Dietary supplements (DS) are commercially available products consumed as an addition to the usual diet and include vitamins, minerals, amino acids (AA), herbs (botanicals) and a variety of other products(1). More than half of adults in the USA(Reference Kennedy, Luo and Houser2,Reference Cowan, Jun and Gahche3) and more than 70 % of US military service members (SM)(Reference Knapik, Trone and Austin4–Reference Austin, Price and Mcgraw6) use DS. Military personnel and civilians report using DS primarily to enhance health(Reference Austin, Price and Mcgraw6–Reference Bailey, Gahche and Miller9). Additional reasons SM report for using DS included (in descending order of prevalence) to provide more energy, improve muscle strength, enhance general performance and for weight loss(Reference Austin, Price and Mcgraw6,Reference Lieberman, Stavinoha and McGraw7) . Many DS users believe supplements can prevent or treat specific conditions like cancer, heart disease, osteoporosis and depression(Reference Neuhouser, Patterson and Levy10–Reference Zick, Blume and Aaronson13), despite limited research supporting these beliefs(Reference Hoffmann, Emons and Brunnhuber14–Reference Nahas and Balla18).
A number of studies have looked at associations between DS use and medical conditions using US nationally(Reference Bender, Levy and Schucker19–Reference Yeh, Davis and Phillips27) or regionally(Reference Satia-Abouta, Kristal and Patterson11,Reference Gunther, Patterson and Kristal28–Reference Lyle, Mares-Perlman and Klein30) representative samples. However, these studies have several limitations. First, all studies have depended on self-reports of medical conditions which could be subject to selective recall bias(Reference Coughlin31). Second, studies have examined a limited number of medical conditions, most notably CVD, cancer, osteoarthritis, hypertension, depression, diabetes and hypercholesterolemia(Reference Satia-Abouta, Kristal and Patterson11,Reference Archer, Stamler and Moag-Stahlberg20,Reference Rashrash, Schommer and Brown22–Reference Lyle, Mares-Perlman and Klein30) . Finally, most studies(Reference Satia-Abouta, Kristal and Patterson11,Reference Archer, Stamler and Moag-Stahlberg20,Reference Rashrash, Schommer and Brown22,Reference Buettner, Phillips and Davis23,Reference Egede, Ye and Zheng25–Reference Gunther, Patterson and Kristal28,Reference Lyle, Mares-Perlman and Klein30) have focused on vitamins, minerals, and herbal products and have not examined the broader range of DS (as defined by the Dietary Supplement Health and Education Act of 1994)(1) that include proteins, AA, combination products, joint health products and fish oils.
The Armed Forces Health Surveillance Branch of the Defense Health Agency (DHA) captures all clinical encounters between medical care providers and armed forces personnel (Air Force, Army, Marine Corps and Navy) at military medical facilities as well as encounters outside of these facilities paid for by the US Department of Defense. This provides an opportunity to examine clinically diagnosed medical conditions (CDMC) for surveillance or for combining with other datasets if approved by the DHA and institutional review boards. The purpose of the present study was to examine associations between DS use and CDMC. We hypothesised that associations would differ depending on the category of DS used and type of CDMC documented in medical records.
Methods
This investigation involved a survey of DS use that was combined with electronic medical records of US military SM. It was part of a larger study examining the health effects of dietary supplements(Reference Knapik, Trone and Steelman32,Reference Calvo33) . The investigation was approved by Naval Health Research Center’s institutional review board and SM consented to participate by electronically signing an informed consent document. Investigators adhered to policies and procedures for the protection of human subjects as prescribed by Department of Defense Instruction 3216.01, and the research was conducted in adherence with provisions of 32 Code of Federal Regulations, Part 219.
Sampling frame and solicitation procedures
Details of the sampling frame, solicitation of SM, subject recruitment flow through the study, statistical power considerations and response bias have been reported elsewhere(Reference Knapik, Trone and Steelman32). Briefly, investigators requested from the Defense Manpower Data Center (DMDC) a random sample of 200 000 SM stratified by gender (88 % male and 12 % female) and branch of service (Army 36 %, Air Force 24 %, Marines 15 % and Navy 25 %). The only inclusion criterion was that the individual be an active duty US military SM. Recruitment of SM in this random sample into the study involved a maximum of eight sequential contacts. The prospective participant was first sent an introductory postal letter with a $1 pre-incentive designed to increase the response rate(Reference Edwards, Cooper and Roberts34,Reference Church35) . The letter also included a description of the survey, a link to a secure website, and a unique number that could be used to access the survey and electronically sign the consent form. As a reminder to those who did not initially complete the survey, a follow-up email message after 10 d and postcard after 3 weeks were sent. If no response was received after sending the postcard, up to five additional email reminders were sent over 8 months, after which contact with the SM ended. All postal and online contacts stated that at any time the SM could decline participation and be removed from the contact list. Recruitment began in December 2018, and no further recruitment was conducted or surveys accepted after August 2019.
Survey description
The survey was designed to obtain type and frequency of DS use and characterise demographics and lifestyle factors of participants. SM were asked to estimate how frequently they consumed DS in the past 6 months (‘never’, ‘once a month’, ‘once a week’, ‘2–6 times/week’ or ‘daily’). Supplement use questions included ninety-six generic DS (e.g. multivitamins/multiminerals (MVM), individual vitamins and minerals, proteins/AA, and herbal products) and sixty-seven brand-name products. The brand-name products listed included some from previous armed forces DS surveys(Reference Knapik, Trone and Austin4,Reference Austin, Price and Mcgraw6,Reference Lieberman, Stavinoha and McGraw7,Reference Austin, Price and McGraw36) , but the list was updated based on a review of DS sold in the Army, Marine Corps, and Air Force Exchange Systems and General Nutrition Center stores on or near military installations. There were also open text fields on the questionnaire where SM could include supplements not on the provided lists. DS category definitions used in this study are provided in Table 1. To characterise participants, there were questions on demographics (gender, age, formal education, height, weight and military service branch) and lifestyle factors (cigarette smoking, aerobic exercise and resistance exercise).
Table 1 Dietary supplement categories in the US military dietary supplement use study

DS, dietary supplement.
Medical data
Once participants were identified by completing the informed consent and survey, the list of participants was sent to the Armed Forces Health Surveillance Branch of the DHA. From the Defense Medical Surveillance System relational database(Reference Rubertone and Brundage37,38) the DHA provided investigators all medical encounters of the volunteers for the 6-month period prior to survey completion. Medical encounters in the Defense Medical Surveillance System were recorded as International Classification of Diseases, Clinical Modification, Revision 10 (ICD-10) codes. Encounters included those within military treatment facilities (i.e. Standard Ambulatory Data Record, Standard Inpatient Data Record and Comprehensive Ambulatory/Professional Encounter Record) as well as those outside these facilities (civilian care) and paid for by the US Department of Defense (reimbursable) (i.e. Tricare Encounter Data-Institutional and Tricare Encounter Data-Noninstitutional).
Statistical analysis
All statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS) version 26, 2019, SPSS Inc., an International Business Machine (IBM) company. BMI was computed from the questionnaire responses as weight/height2 (kg/m2). Weekly duration of aerobic and resistance training (min/week) was calculated by multiplying reported weekly exercise frequency (sessions/week) by the reported duration of training (min/session). Supplements that SM placed in the ‘other’ categories were individually examined and responses placed into their appropriate categories. If the listed DS did not fit in a particular category, it remained in the ‘other’ category. Descriptive statistics determined the number and proportion of SM within each demographic and lifestyle characteristic.
ICD-10 codes are a standard system used worldwide by medical health professionals to classify medical conditions diagnosed in patients during clinical visits or hospitalisation. Codes have a leading letter that provides a broad diagnostic category (e.g. infectious disease, circulatory diseases and injury/poisoning), and this is followed by numbers that provide more specific diagnoses within the broader category. ICD-10 code diagnoses of participants were grouped into twenty-four categories shown in Table 2. One series of codes were grouped by their first three ICD-10 alphanumeric codes into nineteen categories representing the major ICD-10 code groups. A separate category included all ICD-10 codes. Four specific code groupings were developed for depression, hypertension, hypercholesterolemia and osteoarthritis to allow specific comparisons with previous literature(Reference Satia-Abouta, Kristal and Patterson11,Reference Bender, Levy and Schucker19–Reference Lyle, Mares-Perlman and Klein30) .
Table 2 ICD-10 codes for clinically diagnosed medical conditions in the US military dietary supplement use study (n 26 680)

ICD-10, International Classification of Diseases, Clinical Modification, Revision 10; CDMC, clinically diagnosed medical condition.
A CDMC was defined as an ICD-10 code within one of the twenty-four code groupings (Table 2). A participant could have an encounter within more than one category but were included only once within a single category. Within each of the twenty-four CDMC, prevalence (as a %) was calculated by DS category for DS users and non-users. Univariable and multivariable logistic regression determined the odds of a CDMC among users and non-users for each DS category. Univariable logistic regression included only the presence or absence of a CDMC (dependent variable) in the DS category. Multivariable logistic regression adjusted the presence or absence of a CDMC (dependent variable) in the DS category for all demographic and lifestyle characteristics (independent variables).
The prevalence of use in each DS category (Table 1) was also examined by the number of CDMC in the nineteen major code groups, exclusive of any CDMC (Table 2). For each participant, the number of CDMC in the nineteen major code groups were determined and placed into one of four groups: 0 (no CDMC), 1–2, 3–4 and ≥5 CDMC. Differences in the prevalence of DS use by the number of CDMC were examined using Chi-square statistics; linear trends across the number of CDMC were examined using the Mantel–Haenszel statistic. The prevalence of any CDMC was also determined by the number of reported DS. DS number was grouped by 0 (non-user), 1–2, 2–4 and ≥5. Univariable and multivariable logistic regression compared the odds of any CDMC according to the number of DS reported.
Results
From the initial sample frame of 200 000 SM, 73 % (n 146 365) were successfully contacted (i.e. no returned postal mail) and of these, 26 680 (18·2 %) signed the informed consent and completed the survey.
Table 3 shows the demographic and lifestyle characteristics of the participants. Participants were primarily men, 30–39 years of age, with an average (sd) of 33 (8) years. Eighty-six per cent had some formal college education or a college degree. Participants varied substantially in time spent participating in weekly exercise, and there was a relatively low proportion of smokers.
Table 3 Characteristics of sample in the US military dietary supplement use study by demographic and lifestyle characteristics

The overall prevalence of CDMC in the 6-month surveillance period was 70·4 % (95 % CI (69·8, 70·9)). Table 4 shows the association between CDMC and use of any DS, MVM, vitamins/minerals, proteins/AA, and combination products. Users of any DS had elevated risk in 13 of 24 (54 %) CDMC; after adjustment for demographic and lifestyle factors, 12 CDMC (50 %) were statistically significant. Among MVM users, risk was elevated in 21 of 24 (88 %); after adjustment, 11 of 24 (46 %) CDMC were statistically significant. Users of individual vitamins/minerals had elevated risk in 23 of 24 (96 %) CDMC; after adjustment, 22 (92 %) CDMC were statistically significant. Users of protein/AA had lower risk of a CDMC in 19 of 24 (79 %) CDMC; after adjustment, only 6 (25 %) of these remained statistically significant. Among combination product users, risk of a CDMC was lower in 7 of 24 (29 %) CDMC; after adjustment only 3 (13 %) of these remained statistically significant. Also among combination product users, risk of a CDMC was higher in 3 of 24 (13 %) CDMC; after adjustment, 2 (8 %) of these remained statistically significant.
Table 4 Clinically diagnosed medical conditions among users and non-users of any DS, MVM, individual vitamins/minerals, proteins/AA and combination products in the US military dietary supplement use study (yellow indicates higher risk among users, green indicates lower risk among users)
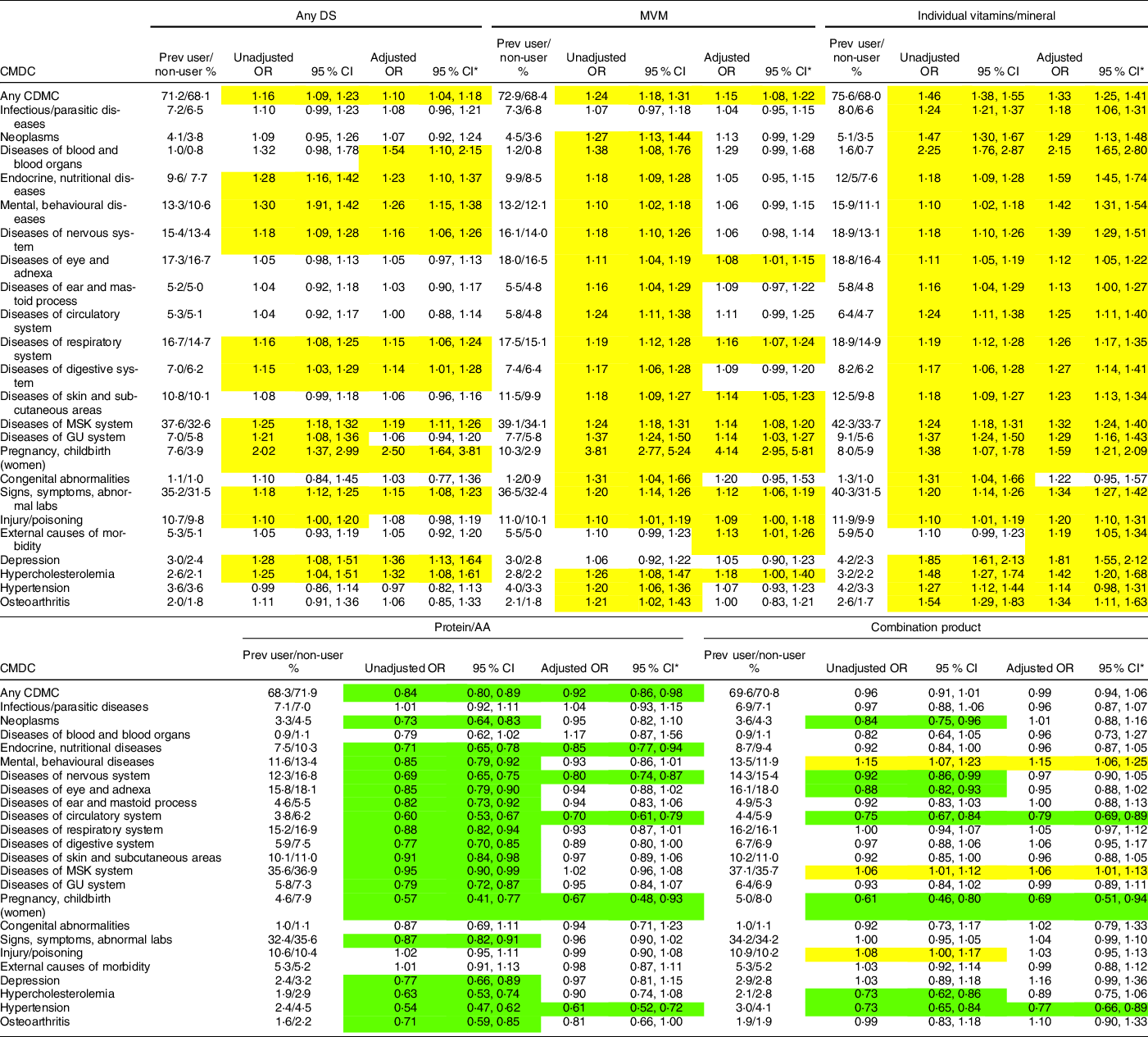
DS, dietary supplement; MVM, multivitamin/multimineral; AA, amino acid; CDMC, clinically diagnosed medical condition; GU, genitourinary; ICD-10, International Classification of Diseases, Clinical Modification, Revision 10 (refer to Table 2 for ICD-10 code groups); MSK, musculoskeletal; Prev, prevalance.
*Adjusted for gender, age, formal education, BMI, weekly aerobic exercise duration, weekly resistance exercise duration, cigarette smoking and military service branch.
Table 5 shows the association between CDMC and use of prohormones, herbal products, joint health products, fish oils, and other DS. Among prohormone users, risk was lower for 1 of 24 (4 %) CDMC, but that CDMC did not remain statistically significant after adjustment. Also among prohormone users, risk was higher in 6 of 24 (25 %) CDMC; after adjustment, risk remained higher in 6 (25 %) CDMC. Users of herbal products had elevated risk in 20 of 24 (83 %) CDMC; after adjustment 14 (58 %) CDMC remained statistically significant. Users of joint health products had elevated risk in 12 of 24 (50 %) CDMC; after adjustment only 4 (17 %) of these remained statistically significant. Also among joint health product users, there was lower risk in 1 (4 %) CDMC and after adjustment, 1 (4 %) remained statistically significant. Among fish oil users, risk was elevated in 9 of 24 (38 %) CDMC; after adjustment, 9 (38 %) CDMC were statistically significant. Among users of other DS, risk of a CDMC was higher in 13 of 24 (54 %) CDMC; after adjustment, 10 (42 %) of these remained statistically significant.
Table 5 Clinically diagnosed medical conditions among users and non-users of prohormones, herbals, joint health products, fish oils and other DS in the US military dietary supplement use study (yellow indicates higher risk among users, green indicates lower risk among users)

Abbreviations: 95%CI = 95% confidence interval; CDMC = clinically diagnosed medical conditions (ICD-10 codes); ICD-10 = International Classification of Diseases, Clinical Modification, Revision 10 [refer to Table 2 for ICD-10 code groups]; GU = Genitourinary; MSK = musculoskeletal; Prev = prevalence.
*Adjusted for gender, age, formal education, BMI, weekly aerobic exercise duration, weekly resistance exercise duration, cigarette smoking, and military service branch.
Table 6 shows the prevalence of DS use by the number of CDMC for each DS category. The greater the number of CDMC, the higher the prevalence of use for any DS, MVM, individual vitamins/minerals, herbal products, joint health products, fish oils and other DS. However, as the number of CDMC increased, the prevalence of protein/AA use decreased. For combination products and prohormones, there was no consistent difference in use prevalence as the number of CDMC increased.
Table 6 Prevalence of DS use by number of CDMC (nineteen major code groups only) in the US military dietary supplement use study

DS, dietary supplement; CDMC, clinically diagnosed medical conditions; MVM, multivitamin/multimineral; AA, amino acid; ICD-10, International Classification of Diseases, Clinical Modification, Revision 10 (ICD-10 code groups; refer to Table 2 for specifics).
Table 7 shows prevalence of any CDMC by the number of DS that SM reported consuming. CDMC tended to increase as the number of DS consumed increased, and this trend was more apparent in the adjusted multivariable analysis.
Table 7 CDMC by number of DS used in the US military dietary supplement use study

CDMC, clinically diagnosed medical conditions (ICD-10 codes) (refer to Table 2 for ICD-10 codes by CMDC group), DS, dietary supplements; ICD, ICD-10, International Classification of Diseases, Clinical Modification, Revision 10.
* Adjusted for gender, age, formal education, BMI, weekly aerobic exercise duration, weekly resistance exercise duration, cigarette smoking and military service branch.
Discussion
This study examined the association between CDMC and categories of DS use in a large sample (>26 000) of military SM. Users of many DS categories had a higher prevalence of CDMC than non-users, even after adjustment for demographics and lifestyle factors. A high prevalence of CDMC was especially apparent among users of individual vitamins/minerals, herbal products and any DS (i.e. when use of any DS was analysed). However, protein/AA users had a lower prevalence of CDMC in some categories. The results for combination product users were mixed, but for many CDMC risk was also lower. The prevalence of DS use increased in a linear manner as the number of CDMC increased among users of any DS, MVM, individual vitamins/minerals, herbal products, joint health products, fish oils and other DS. Contrary to this trend, protein/AA use decreased as the number of CDMC increased; for combination product and prohormones, there was little consistent difference in use with an increasing number of CDMC.
Compared with non-users, the lower risk of CDMC among protein/AA and combination product users may be explained in part by the demographic and lifestyle factors of the users. We previously showed in this same cohort(Reference Knapik, Trone and Steelman32) and in other samples of SM(Reference Knapik, Trone and Austin4,Reference Austin, Price and Mcgraw6,Reference Lieberman, Stavinoha and McGraw7) , that protein/AA and combination products users were more likely to be younger, more physically active men, all factors related to reduced prevalence of medical conditions. Compared with women, men generally use less medical care in military(39,40) and civilian(Reference Bertakis, Azari and Callahan41–Reference Muller44) populations. These gender-related differences remain after consideration of pregnancy-related conditions and socio-economic characteristics(39–Reference Friberg, Krantz and Maatta42). Health care use also increases with age(Reference Ladwig, Marten-Mittag and Formanek43,45,Reference Atella, Mortari and Kopinska46) and more physical activity is associated with a reduced likelihood of medical visits(Reference Wetzler and Cruess47–Reference Langsetmo, Kats and Cawthon49). After controlling for these (and other) factors in multivariate analyses in the current study, the odds for being at greater risk for many types of CDMC was considerably reduced for both protein/AA and combination product users. Nonetheless, a lower risk for some CDMC remained among protein/AA and combination product users, suggesting other factors not examined here might be responsible.
CVD
Among all non-communicable diseases, CVD is the leading cause of mortality and morbidity worldwide(Reference Joseph, Leong and McKee50), and the American Heart Association estimates that about one-third of American adults have at least one type of CVD(Reference Benjamin, Blaha and Chiuve51). In the military, rates of CVD are much lower likely because of SM’s younger age, higher levels of physical activity and lower prevalence of obesity(52). In the current study, SM diagnosed with diseases of the circulatory system reported an overall DS use prevalence (i.e. any DS) similar to those without this CDMC. Nonetheless, risk was higher among users of individual vitamins/minerals, herbals, and fish oils even after adjustment for demographics and lifestyle factors. In agreement, other studies have found that overall DS use was similar among those with self-reported heart disease(Reference Archer, Stamler and Moag-Stahlberg20,Reference Freidman, Birstler and Love24) . However, when looking at different DS categories, individuals self-reporting many different types of CVDs were more likely to report use of vitamins, minerals and herbal substance in some(Reference Archer, Stamler and Moag-Stahlberg20,Reference Rashrash, Schommer and Brown22,Reference Stys, Stys and Kelly53) , but not all(Reference Buettner, Phillips and Davis23,Reference Yeh, Davis and Phillips27) investigations.
The current study also examined specific CVD code groups for hypertension and hypercholesterolemia, risk factors for CVD, in relation to DS use. There was little difference in overall DS use among those diagnosed with hypertension and those not. While the unadjusted prevalence of MVM, individual vitamins/minerals, herbals and fish oils use was higher among those with hypertension, after adjustment only fish oil use remained higher. In general agreement, several studies examining primarily vitamins, minerals and herbal substances(Reference Archer, Stamler and Moag-Stahlberg20,Reference Freidman, Birstler and Love24,Reference Lee and Kim29,Reference Stys, Stys and Kelly53) reported little difference in overall DS use (any DS) among those with and without self-reported hypertension. On the other hand, a study of a Southcentral Wisconsin cohort(Reference Lyle, Mares-Perlman and Klein30) found that self-reported hypertensive individuals were less likely to use MVM.
Overall DS use, as well as use of MVM, individual vitamins/minerals, herbals and fish oils, was higher among those with diagnosed hypercholesterolemia compared with those not using those products. After adjustment, use was still higher in these DS categories among those diagnosed with hypercholesterolemia. Studies have reported that individuals self-reporting hypercholesterolemia(Reference Freidman, Birstler and Love24) or hyperlipidemia(Reference Stys, Stys and Kelly53) had a greater use of any DS, but a study of a Korean cohort(Reference Lee and Kim29) found hyperlipidemic individuals were less likely to be users of DS. Differences in defining the specific type of lipids (i.e. total cholesterol, LDL and TAG) may account for a portion of these between-study differences.
Data from the National Health and Nutrition Examination Survey (NHANES) indicated that while both men and women cite improving or maintaining health as the primary reason for using DS, ‘heart health’ ranks high among specific health reasons(Reference Dickinson, Blatman and El-Dash8–Reference Neuhouser, Patterson and Levy10). Nonetheless, comprehensive narrative and systematic reviews have shown no clear benefit of vitamins, minerals(Reference Jenkins, Spence and Giovannucci54–Reference Ingles, Cruz-Rodriguez and Garcia56) or herbal supplements(Reference Liperoti, Vetrano and Bernabei57–Reference Chrysant59) on CVD prevention or treatment. However, it should be noted that some drugs derived from herbals, like aspirin (from willow bark)(Reference Montinari, Minelli and DeCaterina60) and reserpine (from Rauwolfia sepentina)(Reference Curzon61), have become important in CVD treatment. In contrast to vitamins, minerals, and herbs, several systematic reviews have indicated that fish oil supplements (containing the n-3 fatty acids, EPA and DHA) may be effective for the secondary prevention of fatal and non-fatal cardiovascular events(Reference Wang, Harris and Chung62–Reference Hu, Hu and Manson64). In the current study, SM with diseases of the cardiovascular system, hypertension or hypercholesterolemia were more likely to use fish oils than those without these CDMC even after adjustment for demographics and lifestyle factors.
Cancer
Cancer is the second leading cause of death in the USA with an annual rate of new cancers of 44·2/1000 person-years in 2013–2017(Reference Benjamin, Blaha and Chiuve51,65) . In the military, rates of cancer are much lower than in the civilian sector, likely for reasons previously noted(52). In the current study, after adjustment for demographics and lifestyle factors, only individual vitamins/minerals use was associated with higher risk of neoplasms. These data are largely in agreement with other studies that have examined similar associations. The Vitamins and Lifestyle Study involving a regional cohort in western Washington state(Reference Satia-Abouta, Kristal and Patterson11) found that those self-reporting cancer indicated consuming a similar number of DS compared with those not reporting cancer. Analysis of data from the 2015 National Consumer Survey on the Medication Experience and Pharmacist’s Role(Reference Rashrash, Schommer and Brown22) found that after adjusting for demographics and health status, herbal use was similar among those self-reporting cancer and those not. Data from the Midlife in the US Study(Reference Friedman, Birstler and Love66) showed that those self-reporting cancer were more likely to use DS of any type, but after adjustment for demographics the difference was no longer significant. Secondary analysis of 2017 National Health Interview Survey data(Reference John, Hershman and Falci26) indicated that the odds of vitamin/mineral use was 1·39 (95 % CI (1·29, 1·51)) times higher among self-reported cancer survivors than among individuals not reporting cancer.
Individuals may see use of specific vitamins and minerals as a relatively safe way to take a more active role in their treatment(Reference Velicer and Ulrich67,Reference Ambrosone, Rebbeck and Morgan68) and certain vitamins and minerals (especially vitamins A, C, D and selenium) were once suggested to have some promise for cancer prevention and treatment, largely because of their antioxidant effects(Reference Cotterel and Rai69–Reference Lupulescu71). Cancer cells produce reactive oxygen species to assist in their growth and survival and antioxidants act to reduce reactive oxygen species by donating an electron and moderating oxidative damage(Reference Athreya and Xavier72). While systematic reviews of observational studies suggest some vitamins may decrease mortality incidence or recurrence for some types of cancers(Reference Kanellopoulou, Riza and Damoli73), reviews of randomised controlled trials find no clear beneficial or harmful effects of vitamins or minerals on cancer mortality, remission, recurrence, hospitalised days or progression of lesions(Reference Athreya and Xavier72–Reference Coulter, Hardy and Morton76).
Depression
Depression is a leading cause of years lived with disability across the world with about 350 million people suffering from depressive symptoms(Reference Smith77). Among all mental health disorders in the military in 2016–2020, depressive disorders ranked third after adjustment disorders and anxiety(78). The current study found a higher risk of depression among users of any DS, individual vitamins/minerals, herbal products and other DS, compared with non-users. Previously published data on self-reported depression and DS use has not been consistent. Gunter et al.(Reference Gunther, Patterson and Kristal28) found that self-reported depression was associated with greater use of Saint John’s wort. Friedman et al.(Reference Freidman, Birstler and Love24) found little difference in overall DS use among those self-reporting depression v. those not, while Satia-About et al.(Reference Satia-Abouta, Kristal and Patterson11) found that the number of DS used was higher among men self-reporting depression, but not among women.
There have been numerous systematic reviews of the possible efficacy of vitamins, minerals, herbs and fish oils on treatment of depression. Well controlled randomised trials demonstrate that most vitamins, minerals and herbal products examined singly or in combination have little or no effect on depressive symptoms(Reference Williams, Cotter and Sabina79–Reference Phelan, Molero and Martinez-Gonzalez85). However, supplemental folate(Reference Altaf, Gonzalez and Rubino86–Reference Roberts, Carter and Young88) or zinc(Reference deSilva, dePortela and deFarias-Costa89,Reference Lai, Moxey and Nowak90) may reduce remission rates and/or depressive symptoms on validated symptom scales when combined with standard antidepressant medications (e.g. serotonin/norepinephrine reuptake inhibitors). Most investigated herbal substances have little effect on depressive symptoms(Reference Yeung, Hernandez and Mao91–Reference Sarris, Panossian and Schweitzer93), but systematic reviews of randomised controlled trials involving Saint John’s wort(Reference Linde, Berner and Kriston94,Reference Apaydin, Maher and Shanman95) , saffron(Reference Marx, Lane and Rocks96–Reference Khaksarian, Behzadifar and Behzadifar98) and lavender(Reference Firoozeei, Feizi and Rezaeizadeh99,Reference Shamabadi and Akhondzadeh100) suggest some efficacy. Numerous systematic reviews also suggest that supplemental n-3 fatty acids may modestly reduce symptoms(Reference Luo, Feng and Yang101–Reference Liao, Xie and Zhang105).
Osteoarthritis
Osteoarthritis is a disorder involving deterioration of the articular cartilage and underlying bone and is associated with symptoms of pain and disability(Reference Knapik, Pope and Orr106). Globally, osteoarthritis of the hip and knee ranked as the 11th highest contributor to disability among 291 medical conditions(Reference Cross, Smith and Hoy107), and in the military it was the first or second most common reason for separations from service in 2001 and 2009(Reference Patzkowski, Rivera and Ficke108). The current study found that SM with diagnosed osteoarthritis had similar overall use of DS compared with those without osteoarthritis but were more likely to use MVM, individual vitamins/minerals, prohormones, herbals and joint health products; after adjustment, individual vitamins/minerals, prohormones and joint health product use remained higher. In agreement with the present study, Gunter et al.(Reference Gunther, Patterson and Kristal28) found that those with self-reported osteoarthritis were more likely to use joint health products. Rashrash(Reference Rashrash, Schommer and Brown22) on the other hand found that individuals self-reporting arthritis were more likely to use herbal products, even after adjustment for demographics and other health conditions. Friedman(Reference Freidman, Birstler and Love24) found that those with self-reported arthritis were more likely to use DS of any type, but after adjustment for demographics little difference remained.
Data from systematic reviews of randomised clinical trials indicated that most vitamins, minerals and herbals that have been investigated in osteoarthritic patients do not reduce pain or slow the progression of the disease(Reference Canter, Wider and Ernst109–Reference Diao, Yang and Yu114), although one review suggested there was moderate quality evidence that extracts of Boswellia serrata and avocado-soyabean unsaponifiables may slightly improve pain and function(Reference Cameron and Chrubasik112). Systematic reviews of randomised clinical trials indicated that oral consumption of the joint health products chondroitin and glucosamine reduced osteoarthritis-related pain but had little or no effect on structural changes (e.g. joint space narrowing and cartilage volume)(Reference Knapik, Pope and Hoedebecke115–Reference Singh, Noorbaloochi and MacDonald119).
Number of clinically diagnosed medical conditions in relation to dietary supplement use
We found a higher number of CDMC associated with a higher number of reported DS use in all DS categories other than proteins/AA, combination products and prohormones. This is consistent with the reported use of DS for presumed ‘health enhancement’(Reference Austin, Price and Mcgraw6–Reference Neuhouser, Patterson and Levy10) in that the more medical conditions SM had, the greater use of DS reported in many categories. These data are in agreement with two studies using data from the National Health Interview Study(Reference Bender, Levy and Schucker19,Reference Falci, Shi and Greenlee21) . Data from the 2012 National Health Interview(Reference Falci, Shi and Greenlee21) showed that as number of self-reported chronic conditions increased, there was an increase in use of MVM, individual vitamins, individual minerals and herbal products.
This study was unique in including proteins/AA, combination products and prohormones as a specific category, unlike other studies involving civilians(Reference Satia-Abouta, Kristal and Patterson11,Reference Archer, Stamler and Moag-Stahlberg20–Reference Lyle, Mares-Perlman and Klein30) where there is relatively low number of users of those products. For these DS categories, trends in the association between DS prevalence and the number of CDMC differed considerably from that of other DS categories. Increasing muscle strength is the primary reason protein/AA users report for use of this DS category(Reference Austin, Price and Mcgraw6,Reference Lieberman, Stavinoha and McGraw7) , and judicious use of protein/AA combined with resistance training can improve muscle mass and strength over that of resistance training alone(Reference Morton, Murphy and McKellar120). The lower use of protein/AA with higher numbers of CDMC may be related to the health of users. As the number of co-morbidities increase, individuals may be less able or have less desire to perform activities to increase strength and concurrently reduce use of proteins/AA.
Strength and limitations
The current study recruited a very large stratified random sample of SM from all branches of service allowing results to be generalised to the military population. The medical database used in this study contained virtually complete information on diagnosed medical conditions experienced by SM in the surveillance period. The study controlled for multiple demographic and lifestyle factors that could have confounded the associations. Despite these strengths, there were a number of limitations. First, data regarding the DS use and demographic and lifestyle factors were self-reported and share the usual limitations of these types of data, including recall bias, social desirability, errors in self-observation, and inadequate recall(Reference Podsakoff, MacKenzie and Lee121,Reference Furnham122) . Second, there were a large number of statistical tests examining relationships between DS use and CDMC. The more effects investigated the greater the chance of making a Type 1 error where the null hypothesis will be incorrectly rejected. Third, the study was cross-sectional, so direct casual inferences cannot be made and the relationships are associative only. Finally, medical conditions were those diagnosed in the 6 months prior to questionnaire completion, and some chronic conditions may have been missed if the SM had not reported to a medical care provider in that period.
Conclusions
This study had two major findings. First, there were differences in the risk of CDMC depending on the category of DS use. For many categories of DS, association with many types of CDMC was significant, especially among users of individual vitamins/minerals and herbal products. Contrary to this trend, protein/AA users had a lower risk for many types of CDMC and results for combination products was mixed with higher risk for some CDMC and lower risk for others. Proteins/AA and combination products users were predominately male, younger and more physically active, all factors that likely reduced the likelihood of medical conditions and use of the medical system. The second major finding was that the greater the number of CDMC, the higher the DS use prevalence among users of MVM, individual vitamins/minerals, herbal products, joint health products, fish oils and other DS. Again, contrary to this trend, the greater the number of CDMC, the lower the prevalence of protein/AA use, and there was little consistent difference among combination product and prohormone users. This study contributes to the understanding of the association between DS use and medical conditions by examining medical conditions diagnosed by medical care providers, incorporating the full range of medical conditions and by including categories of DS not previously examined in the literature.
Acknowledgements
Acknowledgements: the authors thank Ms Maureen Humphrey-Shelton for assistance in obtaining references and Dr Michelle Chervak and Ms Lauren Thompson for editorial comments. Financial support: This work was supported by Department of Defense Center Alliance for Nutrition and Dietary Supplement Research of the Defense Medical Research and Development Program, the US Army Medical Research and Development Command (USAMRDC). Authorship: J.J.K. designed the research, analysed data, wrote paper and had responsibility for final content; T.W.D. designed research, conducted research, provided essential material and had responsibility for final content; R.A.S. analysed data and had responsibility for final content; E.K.F. designed research and had responsibility for final content; H.R.L. designed research and had responsibility for final content. All authors have read, edited and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the Naval Health Research Center’s institutional review board (protocol number NHRC.2016.0025). Disclaimer: We are military service member or employee of the US Government. This work was prepared as part of our official duties. Title 17, USC §105 provides that copyright protection under this title is not available for any work of the US Government. Title 17, USC §101 defines a US Government work as work prepared by a military service member or employee of the US Government as part of that person’s official duties. Report No. 20-104 was supported by Defense Health Programme under work unit no. N1335. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the US Government. The study protocol was approved by the Naval Health Research Center Institutional Review Board in compliance with all applicable Federal regulations governing the protection of human subjects. Research data were derived from an approved Naval Health Research Center Institutional Review Board protocol, number NHRC.2016.0025.
Conflicts of interest:
There are no conflicts of interest.










