Children’s and adolescents’ dietary intake is of interest for health practitioners, policy makers and researchers. In large nutritional surveillance or epidemiological studies, there is a need for low-cost, user-friendly and high-quality dietary assessment tools in order to collect data from a large number of participants without the demand of massive resources. Web-based alternatives are thought to fill most of these criteria and a growing number of web-based dietary assessment tools have been developed( Reference Adamson and Baranowski 1 , Reference Thompson, Subar and Loria 2 ). Additionally, children and adolescents prefer new technology platforms over more traditional ones( Reference Mangunkusumo, Duisterhout and de Graaff 3 , Reference Mangunkusumo, Moorman and Van Den Berg-de Ruiter 4 ). Yet, despite the fact that web-based dietary assessment tools for younger age groups are increasingly popular, only a few have been validated thoroughly( Reference Diep, Hingle and Chen 5 – Reference Carvalho, Baranowski and Foster 8 ), thus information regarding the quality of the dietary data is often limited.
It has been suggested that 24 h recalls or food records are the most appropriate methods among the younger age groups( Reference McPherson, Hoelscher and Alexander 9 , Reference Burrows, Martin and Collins 10 ). However, these preferred methods rely on people’s cognitive skills, in addition to their knowledge of foods and how foods are prepared, which is often a limiting element for children and younger people( Reference Livingstone and Robson 11 ). Especially children under 11 years of age may have large difficulties in reporting their food intake without assistance( Reference Baranowski, Islam and Baranowski 12 ). For older children and adolescents, social desirability seems to increasingly affect reporting accuracy in an undesirable manner( Reference Livingstone and Robson 11 ). Hence, capturing the true dietary intake is especially challenging among children and adolescents.
An increasing body of literature emphasizes the importance of a high intake of fruits and vegetables to prevent non-communicable diseases and premature deaths( Reference Boeing, Bechthold and Bub 13 , Reference Wang, Ouyang and Liu 14 ). As chronic diseases develop over years, and dietary habits from childhood seem to persist into adulthood( Reference Mikkila, Rasanen and Raitakari 15 ), being able to capture an accurate intake of these foods among the younger age groups is of great interest. Most fruits and vegetables are rich in the natural pigments carotenoids, which cannot be synthesized by man( Reference Nagao 16 ). More than 700 carotenoids are identified; however, only six (β-carotene, α-carotene, β-cryptoxanthin, lycopene, lutein and zeaxanthin) are present both in the human diet and in a significant concentration in human blood( Reference Maiani, Caston and Catasta 17 ). A dose–response relationship between intakes of these carotenoids and concentrations in plasma has been demonstrated( Reference Rock, Swendseid and Jacob 18 ); hence carotenoids in plasma can be used as objective and valuable markers for consumption of fruits and vegetables rich in them. Nevertheless, as the content of carotenoids in different fruits and vegetables varies widely( Reference Maiani, Caston and Catasta 17 ), plasma concentrations of carotenoids might be better markers for some specific carotenoid-rich foods, rather than a summary marker for all fruits and vegetables.
In the present study the aim was to validate a web-based food recall (WebFR) for children and adolescents, by assessing the degree to which the WebFR could correctly rank individuals according to their intake of carotenoid-rich foods and thus discriminate between participants reporting low intakes of carotenoid-rich foods v. higher intakes. This was done by comparing reported intakes of carotenoid-rich foods with concentrations of carotenoids in plasma.
Methods
Participants and study design
This validation study was carried out from September through December 2013, in Bærum, Norway. Because the WebFR was intended to be used among 4th- and 8th-grade students in an upcoming national dietary survey, a total of 414 pupils from 4th grade (8–9 years) and 8th grade (12–14 years) were invited. Recruitment was done through schools after the parents/guardians had been informed about the study at plenary school meetings and information folders had been given to all schoolchildren and their parents/guardians in paper form or electronically. To be included, children needed Internet access at home and an email account for which their parents/guardians were responsible. A total of 276 schoolchildren returned the signed consent form; nine out of these withdrew from the study before or during the data collection, thus 267 completed all parts of the study. However, five were excluded due to insufficient blood analyses and one was excluded due to incomplete recordings in the WebFR (i.e. no entries had been saved). Finally 261 (63 %) schoolchildren were included in the analyses.
Participants were instructed to complete a four-day food recording using the WebFR; the 4th graders (8–9 years) were recommended to be assisted by their parents/guardians, as children this age have difficulties in reporting their dietary intake accurately( Reference Baranowski, Islam and Baranowski 12 ). A brief demonstration and written instructions were given to all participants. After they had completed the WebFR a small capillary blood sample was drawn from the participants, using the dried blood spot (DBS) method( Reference McDade, Williams and Snodgrass 19 ). Weights and heights of the participants were measured using standard procedures. Upon completion of the study, participants were given a personal gift card containing two cinema tickets. The study was conducted in accordance with the Declaration of Helsinki. Approval was obtained from the Norwegian Data Protection Official for Research in addition to written parental consent and child assent from all participants.
The web-based food recall
The WebFR is a hybrid method combining elements from both a food record and a recall. It is an adaption of the Danish Web-based Dietary Assessment Software for Children (WebDASC)( Reference Biltoft-Jensen, Trolle and Christensen 20 ), altered to Norwegian language, settings and food culture, as described in more detail in a previous paper( Reference Medin, Astrup and Kasin 21 ). The WebFR holds about 550 of the most commonly consumed foods and beverages in Norway, selected from the national survey NORKOST from 2011( 22 ).
Participants were instructed to enter everything they ate and drank during a period of four consecutive days in the WebFR, including one weekend day. They were asked to do the recordings at home, every evening, after they had consumed the last meal of the day. Anything consumed after the completion of a day’s recording could be entered into an open text field in the WebFR the following day.
The WebFR is structured around meals, with photos to estimate portion sizes, and with the possibility to enter food items not found in the food lists in the program in an open field. Foods are selected either from a drop-down list by clicking at different category levels (e.g. fruits and berries → fruits → apple) or by browsing in a search field. Portion sizes are selected by choosing a photo from photo-series of two to four photos, shown simultaneously, illustrating different portion sizes of each food item. Some photos function as proxies for other food items; for example, a glass of milk is exemplified by a glass of apple juice. Questions regarding supplements are included as pop-ups, appearing in the WebFR at the end of each day’s recording.
The interface is designed to be intuitive and joyful, and includes a voice-assisted cartoon character helping the participant complete the recordings. Pop-up reminders in the WebFR are thought to reduce the problem with omissions (i.e. foods eaten, but not reported).
Blood samples
All blood sampling was conducted by the same trained researcher, at the school nurse’s office or other suitable location at school, within 11 d upon the completion of the WebFR, using the DBS method( Reference McDade, Williams and Snodgrass 19 ). A non-fasting blood sample was drawn from the fingertip of each participant and drops of blood were placed directly on to a special filter paper, the DBS cards (Protein SaverTM 903R Cards; Whatman, Sanford, ME, USA), impregnated with a stabilizing agent (Vitas AS, Oslo, Norway). The filter papers were placed in lightproof boxes to air-dry for up to 8 h before they were placed one by one in single airtight and lightproof, foil barrier Ziploc bags containing a silica drying medium. Samples were then placed in a freezer at −70°C at the study centre within a maximum of 14 h from the time of collection and later transferred to the contract laboratory, Vitas AS, for analyses. Concentrations of the carotenoids (β-carotene, α-carotene, β-cryptoxanthin, lycopene, lutein and zeaxanthin) from dried full blood in the DBS cards were all analysed within 4 months after the samples were collected, using standard procedures of HPLC with UV detection( Reference Bastani, Kostovski and Sakhi 23 ). From each dried blood spot, five 3·2 mm discs were punched out, placed in vials and mixed with added distilled water. Further, proteins were precipitated, and carotenoids were extracted with isopropanol containing an internal standard (β-Apo-8 carotenal; Sigma-Aldrich, St. Louis, MO, USA), before being mixed thoroughly and then centrifuged. Subsequently, an aliquot of the isopropanol phase was analysed using an 1100-series HPLC-UV system with a 1260 diode array detector (453 nm; Agilent Technologies, Palo Alto, CA, USA). Separation was performed on a 3 mm YMC C30 column (150 mm×4·6 mm internal diameter; YMC, Kyoto, Japan). The measured concentrations of carotenoids from dried whole blood (DBS) were subsequently converted to plasma values by multiplying with a correction factor of 2( Reference McDade, Williams and Snodgrass 19 ). This was done in order to compare our whole-blood values with plasma values in other studies.
Construction of the carotenoid-rich foods variables
The contents of β-carotene, α-carotene, β-cryptoxanthin, lycopene, lutein and zeaxanthin vary largely between different sorts of fruits and vegetables. Hence, considering all fruits and vegetables as foods rich in carotenoids may be imprecise. Therefore the carotenoid-rich food variables were constructed. This was done by selecting the specific foods and beverages that both were consumed in the present study and had a high content of carotenoids. The selection of the carotenoid-rich foods was based on the carotenoid concentrations presented in the US Department of Agriculture’s National Nutrient Database( 24 ) and papers covering the applicable foods eaten in the present study( Reference Maiani, Caston and Catasta 17 , Reference Leth, Jakobsen and Andersen 25 – Reference Lashmanova, Kuzivanova and Dymova 30 ). Foods are presented in descending order from the most eaten for the following variables that were constructed.
-
1. ‘β-Carotene-rich foods’: carrot, broccoli, pepper, lettuce, leek, spinach, dried apricot, cantaloupe melon, parsley, chilli (all with a mean value of at least 900 μg β-carotene/100 g food).
-
2. ‘α-Carotene-rich foods’: carrot (with a mean value of at least 3500 μg α-carotene/100 g food).
-
3. ‘β-Cryptoxanthin-rich foods: orange juice, clementine, pepper, orange, corn (maize), mango, watermelon, popcorn, pineapple, peach, basil, chilli (all with a mean value of at least 65 μg β-cryptoxanthin/100 g food).
-
4. ‘Lycopene-rich foods’: canned tomato, fresh tomato, ketchup, fresh cherry tomato, tomato puree, watermelon, boiled tomato, tomato soup powder (all with a mean value of at least 4500 μg lycopene/100 g food).
-
5. ‘Lutein+zeaxanthin-rich foods’: broccoli, pepper, lettuce, corn (maize), cornflakes, corn meal, leek, popcorn, peas, corn flour, Brussels sprouts, spinach, basil, parsley (all with a mean value of at least 800μg lutein+zeaxanthin/100 g food).
-
6. ‘Total carotenoid-rich foods’: the sum of all the foods included in the single carotenoid-rich food variables.
Other variables
Height and weight were measured by trained personnel to the nearest 1 mm and 0·1 kg, respectively, according to standard procedures, in light clothing and without shoes. A digital scale (TANITA TBF-300; Tanita Corporation, Tokyo, Japan) was used during weighing. Overweight and obesity among the study participants were defined using age- and sex-specific ISO-BMI cut-offs( Reference Cole, Bellizzi and Flegal 31 ).
Information regarding sex and age of the participants, parental education level, parental ethnicity and family structure were provided by the parents/guardians in the written consent form.
Statistical analyses
Microsoft® Excel version 2010 and the food database software system KBS (database AE-10, version 7.2; Department of Nutrition, University of Oslo, Norway) were used to create the variables. The statistical software package IBM SPSS Statistics Version 21.0 (2012) was used in all statistical analyses.
Descriptive analyses were performed to describe participant characteristics, concentrations of carotenoids in plasma and the reported food intake. Food intake data for single carotenoid-rich foods had a skewed distribution and were insensitive to transformation because of an abundance of zero values. Yet, mean values are presented in Table 2, in addition to the median, in order to increase the comparability with other studies. As concentrations of carotenoids in plasma can provide only an indirect measure of dietary intakes, any direct comparisons with absolute intakes were impossible. Hence, only correlations and cross-classifications were used. Spearman’s rank correlations were used to evaluate the relationship between concentrations of carotenoids in plasma and the reported intakes of fruits, vegetables, juice and the carotenoid-rich foods. Serra-Majem and co-workers’ definition from 2009 was used to determinate the strength of the correlation coefficients( Reference Serra-Majem, Frost Andersen and Henrique-Sanchez 32 ). The Fisher Z method was used to calculate 95 % confidence intervals for Spearman’s correlation coefficients.
Possible differences between groups were also investigated. Spearman’s rank correlations between the reported intake of vegetables and the concentration of total carotenoids in plasma, and correlations between the reported intake of carotenoid-rich foods and the corresponding concentration of single and total carotenoids in plasma, were also calculated separately for the following groups for comparison: overweight or obese v. normal-weight subjects; 4th graders (8–9 years) v. 8th graders (12–14 years); girls v. boys; children having at least one parent/guardian of ethnic Norwegian origin v. others; low parental education v. high. Moreover, Fisher’s Z-test was used to test if there were any statistically significant differences in the correlation coefficient between these groups.
In order to get insights into the level of agreement between the reported food intakes in the WebFR and the objective measurement of carotenoids in plasma, cross-classification analyses were conducted. To what degree the WebFR allocated individuals into the correct quartiles of intake compared with the corresponding quartiles of single and total carotenoids in plasma was investigated. Proportions of participants classified into the same, adjacent or opposite quartiles were determined.
Sample size
The sample size was based on a significance level of 0·05 and 80 % power, using a table of sample size for correlations( 33 ). We expected correlations between foods and concentrations of carotenoids in plasma in the range of 0·3–0·4( Reference Burrows, Warren and Colyvas 34 – Reference Byers, Trieber and Gunter 36 ). A sample of n 67 was needed to detect a correlation of 0·3, and n 37 to detect a correlation of 0·4. To be able to analyse results by different subgroups (i.e. age, sex), we set the sample size to 240 participants.
Results
Slightly fewer boys and 4th graders (8–9 years) participated in the study, compared with girls and 8th graders (12–14 years; Table 1). The majority of the study participants had a normal body weight, parents/guardians with a high education level and Norwegian ethnicity. Furthermore, more than half of the participants used some kind of supplements; however, only 2·6 % (n 4) of these used carotenoid-containing supplements and were not excluded from the analyses.
Table 1 Characteristics of children and adolescents participating in a validation study of a web-based food recall (WebFR) in Norway, September–December 2013

* Information on parental educational level for one or both parents/guardians was available for 250 cases. Information regarding only one parent/guardian was available for sixteen out of these 250 cases, and these were included in the table based on the one available parent/guardian’s educational level.
† Both parents/guardians’ education is maximum high school level.
‡ One parent/guardian’s education is maximum high school level and the second parent/guardian’s education is at university-college or university level.
§ Both parents/guardians’ education is at university-college or university level.
|| Western world defined as: Western European/European Economic Area countries, North America, Australia and New Zealand.
The single and total carotenoid concentrations in plasma are shown in Table 2, in addition to intakes of vegetables, fruits, juice and carotenoid-rich foods recorded in the WebFR. Median intakes of vegetables, fruits and juice combined, and all carotenoid-rich foods combined, were 225·1 and 80·6 g/d, respectively. That is, more than half of all vegetables, fruits and juice recorded in the WebFR were not considered as carotenoid-rich foods in the present study. Lycopene, found mostly in tomato and tomato products, was the most abundant single carotenoid in plasma (i.e. median of 0·74 µm), and the reported intake of lycopene-rich foods was higher than for the other carotenoid-rich foods, demonstrated by a median value of 23·5 g/d.
Table 2 Plasma concentrations of carotenoids and recorded food intakes in the web-based food recall (WebFR) among 4th graders (8–9 years) and 8th graders (12–14 years) in Norway (n 261), September–December 2013
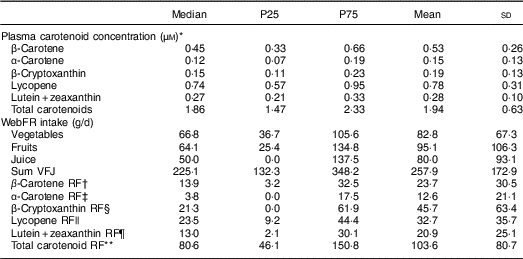
P25, 25th percentile; P75, 75th percentile; sum VFJ, sum of vegetables, fruits and juice; RF, rich foods.
* The dried blood spot method was used to collect samples of non-fasting whole blood. A fixed haematocrit value of 2 was used to convert whole-blood values to plasma values.
† Foods included (presented in descending order from the most eaten): carrot, broccoli, pepper, lettuce, leek, spinach, apricot, cantaloupe melon, parsley, chilli.
‡ Foods included: carrot.
§ Foods included (presented in descending order from the most eaten): orange juice, clementine, pepper, orange, corn (maize), mango, watermelon, popcorn, pineapple, peach, basil, chilli.
|| Foods included (presented in descending order from the most eaten): canned tomato, fresh tomato, ketchup, fresh cherry tomato, tomato puree, watermelon, boiled tomato, tomato soup powder.
¶ Foods included (presented in descending order from the most eaten): broccoli, pepper, lettuce, corn (maize), cornflakes, corn meal, leek, popcorn, peas, corn flour, Brussels sprouts, spinach, basil, parsley.
** Foods included: all foods included in the carotenoid-rich foods for the single carotenoids.
Spearman’s rank correlations between concentrations of single and total carotenoids in plasma, and intakes of vegetables, fruits, juice and the carotenoid-rich foods, are presented in Table 3. Plasma concentration of all carotenoids, except lutein+zeaxanthin, correlated considerably more strongly with reported intake of the corresponding carotenoid-rich foods (r=0·30 to 0·44) than with reported intake of all vegetables, fruits, juice, or these combined (r=−0·04 to 0·32). The strongest correlations were found between plasma concentrations of α- and β-carotene and reported intakes of α- and β-carotene-rich foods.
Table 3 Spearman’s rank correlation coefficients, and corresponding 95 % confidence intervals, calculated for carotenoids in plasmaFootnote * and recorded food intakes in a web-based food recall (WebFR) among 4th graders (8–9 years) and 8th graders (12–14 years) in Norway (n 261), September–December 2013

Sum VFJ, sum of vegetables, fruits and juice; RF, rich foods.
* The dried blood spot method was used to collect samples of non-fasting whole blood. A fixed haematocrit value of 2 was used to convert whole-blood values to plasma values.
† Foods included (presented in descending order from the most eaten): carrot, broccoli, pepper, lettuce, leek, spinach, apricot, cantaloupe melon, parsley, chilli.
‡ Foods included: carrot.
§ Foods included (presented in descending order from the most eaten): orange juice, clementine, pepper, orange, corn (maize), mango, watermelon, popcorn, pineapple, peach, basil, chilli.
|| Foods included (presented in descending order from the most eaten): canned tomato, fresh tomato, ketchup, fresh cherry tomato, tomato puree, watermelon, boiled tomato, tomato soup powder.
¶ Foods included (presented in descending order from the most eaten): broccoli, pepper, lettuce, corn (maize), cornflakes, corn meal, leek, popcorn, peas, corn flour, Brussels sprouts, spinach, basil, parsley.
** Foods included: all foods included in the carotenoid-rich foods for the single carotenoids.
The correlation coefficients between concentrations of total carotenoids in plasma and reported intakes of vegetables were significantly different between 4th graders (8–9 years) and 8th graders (12–14 years): r=0·47 and r=0·14, respectively (Table 4). No other significant differences in correlations between reported intake and the biomarker were found for weight status, age, sex, parental ethnicity or parental education.
Table 4 Spearman’s rank correlation coefficients calculated for concentration of total carotenoids in plasma and recorded vegetable intake in a web-based food recall (WebFR) among 4th graders (8–9 years) and 8th graders (12–14 years) in Norway (n 261), September–December 2013

* Fisher’s Z-test was used to compare the correlation coefficient between groups.
† Both parents/guardians’ education is maximum high school level.
‡ At least one parent/guardian’s education is university-college or university level.
Cross-classifications of participants by quartiles of reported food intakes and concentrations of carotenoids in plasma are shown in Table 5. The percentage of participants categorized in the same or adjacent quartile was higher for the carotenoid-rich food variables, except for lutein+zeaxanthin, than for the variables including vegetables, fruits and juice, or the sum of these (71·6–76·6 % v. 61·3–72·0 %, respectively). Furthermore, cross-classification of participants by quartiles of plasma concentrations of carotenoids and reported intakes of all carotenoid-rich foods showed that 3·8–10·0 % of the participants were grossly misclassified; β-cryptoxanthin-rich foods, followed by β-carotene-rich foods, were the least grossly misclassified. Moreover, cross-classifications of participants by quartiles of the total concentration of plasma carotenoids and reported intakes of vegetables, fruits and juice, separately and combined, showed that 7·7–14·9 % of the participants were grossly misclassified.
Table 5 Cross-classification between quartiles of carotenoids in plasmaFootnote * and quartiles of recorded food intakes in a web-based food recall (WebFR) among 4th graders (8–9 years) and 8th graders (12–14 years) in Norway (n 261), September–December 2013

SAQ, correctly classified: classified into the same or adjacent quartile; OQ, grossly misclassified: classified into opposing quartiles; sum VFJ, sum of vegetables, fruits and juice; RF, rich foods.
* The dried blood spot method was used to collect samples of non-fasting whole blood. A fixed haematocrit value of 2 was used to convert whole-blood values to plasma values.
† Foods included (presented in descending order from the most eaten): carrot, broccoli, pepper, lettuce, leek, spinach, apricot, cantaloupe melon, parsley, chilli.
‡ Foods included: carrot.
§ Foods included (presented in descending order from the most eaten): orange juice, clementine, pepper, orange, corn (maize), mango, watermelon, popcorn, pineapple, peach, basil, chilli.
|| Foods included (presented in descending order from the most eaten): canned tomato, fresh tomato, ketchup, fresh cherry tomato, tomato puree, watermelon, boiled tomato, tomato soup powder.
¶ Foods included (presented in descending order from the most eaten): broccoli, pepper, lettuce, corn (maize), cornflakes, corn meal, leek, popcorn, peas, corn flour, Brussels sprouts, spinach, basil, parsley.
** Foods included: all foods included in the carotenoid-rich foods for the single carotenoids.
Discussion
In the present study we assessed to what degree the WebFR could rank individuals according to their reported intake of carotenoid-rich foods. Results showed that correlations between concentrations of carotenoids in plasma and reported intakes of carotenoid-rich foods, except for lutein- and zeaxanthin-rich foods, were acceptable (0·30–0·44). The strongest correlations were found between plasma concentrations of α- and β-carotene and reported intakes of α- and β-carotene-rich foods. Correlations between concentrations of carotenoids in plasma and reported intakes of vegetables, fruits and juice were weaker. Cross-classifications between carotenoids in plasma and reported food intakes also showed better results for carotenoid-rich foods, compared with vegetables, fruits and juice. β-Carotene-rich foods and the corresponding carotenoids in plasma had the best agreement, with 76·6 % correctly classified. Moreover, reported intake of foods rich in β-cryptoxanthin and the corresponding carotenoid in plasma had the least gross misclassifications (3·8 %).
Only a small number of high-quality validation studies for web-based dietary assessment tools tailored for children and adolescents are found in the literature( Reference Diep, Hingle and Chen 5 – Reference Carvalho, Baranowski and Foster 8 ). Furthermore, to the best of our knowledge, just one validation study of web-based tools developed for this age group has used biomarkers for fruit and vegetable consumption as the reference method( Reference Biltoft-Jensen, Bysted and Trolle 6 ). Biltoft-Jensen et al. validated the Danish version of the WebFR, the WebDASH, using plasma carotenoid concentrations and compared them with the estimated intakes of carotenoids from foods and total intakes of food groups. Compared with our study, they found considerably stronger correlations between plasma concentrations of carotenoids and reported intakes of vegetables, fruits and juice (e.g. a correlation coefficient of 0·58 between total plasma carotenoids and total intake of vegetables, fruits and juice); moreover, only 1 % were grossly misclassified in the reported cross-classification analysis between fruits, juice and vegetables and the total plasma carotenoid concentrations, compared with 8·8 % in our study. This discrepancy between these similar tools, the WebFR and the WebDASH, was surprising. However, the WebDASH validation study was part of an intervention study focusing on a healthy diet rich in vegetables and fruits, and the participants had their lunch weighed and photographed before and after eating, for five days( Reference Biltoft-Jensen, Bysted and Trolle 6 ). We argue that these factors in the WebDASH study may have enhanced the participants’ abilities to remember and register the fruits and vegetables they ate, and thus increased the recording accuracy of these foods, compared with our study. Furthermore, in the study of Biltoft-Jensen et al. it is stated as a limitation that their sample had little ethnic, social and cultural diversity( Reference Biltoft-Jensen, Bysted and Trolle 6 ); this was not the case in our study, which had more ethnic diversity than in the general population. This might explain part of the discrepancy between the studies.
Other studies among healthy children and adolescents have validated FFQ using plasma carotenoids as an objective reference method; they report Pearson’s correlation coefficients between self-reported or parental-assisted self-reported total intake of vegetables and concentration of total carotenoids or β-carotene in plasma in the range of 0·15–0·26( Reference Nguyen, Scherr and Linnell 37 , Reference Slater, Enes and Lopez 38 ). In comparison, we found a similar Spearman’s correlation coefficient (r=0·28) between total intake of vegetables and concentration of total carotenoids in plasma.
To our knowledge, the present study is the first validation study among children and adolescents in which clear, predefined, carotenoid-rich food variables have been used. By excluding foods with a lower content of carotenoids, correlation coefficients were strengthened and potential attenuating effects on the true correlations have most likely been reduced. This indicates that plasma concentrations of carotenoids might be better markers for carotenoid-rich foods, rather than a summary marker for all fruits and vegetables; which is similar to the flavonoids being considered as better markers for specific foods or food groups than for total fruit and vegetable consumption( Reference Scalbert and Williamson 39 ). Also, in agreement with our conclusions, Jin and co-workers argue that concentrations of carotenoids are not the ideal biomarkers of the sum of fruits and vegetables; however, whereas their approach is to improve the prediction of total fruit and vegetable intake by creating a wider, integrated biomarker combining and including vitamin C, plasma carotenoids and ferric-reducing antioxidant power( Reference Jin, Gordon and Alimbetov 40 ), we narrow down on what the single carotenoids can predict. Both approaches seem to be superior to simply comparing carotenoids with the sum of fruits and vegetables.
The discrepancy between the time frame that is being reflected by the plasma carotenoids, and the time frame for the recordings in the WebFR, is a limitation in the current study. Although concentrations of plasma carotenoids can be affected shortly after a carotenoid-rich meal, it has been demonstrated that diurnal variations are small, and plasma concentrations do not change significantly from the fasting state and for several hours after ingestion of a meal( Reference Cantilena, Stukel and Greenberg 41 ). Moreover, carotenoids have been shown to have a half-life of some weeks( Reference Rock, Swendseid and Jacob 18 , Reference Micozzi, Brown and Edwards 42 , Reference Burri, Neidlinger and Clifford 43 ) and will to a great extent reflect what has been consumed over the last weeks. Therefore, intakes covering a few days, as measured by the WebFR, do not cover the exact same time frame as the plasma carotenoids reflects; this may have weakened the correlations in our study.
The strongest correlations found were between plasma concentrations of α- and β-carotene and reported intakes of α- and β-carotene-rich foods; a slightly weaker correlation was seen between plasma concentration of lycopene and lycopene-rich foods, and a clearly weak correlation between plasma concentration of the sum of lutein and zeaxanthin and the corresponding foods rich in these. Variations in the carotenoids’ ability to reflect the true intake, and not dissimilarities in how accurately these different carotenoid-rich foods have been reported, may partly explain these results. Lycopene and especially lutein/zeaxanthin have been described to have a longer half-life than the other carotenoids( Reference Rock, Swendseid and Jacob 18 ) and may consequently have reflected the period of recording in the WebFR even more poorly than the other carotenoids. Moreover, differences in release from the food matrix, solubility and absorption from the intestine, in addition to inter-individual plasma response depending on chylomicron clearance, also play an important role( Reference Faulks and Southon 44 ); for instance, it has been suggested that β-carotene inhibits absorption of lutein( Reference Yeum, Booth and Sadowski 45 ). Hence, a speculation is that such an inhibiting effect may have contributed to weaken the correlations between plasma concentrations of lutein/zeaxanthin and the reported intakes of foods rich in these, to some extent.
A significantly lower correlation coefficient was found between reported vegetable intake and total plasma concentration of carotenoids for the 8th graders (12–14 years) compared with the youngest (8–9 years), and may indicate less misreporting among the youngest children. Only parents/guardians of the 4th graders (8–9 years) in the present study were instructed to assist their children, as this is regarded as important to reduce misreporting in this age group( Reference Baranowski, Islam and Baranowski 12 ). Misreporting due to limited cognitive abilities among children is believed to be reduced by increasing age, but misreporting due to social desirability increases with age( Reference Livingstone and Robson 11 ). This may partly explain the significant differences between the age groups.
There are additional factors that may influence the strengths of correlations and the degree of misclassifications. Overweight and obesity are associated with lower concentrations of carotenoids in plasma( Reference Burrows, Warren and Colyvas 34 ). Furthermore, the use of dietary supplements has been shown to increase plasma concentrations of carotenoids( Reference Micozzi, Brown and Edwards 42 ). These possible confounding factors may therefore introduce bias and should be taken into account when interpreting associations between reported intakes and plasma values. However, in our study, there were very few participants using supplements containing carotenoids (n 4). The absence of significant differences between users and non-users of supplements containing carotenoids may be explained by lack of power. Furthermore, the absence of statistically significant differences in correlations for overweight or obese participants, compared with normal-weight participants, may also be explained by the limitations of the sample size; there were thirty-seven overweight or obese participants, thus only large dissimilarities could have been detected in the study.
Our study participants were instructed to complete the recordings in the WebFR at the end of each of the four recording days. It has been demonstrated extensively by Baxter and co-workers that misreporting increases with time( Reference Baxter, Guinn and Royer 46 , Reference Baxter, Hardin and Guinn 47 ). Thus, recall bias is likely to have weakened recording accuracy and subsequently weakened the correlation coefficients and increased the misclassification in the present study. For that reason, a limitation of our study is the lack of information concerning the time of entries in the WebFR. In addition, complete data on parental assistance is also lacking; we do know that not all of the youngest children were assisted when recording food entries, despite their caregivers being instructed to do so. In theory, this lack of parental assistance may have contributed to misreporting in the present study.
All sampling was conducted by the same researcher, according to a strict protocol, and all participants had their blood sample drawn within 11 d upon completion of the recordings in the WebFR. The highly standardized procedures used in the study have resulted in a high internal validity.
In urban and semi-urban areas in Norway parental educational levels are higher, the number of non-ethnic Norwegians higher and the proportion of overweight and obese individuals lower, than in the general Norwegian population. Parental educational level of university-college or university was high in our study population, compared with the average of 42 % among the general Norwegian population in the age group 25–59 years( 48 ). In addition, the proportion of non-ethnic-Norwegian parents/guardians was larger in the present study than the average in Norway (i.e. 23 % v. 14 %, respectively)( 49 ) and the number of overweight and obese participants was slightly lower than what is reported for Norwegian children and adolescents in general (i.e. 14 % v. 16 %)( 50 ). Thus, our study sample resembles the population from urban and semi-urban areas in Norway and we argue that the results from our study are most likely representative for children and adolescents living in such areas in Norway.
In the present validation study of a WebFR for children and adolescents, the correlations between reported intakes of carotenoid-rich foods and concentrations of carotenoids in plasma were acceptable. Poor correlations were found between reported intake of vegetables and the plasma carotenoids; however, these levels are in line with most other comparable studies. Nevertheless, the cross-classification analyses demonstrated that the WebFR was able to rank participants according to their reported intakes of foods rich in α-carotene, β-carotene, β-cryptoxanthin and lycopene, and it is therefore a satisfactory tool for classifying the intake of these foods. Additionally, the WebFR reduces the burden of data handling and may be perceived as more user-friendly among the younger age groups, compared with traditional dietary assessment tools. In conclusion, the WebFR is a promising tool to be used in future studies of dietary assessment among children and adolescents.
Acknowledgements
Financial support: This study was funded by the Institute of Basic Medical Sciences, University of Oslo, with supplementary funds from the Throne Holst Nutrition Research Foundation. The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: The authors’ roles in the study were as follows. A.C.M. and L.F.A.: conception and design; A.C.M.: acquisition of data; A.C.M., M.H.C. and L.F.A.: analysis and interpretation of data; A.C.M.: drafted and wrote the manuscript; A.C.M., M.H.C. and L.F.A.: critically revised the manuscript; L.F.A.: supervision and obtained funding. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Norwegian Data Protection Official for Research (NSD). Written informed parental consent and child assent was obtained from all participants.








