The metabolic syndrome (MetS) is a cluster of CHD risk factors: diabetes and elevated fasting blood glucose, abdominal obesity, low HDL cholesterol (HDL-C) and high blood pressure( Reference Alberti, Zimmet and Shaw 1 – 3 ). It is estimated that 20–25 % of the world’s adult population and approximately 34 % of the US population have MetS( Reference Ervin 4 ). Those with elevated MetS risk factors are twice as likely to die from MetS and three times as likely to have a heart attack or stroke compared with people without MetS. In addition, people with MetS have a fivefold greater risk of developing type 2 diabetes( Reference Stern, Williams and Gonzalez-Villalpando 5 ).
According to the American Heart Association( Reference Roser, Go and Lloyd-Jones 6 ), the following factors have been reported as being directly associated with incident MetS in prospective or retrospective cohort studies: age, low socio-economic status, smoking, low levels of physical activity, intake of soft drinks, Western dietary pattern, heavy alcohol consumption, long-term stress at work, obesity or BMI. Four behavioural risk factors targeted in primary care to prevent and manage non-communicable diseases, including MetS and its sequelae, type 2 diabetes and CVD, are tobacco use, physical inactivity, unhealthy diet and alcohol( 7 ). One of the four behavioural risk factors, physical inactivity, is not only a leading risk factor for mortality but also a significant contributor to rising health-care costs. Each year, approximately 3·2 million deaths and 32·1 million disability-adjusted life years, representing about 2·1 % of global disability-adjusted life years, are attributable to insufficient physical activity( 8 ). People who are insufficiently physically active have a 20–30 % increased risk of all-cause mortality compared with those who engage in at least 30 min of moderate-intensity physical activity on most days of the week( 9 ). Participation in 150 min of moderate physical activity each week or equivalent is estimated to reduce the risk of IHD by approximately 30 %, the risk of diabetes by 27 % and the risk of breast and colon cancer by 21–25 %( 8 , 9 ). In the National Health and Nutrition Examination Survey (NHANES) 2007–2008, 32 % of US men and 36 % of US women were obese and an additional 40 % of men and 28 % of women were overweight. About one in twenty Americans had a BMI of >40 kg/m2 (class III obesity)( Reference Flegal, Carroll and Ogden 10 ). Compared with adults with a BMI of 20·0–24·9 kg/m2, those with a BMI of 30·0–34·9 kg/m2 and ≥35·0 kg/m2 had 25 % and 44 % higher mean annual total (inpatient and outpatient) health-service costs, respectively( Reference Quesenberry, Caan and Jacobson 11 ).
Although a large number of studies have reported associations between MetS and physical activity behaviours using questionnaires, little research has been conducted about the relationship of MetS risk with objectively measured physical activity. A literature search using the keywords ‘objectively measured physical activity’, ‘accelerometer’ and ‘MetS’ in PubMed yielded only fourteen articles. Studies reporting associations between daily steps and MetS yielded only two articles( Reference Camhi, Sisson and Johnson 12 , Reference Sisson, Camhi and Church 13 ). The remaining articles reported associations between intensity-related physical activity and MetS. Furthermore, to our knowledge, no studies have been published about the associations of food consumption and serum vitamins with MetS risk stratified by levels of physical activity measured by daily steps taken.
However, diet is indispensible in every day of our lives and one of the four major risk factors for non-communicable diseases( 7 ). Also diet can be an immediate factor affecting the action of other factors. This means that diet is a key element in maintaining or increasing physical activity and in disease prevention or treatment. In addition, serum vitamin concentrations are closely connected with physical activity, usual food consumption and health status. For example, low levels of serum vitamins are closely associated with insufficient physical activity, insufficient consumption of fruit and vegetables, and poor health status such as overweight and MetS as well as undernutrition. In this respect, it is valuable to measure these three factors (food consumption, serum vitamin status, MetS risk) by objectively measured physical activity level. Therefore the objective of the present study was to examine the associations of food consumption, serum vitamins and MetS risk with physical activity level in middle-aged adults.
Methods
Data collection
The present study used cross-sectional data from the participants of NHANES 2005–2006. NHANES is one of a series of health-related programmes conducted by the Centers for Disease Control and Prevention’s National Center for Health Statistics. The NHANES target population is the civilian, non-institutionalized US population and the survey is a stratified, multistage probability sample design. NHANES 2005–2006, one of the continuous NHANES, refers to the two-year cycles of data produced since 1999. Each cycle is divided into five sections labelled by collection method: Demographics, Dietary, Examination, Laboratory and Questionnaire. All of the NHANES five-section data files can be linked by using the common survey participant identification number. Details of NHANES 2005–2006 can be found online( 14 ). We combined the five-section data to analyse associations between level of daily steps and MetS risk and to examine differences in food consumption and levels of serum vitamins by the level of daily steps. Individuals selected for the present study were adults aged 40–70 years and at high risk for MetS. NHANES 2005–2006 contains data for 10348 individuals of all ages. Among the participants, women who were pregnant or lactating and individuals whose accelerometer was not in calibration were excluded. Moreover, missing measurements that existed in Dietary, Examination and Laboratory data were removed. Therefore, the final analytical sample size (of men and women, respectively) for each analysis was as follows: (i) in sociodemographic characteristics and MetS risk analysis, n 948 and n 982; (ii) in food group consumption analysis for 2 d dietary data, n 838 and n 896; and (iii) in serum vitamins analysis, n 747–813 and n 789–868.
Accelerometer-determined daily steps
Daily steps (steps/d) were obtained from the physical activity monitor data in the NHANES 2005–2006 Examination section. The physical activity monitor component was added to NHANES in 2003, with NHANES 2005–2006 being the first release of accelerometer-determined step data along with the more commonly collected and reported intensity and duration data based on accelerometer-determined activity counts. The primary objective of the physical activity monitor component is to collect objective information on physical activity. The device used was the ActiGraph AM-7164 (formerly the CSA/MTI AM-7164), manufactured by ActiGraph LLC (Fort Walton Beach, FL, USA). This device is powered by a small watch battery with a small electric signal emitted during movement. The device is programmed to detect and record the magnitude of acceleration or intensity of movement; acceleration data are stored in memory according to a specified time interval. A 1 min time interval or ‘epoch’ was used to aggregate accelerometer data. As the activity monitors are not waterproof, activities such as swimming and water aerobics are not recorded. Therefore, a bias can exist because the physical activity assessment is not for a whole day if the wearer stays in a water environment. However, to account for day-to-day variations and reliably estimate habitual physical activity in adults, Mathews et al. suggested 3 to 5 d of monitoring (optimally 7 d)( Reference Matthews, Ainsworth and Thompson 15 , Reference Willett 16 ). In the present study, participants (948 men and 982 women) who had at least 3 d of accelerometer data were used in the final analysis. Additionally, 98 % of the participants had seven valid days of accelerometer data. A detailed description of the monitors and studies that have used this device is posted on the ActiGraph website( 17 , 18 ). Steps were recorded by the ActiGraph accelerometer and summed as total steps taken and steps/min for 24 h (1440 min).
Metabolic syndrome definition
According to the updated guideline of the US National Cholesterol Education Program Adult Treatment Panel III, MetS was defined as the presence of three or more of the following five components: (i) waist circumference (WC) ≥102 cm for men and ≥88 cm for women; (ii) TAG ≥150 mg/dl or taking medication to treat hypertriacylglycerolaemia; (iii) HDL-C <40 mg/dl for men and <50 mg/dl for women or taking medication to treat low HDL-C; (iv) blood pressure ≥130/85 mmHg or taking medication to treat hypertension; and (v) fasting blood glucose ≥100 mg/dl or taking medication to treat hyperglycaemia( Reference Grundy, Cleeman and Daniels 19 ). The MetS data were obtained from the NHANES 2005–2006 Examination, Laboratory and Questionnaire section files.
Food group consumption
In the dietary recall survey of NHANES 2005–2006, two dietary interviews were administered to all sample persons. The primary dietary interview was administered in person in the mobile examination centre (called ‘the MEC in-person interview’). A follow-up dietary interview was conducted by telephone 3 to 10 d later from the home office (called ‘the phone follow-up interview’). The two non-consecutive days of 24 h dietary recall do not include the same day of the week (if the in-person MEC interview was conducted on Monday, then Monday would not be an available day for scheduling a phone follow-up interview). Using a computer-assisted dietary data entry system, all foods collected from the 24 h dietary recall of the respondents were coded with an 8-digit, US Department of Agriculture food code. Four data files were produced from the information collected in the dietary interview by the 24 h dietary recall method: two Individual Foods files and two Total Nutrient Intake files. Each file includes 1 d of intake data. Therefore, we used the two Individual Foods files to estimate the average daily intake of each food group. We classified all foods reported in the two Individual Foods files (first day: 4052 foods; second day: 3808 foods) into nine food groups according to the US Department of Agriculture food classification: (i) milk and milk products; (ii) meat, poultry, fish and mixtures; (iii) eggs; (iv) legumes, nuts and seeds; (v) grain products; (vi) fruits; (vii) vegetables; (viii) fats, oils and salad dressings; and (ix) sugars, sweets and beverages( 20 , 21 ). Then the average consumption of these nine food groups was calculated per person per day for each group by tertile of accelerometer-determined steps/d.
Serum vitamins
Vitamin C, vitamin B12, α-carotene, trans-β-carotene, cis-β-carotene, β-cryptoxanthin, γ-tocopherol, lutein+zeaxanthin, trans-lycopene, total lycopene, vitamin A, vitamin E and vitamin D in the NHANES 2005–2006 Laboratory file were used for the estimation of serum vitamin status by the level of daily steps. Detailed procedures for phlebotomy, subsequent handling of blood samples and analysis of samples, as well as reporting procedures for each of the serum vitamins, can be found online( 14 ). The assay methods used for measurement of serum analytes are detailed in the NHANES documentation and included the following: HPLC with electrochemical detection for vitamin C; RIA for serum vitamin B12; the two-step Diasorin procedure for vitamin D; and HPLC with photodiode array detection for vitamins A and E, γ-tocopherol and the carotenoids (α-carotene, trans-β-carotene, cis-β-carotene, β-cryptoxanthin, lutein+zeaxanthin, trans-lycopene and total lycopene)( 14 ).
Statistical analysis
Data analysis was performed separately for men and women because it is assumed that men and women have different daily steps( Reference McCormack, Giles-Corti and Milligan 22 – Reference Hirvensalo, Telama and Schmidt 24 ) and sociodemographic characteristics( Reference Ng, Kowal and Kahn 25 , Reference Gallo, Mackenbach and Ezzati 26 ). Three categories of independent variables were created by tertile of accelerometer-determined steps/d (in men and women, respectively): tertile 1 (sedentary), <6802, <5785; tertile 2 (intermediate), 6802–10698, 5785–9225; tertile 3 (active): ≥10699, ≥9226. Means and standard errors were calculated. In sociodemographic characteristics analysis, ANOVA with Bonferroni post hoc testing was used for multiple comparisons to determine mean differences. In food group consumption and serum vitamins analysis, univariate general linear model ANCOVA with Bonferroni post hoc testing was used. Covariates included age, BMI and total energy intake. Logistic regression was used to estimate the odds ratios and 95 % confidence intervals for MetS risk. Covariates included age, BMI, total energy intake, poverty income ratio, ethnicity and education level, and the active steps/d group was considered to be a reference. All reported P values are based on two-sided tests and measured to a significance level of 0·05. The sample weights, WTMEC2YR (full sample 2-year MEC exam weight), WTINT2YR (full sample 2-year interview weight) and WTDR2D (dietary 2-day sample weight), were used in all analyses to obtain unbiased national estimates. Statistical analyses were conducted using the Complex Samples module in the statistical software package IBM SPSS Statistics, version 19.
Results
Characteristics of the population
Table 1 shows the sociodemographic characteristics and MetS components by physical activity group and sex. As expected, the daily steps taken were inversely associated with age in men and women. The poverty income ratio was the highest in the intermediate activity group and the lowest in the sedentary group for men and women. For ethnicity, Black men and Black women had the highest prevalence in the sedentary group while White men and Hispanic/Others women had the lowest prevalence in the sedentary group. Conversely, Black men and Black women had the lowest prevalence in the active group. For education, among men, prevalence in the sedentary group decreased with education. The highest prevalence in the active group was observed for men with 9–12 years of education. Among women, the highest prevalence in the sedentary group was observed for those with 9–12 years of education. Prevalence in the active group increased with educational attainment in women.
Table 1 Sociodemographic characteristics and MetS components by tertile of daily steps in adults aged 40–70 years, NHANES 2005–2006
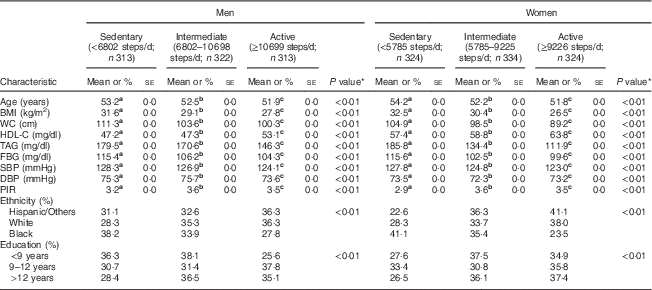
MetS, metabolic syndrome; NHANES, National Health and Nutrition Examination Survey; WC, waist circumference; HDL-C, HDL cholesterol; FBG, fasting blood glucose; SBP, systolic blood pressure; DBP, diastolic blood pressure; PIR, poverty income ratio.
a,b,cMean values within a row with unlike superscript letters were significantly different (multiple comparisons between tertiles within each sex by Bonferroni post hoc test; P<0·05).
*P value for difference between tertiles within each sex calculated from ANOVA.
BMI, WC and other components of MetS such as HDL-C, TAG, fasting blood glucose and blood pressure were significantly inversely associated with daily steps. Overall, men had higher values than women for WC, and women had higher values than men for BMI, HDL-C and TAG. The increased values for the MetS components seen with lower level of daily steps were greater in women than men. Compared with other components of the MetS, TAG values decreased the greatest with higher daily steps in men and women.
Food group consumption
As shown in Table 2, compared with the sedentary group, the active men consumed significantly more grain products, fruits and vegetables, whereas the active women consumed significantly more legumes and vegetables.
Table 2 Food group consumption by tertile of daily steps in adults aged 40–70 years, NHANES 2005–2006
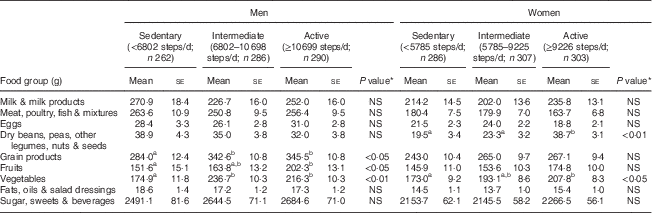
NHANES, National Health and Nutrition Examination Survey.
Covariates: age, BMI, total energy intake.
a,bMean values within a row with unlike superscript letters were significantly different (multiple comparisons between tertiles within each sex by Bonferroni post hoc test; P<0·05).
*P value for difference between tertiles within each sex calculated from general linear model ANCOVA.
Serum vitamin status
As shown in Tables 3 and 4, serum vitamin concentrations were significantly associated with daily steps in both men and women. For both sexes, the active group had the highest concentrations and the sedentary group had the lowest concentrations for vitamin C, α-carotene, trans-β-carotene, cis-β-carotene, β-cryptoxanthin, lutein+zeaxanthin, trans-lycopene, total lycopene and vitamin D. γ-Tocopherol was the highest in the sedentary group in men and women. No differences were observed by activity group for vitamin B12, vitamin A and vitamin E among men or women.
Table 3 Serum vitamin concentrations by tertile of daily steps in men aged 40–70 years, NHANES 2005–2006
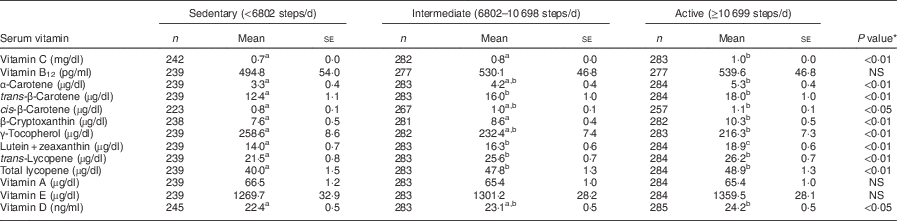
NHANES, National Health and Nutrition Examination Survey.
Covariates: age, BMI, total energy intake.
a,b,cMean values within a row with unlike superscript letters were significantly different (multiple comparisons between tertiles by Bonferroni post hoc test; P<0·05).
*P value for difference between tertiles calculated from general linear model ANCOVA.
Table 4 Serum vitamin concentrations by tertile of daily steps in women aged 40–70 years, NHANES 2005–2006
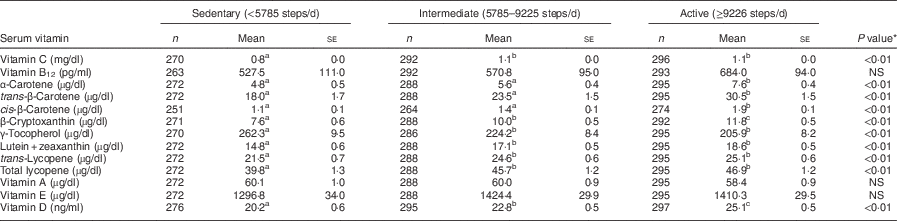
NHANES, National Health and Nutrition Examination Survey.
Covariates: age, BMI, total energy intake.
a,b,cMean values within a row with unlike superscript letters were significantly different (multiple comparisons between tertiles by Bonferroni post hoc test; P<0·05).
*P value for difference between tertiles calculated from general linear model ANCOVA.
Metabolic syndrome risk
Table 5 shows odds ratios and 95 % confidence intervals for MetS risk components. The active group was the referent group. The sedentary group had an approximate 1·9- and 2·5-fold risk for MetS in men and women, respectively. Sedentary behaviour was 1·5 to 1·8 times more likely to develop the risks for four of the five components of MetS in men and increased the risks of the five components by 1·6- to 3·9-fold in women. Among participants in the sedentary group, men had the highest risks for hyperglycaemia (OR=1·79) and hypertriacylglycerolaemia (OR=1·66), and women had the highest risks for hypertriacylglycerolaemia (OR=3·97) and abdominal obesity (OR=2·73).
Table 5 MetS risk by tertile of daily steps in adults aged 40–70 years, NHANES 2005–2006
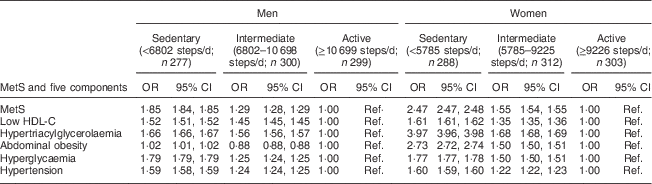
MetS, metabolic syndrome; NHANES, National Health and Nutrition Examination Survey; HDL-C, HDL cholesterol; Ref., referent category.
Covariates: age, BMI, total energy intake, poverty income ratio, ethnicity, education.
Discussion
Many studies have been reported that a lack of physical activity is a major risk factor for MetS( Reference Carnethon, Gidding and Nehgme 27 – Reference Ferreira, Henry and Twisk 29 ). The present study also found that lower daily steps were significantly associated with higher risk of MetS. Compared with the active group, OR for MetS were elevated for both men (sedentary, OR =1·85; intermediate, OR=1·29) and women (sedentary, OR=2·47; intermediate, OR=1·55). The OR for components of MetS were also significantly higher than 1·0 for sedentary and intermediate activity groups in men and women. This underscores the importance of regular physical activity in the prevention of MetS and its components in men and women. With regard to the risk difference between men and women, previous studies have reported that the association between sedentary behaviours and MetS is stronger in women than in men, and the risk of MetS is increased more in women than in men with prolonged time spent in sedentary behaviours( Reference Bertrais, Beyeme-Ondoua and Czernichow 30 – Reference Ford, Kohl and Mokdad 32 ). However, it is debatable whether women are more susceptible to developing MetS than men as some studies suggest that men are more likely to develop MetS than women. According to a recent study by Fernádez-Bergés et al.Reference Fernández-Bergés, Cabrera de León and Sanz (33) , in persons aged up to 55 years, MetS is more frequent in men but becomes more frequent in women in persons aged over 65 years.
In addition, the present study showed that there were significant differences between men and women in the OR of the five MetS components. Men had a high risk to develop hyperglycaemia and hypertriacylglycerolaemia, while women had a high risk to develop hypertriacylglycerolaemia and abdominal obesity. In a study of 1958 older adults from the Australian Diabetes Obesity and Lifestyle StudyReference Gardiner, Healy and Eakin (34) , Gardiner et al. found that overall sitting time was detrimentally associated with greater risk of having high TAG levels in both sexes, abdominal obesity in women and low HDL-C in men. Also, Travers et al. Reference Travers, Jeffers and Eckel (35) and Williams et al. Reference Williams, Zimmet and Shaw (36) reported that the prevalence of diabetes and elevated fasting glucose was higher in men than in women. In the present study, the OR of women for abdominal obesity was two times higher than that of men. Women’s WC itself is naturally smaller than that of men, but the increase was greater in women than in men with lower daily steps (Table 1). According to a WHO report, women have a greater relative risk of CVD at lower WC than do men generally (37) . In a follow-up study of 18 892 middle-aged Finnish men and women by Hu et al. Reference Hu, Tuomilehto and Silventoinen (38) , the age- and study year-adjusted hazard ratios of CVD across quartiles of WC were 1·0, 1·15, 1·37 and 1·97 in men, and 1·0, 1·41, 1·37 and 2·18 in women, respectively. These results and those observed in the present study indicate that while the mean of WC is smaller in women than men, the variation of WC is greater with lower daily steps and has a greater influence on the development of MetS in women than in men.
In examining the serum vitamin concentrations by daily step levels, higher concentrations of vitamin C, α-carotene, trans-β-carotene, cis-β-carotene, β-cryptoxanthin, lutein+zeaxanthin, trans-lycopene, total lycopene and vitamin D were significantly associated with higher daily steps in both sexes. These vitamins called antioxidants help protect the body against oxidative stress. The oxidative stress induced by free radicals is involved in the aetiology of a wide range of chronic diseases such as CVD, diabetes, renal disease and cancerReference Ames and Gold (39) . It has been reported that antioxidant nutrient status and exercise training have an interactive effect on oxidative stress and antioxidant enzyme activities( Reference Benderitter, Hadj-Saad and Lhuissier 40 , Reference Chang, Huang and Tseng 41 ). This suggests that eating enough antioxidants in foods or moderate exercise may lead to increased blood vitamins and minerals and decreased oxidative stress, which can prevent development of oxidative stress-related diseases like obesity, MetS, type 2 diabetes and cancer. According to epidemiological studies, serum concentrations of β-carotene and vitamin C were lower in obese individuals in the SUpplémentation en VItamines et Minéraux AntioXydants (SU.VI.MAX) study, which was conducted in 1821 women aged 35–60 years and 1307 men aged 45–60 yearsReference Galan, Viteri and Bertrais (42) . Coyne et al. Reference Coyne, Ibiebele and Baade (43) found that mean serum concentrations of α-carotene, β-carotene and sum of five carotenoids (α-carotene, β-carotene, β-cryptoxanthin, lutein and lycopene) were significantly lower in persons with MetS than persons without MetS in a study conducted on 1523 adults aged 25 years and over. α-Carotene, β-carotene and total carotenoids also decreased significantly with increased number of components of MetS. Alipanah et al. Reference Alipanah, Varadhan and Sun (44) determined that low total serum carotenoid (α-carotene, β-carotene, β-cryptoxanthin, lutein+zeaxanthin, lycopene) concentrations were associated with low walking speed and greater decline of walking speed during 3 years of follow-up. Cesari et al. Reference Cesari, Pahor and Bartali (45) also determined that plasma antioxidant concentrations correlated positively with physical performance and strength in the part of the Invecchiare in Chianti (ageing in the Chianti area; InCHIANTI) study that was conducted in 986 people aged 65 years and older. Also, Shardell et al.Reference Shardell, Alley and Hicks (46) observed a modest decrease in mortality for the second and third quartiles of lycopene intake in NHANES III. In another NHANES study, Patel et al.Reference Patel, Rehkopf and Leppert (47) determined that serum trans-lycopene was negatively associated with all-cause mortality. Specifically, higher levels of trans-lycopene were associated with a 20 % decreased risk for mortality. Interestingly, in the present study, higher levels of serum vitamin C and serum carotenoids (α-carotene, β-carotene, β-cryptoxanthin, lutein+zeaxanthin, trans-lycopene, total lycopene) were significantly associated with higher levels of walking and lower risks of MetS. Those people also consumed more foods rich in vitamin C and carotenoids, such as fruits and vegetables, as shown in Table 2. Namely, the results of our study are closely in agreement with the above findings.
Some studies have been conducted on the relationship among vitamin D, physical activity and multiple pathological conditions including MetS, CVD and type 2 diabetes. Gagnon et al. Reference Gagnon, Lu and Magliano (48) found that lower serum vitamin D concentration was associated with increased MetS risk and higher WC, serum TAG, fasting blood glucose and insulin resistance. Thomas et al. Reference Thomas, ó Hartaigh and Bosch (49) found that physical activity was positively associated with 25-hydroxyvitamin D (25(OH)D) level and optimal 25(OH)D levels (≥75 nmol/L) substantially lowered all-cause and CVD mortality in individuals with MetS. Also, Ardestani et al. Reference Ardestani, Parker and Mathur (50) confirmed that there was a significant interaction between 25(OH)D level and self-reported hours of moderate-to-vigorous physical activity and a direct relationship between 25(OH)D levels and VO2max in men and women over a broad age range (20–73 years) and various serum 25(OH)D levels (10–82 ng/ml) regardless of seasonal variations of 25(OH)D. Furthermore, higher levels of physical activity are generally associated with better serum vitamin D status, presumably because more outdoor physical activity can increase sun exposure and vitamin D production in the skinReference Willett (16) . If the above research results are synthesized with the results observed in the present study, physical activity increases serum 25(OH)D level and increased serum 25(OH)D will lead to both increased VO2max and reduced risks for MetS.
Perhaps the most interesting result from the present study is that γ-tocopherol was significantly inversely related with daily steps, unlike other serum vitamins. Significantly lower levels of γ-tocopherol in the active group were observed in both sexes. This result can be explained in two ways: physical activity and physical condition. Chorell et al. Reference Chorell, Svensson and Moritz (51) detected decreased levels of γ-tocopherol in highly fit individuals, suggesting that this result could be explained by an increased intracellular uptake and utilization of γ-tocopherol in highly fit individuals due to adaption to the oxidative stress during regular physical training. Also, Ford et al. Reference Ford, Mokdad and Ajani (52) reported that γ-tocopherol concentration was positively associated with concentrations of glucose and glycosylated Hb. In the study of Bates et al. Reference Bates, Mishra and Prentice (53) using data from the British National Diet and Nutrition Survey, higher plasma γ-tocopherol was significantly directly correlated with indices of obesity such as body weight, BMI, WC and waist-to-hip ratio. This correlation was higher in adults aged 35 years and over than in young people, especially in women aged 65 years and over. In the analysis of a prospective, nested case–control study conducted by Hak et al. Reference Hak, Stampfer and Campos (54) , it was shown that men with high plasma γ-tocopherol levels were more likely to have an increased risk of non-fatal and fatal myocardial infarction. Therefore, for the present study, it is plausible that the γ-tocopherol concentration of the active group was the lowest as an adaption to oxidative stress caused by aerobic exercise such as walking and running to increase daily steps. On the other hand, a possible reason why the sedentary group had the highest concentration of γ-tocopherol may be because the sedentary group had some other high-risk condition that can lead to CVD, type 2 diabetes and MetS outcomes. However, other authors have shown that the decreased plasma status of γ-tocopherol and some carotenoids during the acute phase of myocardial infarction normalized the year after the myocardial infarction event in a case–control and follow-up studyReference Ruiz Rejon, Martin-Pena and Granado (55) . Their results are contrary to ours. We can interpret this strange result as follows. The mean values of γ-tocopherol in the cases and controls were too low. According to the geometric mean and selected percentile values of serum γ-tocopherol concentration for the total US population aged 6 years and older in NHANES 2005–2006 (56) , the mean serum γ-tocopherol concentration of control subjects was only about the 5th percentile valueReference Ruiz Rejon, Martin-Pena and Granado (55) , whereas the mean serum γ-tocopherol concentrations in the present and aforementioned( Reference Chorell, Svensson and Moritz 51 – Reference Hak, Stampfer and Campos 54 ) studies were about the 50th percentile value.
Regarding the food group consumption results, the male active group consumed significantly more grain products, fruits and vegetables and female active group consumed significantly more legumes and vegetables than comparison groups. Accordingly, a recent study of 1359 middle-aged French persons in the SU.VI.MAX study showed that leisure-time physical activity such as walking and gardening was positively associated with eating healthy foods such as fruits and vegetables in both sexesReference Charreire, Kesse-Guyot and Bertrais (57) . In the Pelotas Birth Cohort Study designed to identify dietary patterns and relationships with sociodemographic and lifestyle factors of 4202 young Brazilian adults, those with a higher adherence to the vegetable/fruit dietary pattern had higher leisure-time physical activity( Reference Olinto, Willett and Gigante 58 ). Also, a cross-sectional study of older adults aged 70 years and over showed that higher levels of physical activity assessed by accelerometry were significantly correlated with the ease of purchasing fresh fruits, vegetables and low-fat products( Reference Thompson, Bentley and Davis 59 ). Georgiou et al.( Reference Georgiou, Betts and Hoos 60 ) identified that exercisers considered it more important to eat nutritious foods, ate more nutrient-dense low-fat foods, and more frequently met the Food Guide Pyramid-recommended grain and fruit intakes, than non-exercisers. Therefore, it appears that more active people eat more healthy foods and nutritious foods. The importance of fruit and vegetable consumption cannot be overemphasized because fruits and vegetables are an excellent source of various bioactive compounds including vitamins, minerals and antioxidants such as carotenoids, which have a cardioprotective effect. Also, higher levels of dietary fibre in fruits and vegetables may decrease the development of MetS by reducing blood cholesterol and lipid oxidation, and by modulating oxidative stress. All of these things make it clear that the avoidance of sedentary behaviours, healthy eating and good health are mutually connected.
The strength of the present study is that it is unique in identifying differences in food group consumption and serum vitamin status to analyse MetS risks by objectively measured daily steps in a representative sample of the US civilian non-institutionalized population. On the other hand, the weakness of the study is that detailed analysis about food consumption (e.g. separate sweets and beverages from the group of ‘sugar, sweets and beverages’) was not performed. Thus the study was unable to provide information on the detailed food intake affecting serum vitamin levels. A second limitation of the present study is that exact causality of the relationships among food intake, serum vitamin concentrations, daily steps and MetS cannot be suggested because of the cross-sectional design. However, as the primary study objective was to evaluate the association between daily steps and MetS and the risk conditions, and to find some difference in food intake and serum vitamin status by daily steps, the study was effective in providing new information regarding lifestyle behavioural risks for MetS. Most importantly, MetS is lowest and serum vitamin status is highest among those with the highest daily steps. Consequently, it appears that increasing daily walking is a helpful way to decrease MetS and its risk components.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: J.E.C. contributed to the research questions, data analysis, and manuscript writing and editing. B.E.A. contributed to the research questions and manuscript writing and editing. Ethics of human subject participation: The NHANES 2005–2006 was approved by the NCHS (National Center for Health Statistics) Research Ethics Review Board (protocol #2005–06).








