( Reference Czeizel 8 ), Europe( Reference Khoshnood, Loane and Walle 9 ) and China( Reference Gong, Wang and Wang 10 ). Considering that the neural tube closes by day 28, before most women notice that they are pregnant, folic acid supplementation must be started preconceptionally. Indeed, recent studies confirmed that starting folic acid supplementation before conception decreases the incidence and severity of NTD( Reference De-Regil, Fernandez-Gaxiola and Dowswell 11 , Reference Bergman, Otten and Verheij 12 ). In response to this accumulating evidence, the WHO and governmental organizations in many countries have recommended folic acid supplementation and folate-rich diets for women of childbearing age( Reference Gomes, Lopes and Pinto 13 ) and have noted that periconceptional folic acid supplementation prevents 50–75 % of cases of NTD.
In 2000, the Ministry of Health and Welfare (MHW) of Japan announced a recommended daily intake of 0·4 mg folic acid as a dietary supplement from one month before conception to the end of the third month of pregnancy( 14 ). Despite this announcement and nationwide campaigns, the incidence of spina bifida remained unchanged at approximately 5–6 per 10 000 births in the succeeding 10 years( 15 ). Kondo et al.( Reference Kondo, Morota and Ihara 16 ) identified four risk factors for spina bifida, including no intake of folic acid supplements, the presence of spina bifida within third-degree relatives, taking antiepileptic drugs without folic acid and birth weight ≤2500 g in the newborn, among which not taking folic acid supplements increased the risk by a factor of 2·5.
Several studies have indicated that the low uptake rate of supplementation is due to a lack of knowledge about folic acid( Reference French, Barr and Levy-Milne 17 – Reference de Jong-Van den Berg, Hernandez-Diaz and Werler 20 ). In Japan, less than 15 % of women knew about the relationship between folic acid and NTD in 2005( Reference Kondo, Kamihira and Shimosuka 21 ). Since then, knowledge of the NTD risk due to low folic acid intake has increased to 81·3 %, but most women take folic acid supplements only after conception( Reference Sato, Nakanishi and Chiba 22 ). This pattern indicates that the public health messages delivered to date have been ineffective and have failed to change the behaviour of women in preventing NTD. In the present study, the current status of awareness about folic acid and its periconceptional use among pregnant women was studied to clarify the factors involved and means to effectively increase the periconceptional intake of folic acid.
Methods
Participants
The present cross-sectional study was conducted at the Osaka Medical Center and Research Institute for Maternal and Child Health from September 2014 to December 2015. Approximately 1700 deliveries per year occur at the institute. With the exception of those in emergency situations, the pregnant women who visited the institute hospital during the study period were recruited. Inclusion criteria were as follows: pregnant woman who consulted the institute hospital during the study period and not having a severe medical disorder. Exclusion criteria were as follows: patient had a critical condition and/or was not proficient in Japanese. Filling out the questionnaire was considered consent for participation. The study was conducted in Osaka Prefecture, which has a population of approximately 8·8 million and is the second largest prefecture in Japan. The population represents average socio-economic condition of the Japanese. While the incidence of NTD in Osaka was not available, the incidences of anencephaly, spina bifida and encephalocele in Japan were reported to be 0·37, 5·18 and 0·83, respectively, per 10 000 births in 2012( 15 ).
Questionnaire
During their first routine visit, participants were asked to complete a questionnaire regarding their knowledge of folic acid and their use of folic acid supplements. The time of commencing usage of folic acid was chosen from five choices: pre-pregnancy before one month, just after conceptionally, first trimester, second trimester and third trimester. The end of usage of folic acid was chosen from four choices: just after recognition of pregnancy, first trimester, second trimester and third trimester. Although the questionnaire was not anonymous when completed, identifying information was removed after acquiring personal information including age, gestational age, gravidity and parity from the electronic medical records of the participants. Miscarriage was counted as the number of pregnancies minus parity.
The questionnaire comprised ten items in two parts. The first part included questions concerning knowledge about folic acid, sources of information, and awareness of the MHW recommendations and the statement in the Maternal and Child Health (MCH) Handbook, which is provided by local government offices when pregnancies are registered and comprises a record of pregnancy, delivery and child health-care, as well as information about the protective effects of folic acid against NTD. The second part included questions regarding folic acid intake during the current pregnancy, the source of folic acid (food, fortified food or supplements) and the brand name of any folic acid supplements used. The time of commencing usage of folic acid was chosen from five choices (i.e. pre-pregnancy before one month, just after conceptionally, first trimester, second trimester, third trimester). The end of usage of folic acid was chosen from four choices (i.e. just after recognition of pregnancy, first trimester, second trimester, third trimester).
Statistical analysis
Data were analysed using JMP® statistical software version 12 (SAS Institute Inc.). A χ 2 analysis was performed to compare differences in the proportions of categorical variables between groups. The χ 2 test for linear trend was used to analyse the trends of periconceptional use of folic acid supplements with respect to age, gestational age, number of pregnancies and parity. To examine the association between awareness and intake of folic acid supplements, we performed univariate and multivariate regression analyses. The results of these analyses were expressed as OR and their 95 % CI. Variables without significant multicollinearity were included in the multivariate model after being identified using Spearman’s rank correlation. The OR was considered significant if its 95 % CI excluded 1·00. The level of statistical significance was set at P<0·05.
Results
Participant characteristics
A total of 2012 pregnant women received the questionnaire, of whom 1862 filled out the form. The mean age of the participants was 32·9 (sd 5·6) years and the mean gestational age was 18·6 (sd 8·8) weeks. Data regarding pregnancy history were available for 1215 participants.
Awareness and use of folic acid
The age and obstetric characteristics of the participants together with their knowledge about folic acid are shown in Table 1. Of 1862 women, 1700 (91·2 %) had heard the words ‘folic acid’ and 1311 (70·4 %) knew about the protective effects of folic acid against NTD. Six hundred women (32·2 %) properly understood the requirements of 400 µg/d for NTD prevention. Since few participants recalled the manufacturer of their supplements, we could not collect dose data. Presumably, they took the required amount mentioned above.
Table 1 Clinical characteristics of participants: pregnant women who consulted the Osaka Medical Center and Research Institute for Maternal and Child Health (Japan), September 2014 to December 2015

NTD, neural tube defects; MHW, Ministry of Health and Welfare; MCH Handbook, Maternal and Child Health Handbook.
* Data regarding pregnancy history were available for 1215 participants in total, thus: N 1108 for knowledge of folic acid; N 874 for knowledge of NTD protection by folic acid; N 805 for knowledge of the recommendations of the Japanese MHW; N 530 for knowledge of the statement in the MCH Handbook; and N 400 for knowledge of the folic acid dose for NTD prevention.
Unexpectedly, only 382 participants (20·5 %) took folic acid supplements periconceptionally (i.e. one month at the latest prior to conception). As shown in Table 2, we observed a significant linear trend of more frequent periconceptional intake in older age groups. Nevertheless, a significant reverse trend was observed between the percentage of women using folic acid and parity. Periconceptional intake was more frequent in women who experienced miscarriage compared with those without miscarriage history. The percentage of participants who took folic acid from supplements and/or fortified food was 28·6%.
Table 2 Relationship between clinical characteristics and folic acid intake among pregnant women who consulted the Osaka Medical Center and Research Institute for Maternal and Child Health (Japan), September 2014 to December 2015
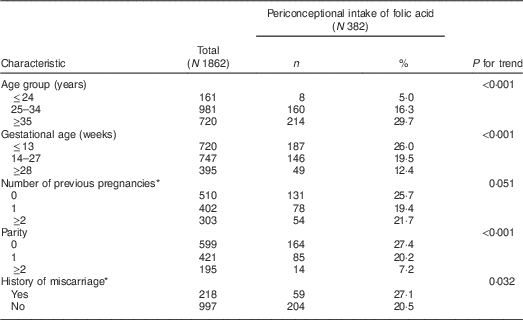
* Data regarding pregnancy history were available for 1215 participants in total, thus: N 263 for periconceptional intake of folic acid.
Sources of information about folic acid
Table 3 shows the sources of information about folic acid reported by the participants. The most common source was the Internet. Women who obtained information from medical staff, discussions with other pregnant women or the Internet were prevalent among folic acid takers. On the contrary, the percentage of women who obtained information from the MCH Handbook was significantly smaller in takers than in non-takers. Newspapers/magazines and the MCH Handbook were the main sources of information for non-takers, whereas the frequency of learning about folic acid in school or shops (pharmacies) did not differ between the groups.
Table 3 Sources of information about folic acid according to folic acid supplementation status among pregnant women who consulted the Osaka Medical Center and Research Institute for Maternal and Child Health (Japan), September 2014 to December 2015

MCH Handbook, Maternal and Child Health Handbook.
Univariate and multivariate analyses
The univariate and multivariate analyses of the cohort were performed for the characteristics related to the intake of folic acid pre-pregnancy through early pregnancy as well as the awareness about folic acid supplementation (Table 4). In the univariate analysis for periconceptional folic acid intake, age 25–34 and ≥35 years, parity of ≥2 and history of miscarriage were the significant background factors. Regarding knowledge of folic acid, the positive factors were awareness of NTD prevention, dosage, the MHW recommendations and the statement in the MCH Handbook.
Table 4 Results of univariate and multivariate analyses of periconceptional folic acid intake in relation to age, clinical characteristics and awareness about folic acid supplementation among pregnant women who consulted the Osaka Medical Center and Research Institute for Maternal and Child Health (Japan), September 2014 to December 2015

NTD, neural tube defects; MHW, Ministry of Health and Welfare; MCH Handbook, Maternal and Child Health Handbook; Ref., reference category.
In the multivariate analysis, the number of previous pregnancies was excluded because of significant multicollinearity and the final model adjusted for variables including age, gestational age, parity, miscarriage, awareness of NTD prevention by folic acid, awareness of the MHW (currently the Ministry of Health, Labour and Welfare (MHLW)) recommendations, awareness of the statement in the MCH Handbook, and knowledge of the amount of folic acid required to prevent NTD. The following variables were associated with the periconceptional intake of folic acid: age ≥35 years (OR=2·80; 95 % CI 1·24, 6·29), history of miscarriage (OR=1·76; 95 % CI 1·20, 2·58), awareness of NTD prevention by folic acid (OR=1·75; 95 % CI 1·11, 2·77), knowledge of the dose of folic acid needed to prevent NTD (OR=2·64; 95 % CI 1·92, 3·62) and awareness of the MHW recommendations (OR=2·20; 95 % CI 1·47, 3·31). OR decreased as parity increased. A parity of 1 (OR=0·63; 95 % CI 0·45, 0·87) or ≥2 (OR=0·18; 95 % CI 0·10, 0·32) was negatively associated with periconceptional folic acid intake.
Discussion
The present study investigated the current status of periconceptional folic acid supplementation among pregnant Japanese women and the factors associated with awareness and periconceptional intake.
Our results showed that only 20·5 % of the pregnant women in the study took folic acid periconceptionally, even though 70·4 % of participants had knowledge about the protective effect of folic acid against NTD. Periconceptional folic acid use was strongly related to age ≥35 years, primiparity and awareness about the benefit of folic acid. Approximately 90 % of participants had heard of folic acid, which is comparable to the rate observed in a Canadian study (95 %)( Reference French, Barr and Levy-Milne 17 ) and even higher than that reported in the Australian population (77 %)( Reference Chan, van Essen and Scott 23 ) (Table 5). Knowledge of the preventive effect of folic acid against NTD (70 %) was less prevalent than general awareness about folic acid, but the percentage was higher than that reported in Canada (25 %)( Reference French, Barr and Levy-Milne 17 ) and Australia (39 %)( Reference Chan, van Essen and Scott 23 ). The rate of periconceptional folic acid intake in the present study was approximately 20 %, which is comparable to that in Australia (23 %)( Reference Forster, Wills and Denning 24 ) but less than that in the Netherlands (51 %)( Reference de Walle and de Jong-van den Berg 25 ) and Canada (58 %)( Reference Nelson, Leon and Evans 26 ). Very low intake rate and awareness was reported in the Middle East. For example, only 4 % of women who participated in the study used folic acid during their preconceptional period in Saudi Arabia( Reference Al-Akhfash, Abdulla and Osman 27 ). It was also reported that 48·4 % of the participating women had low or lack of knowledge about folic acid in Iran( Reference Riazi, Bashirian and Amini 28 ). Although the proportion of periconceptional intake of folic acid supplements among Japanese women varied from 7·4 %( Reference Obara, Nishigori and Nishigori 29 ) to 32 %( Reference Kondo, Morota and Date 18 ) in different studies, the proportion of supplemental intakes has been gradually and steadily increasing year by year in Japan.
Table 5 Comparison of study results on acid awareness and supplementation with folic acid

NTD, neural tube defects.
Overall, half of our study participants did not take folic acid despite having knowledge of its benefits. Young (age ≤24 years) and multiparous women were most likely to be unaware of the preventive effect of folic acid against NTD and not take folic acid supplements. Knowledge of the benefits was correlated with intake. Taken together, these findings suggest that promoting the use of folic acid supplements in young and multiparous women should be prioritized.
The sources of information about folic acid consulted by the study participants differed significantly between the taker and non-taker groups. Takers obtained the information from the Internet, medical staff and communication with other pregnant women, and thus seemed more proactive in obtaining guidance by searching the Internet and asking advice. By contrast, non-takers were passive, and their information sources were less interactive media such as newspapers, magazines, television or the MCH Handbook. Although reports from Canada and Arabian Qatar( Reference French, Barr and Levy-Milne 17 , Reference Bener, Al Maadid and Al-Bast 30 ) indicate that women are advised to take folic acid by doctors, in the present study, the percentage of pregnant women who reported being informed about folic acid by medical staff was only 20 %. Given that significantly more women obtained information from medical staff in the taker group compared with non-takers, Japanese medical staff should more intensely recommend folic acid supplementation to their patients.
The MCH Handbook was first distributed by the Japanese MHLW in 1947 and is an effective tool for maternal and child health promotion( 31 ). It consists of records of pregnancy, delivery, child development, immunizations and child growth, and information about optimal diet and nutrition, including a statement recommending periconceptional folic acid intake. In the present study, only 8 % of the takers were informed about folic acid by the handbook. Clearly, the distribution of information in a handbook issued after pregnancy is too late for index pregnancies; such information should be delivered before pregnancy via the Internet or health-related education programmes in schools directed specifically at groups of young women of reproductive age and women planning to conceive. A few of studies have demonstrated a positive correlation between education level and awareness of periconceptional use of folic acid supplements( Reference Nelson, Leon and Evans 26 , Reference Buttriss 32 , Reference Timmermans, Jaddoe and Mackenbach 33 ).
The mandatory fortification of foods with folic acid has been introduced in more than eighty countries( 34 , Reference Williams, Rasmussen and Flores 35 ) and has resulted in a significant decline in the prevalence of spina bifida and anencephaly( Reference De Wals, Tairou and Van Allen 36 ). Such fortification does not occur in Japan even though the average dietary intake of folic acid is small in the Japanese population and serum folate levels are low( Reference Matsuzaki, Haruna and Ota 37 ). Therefore, mandatory fortification is needed in this country as well. However, in the USA, only ~8 % of women took a sufficient amount of folic acid after food fortification, probably due to their carbohydrate-deficient diet and their use of multivitamin supplements with low folic acid content( Reference Massi Lindsey, Silk and Von Friederichs-Fitzwater 38 ). Thus, besides mandatory fortification, additional strategies targeting young women are required. Social networking sites are expected to be useful and helpful in this respect, since those who are in their twenties to forties use this medium( 39 ).
Therefore, encouraging the government to start to mandate fortification with folic acid is needed. On the other hand, in the USA where the fortification of grains was mandated in 1998, fewer than 8 % of women reach the daily recommended level of folic acid with grain fortification alone( Reference Massi Lindsey, Silk and Von Friederichs-Fitzwater 38 ). The reason for this is attributed to diet and the usage of multivitamins, and these are also likely to occur in Japan. Besides mandatory fortification, additional strategies need to be targeting both young women and men. According to the fact that about 50 % of those in their twenties to forties use social networking sites, this would be a useful tool to spread the information( 39 ).
Our study had limitations. First, it was conducted in a single tertiary medical centre, which suggests that the results may not represent the general population. Second, the questionnaire surveyed all participants about their current pregnancy but did not survey multiparous participants about their history of folic acid use in previous pregnancies. Third, the study was cross-sectional and thus we were unable to draw any causal inferences from the results. Finally, the present study did not collect data about education level or family income, which would influence the intake of folic acid supplements( Reference Chan, van Essen and Scott 23 , Reference Xing, Tao and Hao 40 ).
Conclusions
Awareness about folic acid was relatively high among pregnant Japanese women, but awareness about the prevention of NTD by folic acid and the need for periconceptional intake is insufficient. A thorough educational programme directed at women of childbearing age is necessary to promote periconceptional folic acid supplementation in countries in which the fortification of foods with folic acid does not occur.
Acknowledgements
Acknowledgements: The authors thank the medical staff who assisted with this research in Osaka Medical Center and Research Institute for Maternal and Child Health. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: S.Y. designed the study and analysed the data. Y.W. organized the distribution of questionnaires in the outpatient clinic. S.Y. and Y.W. interpreted the data and wrote the paper. Ethics of human subject participation: Ethical approval for the study was obtained from the Ethics Committee of Osaka Medical Center and Research Institute for Maternal and Child Health.








