Pain is defined by the International Association for the Study of Pain as ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage’( 1 ). Pain, and conditions with pain as a prominent symptom, including chronic widespread pain, musculoskeletal pain (e.g. lower-back pain), arthritis and headache, are the most common reason for primary care and medical consultations( Reference Ehrlich 2 – Reference Stovner, Hagen and Jensen 4 ). The prevalence of chronic pain ranges from 8 to 60 % and over, depending on the population studied( Reference Phillips 5 ). Recent reviews of painful conditions have reported that the prevalence of fibromyalgia varies from 2 to 8 % in the general population( Reference Clauw 6 ); while the global prevalence of lower-back pain is 9·4 %( Reference Hoy, March and Brooks 7 ), of rheumatoid arthritis is 0·24 %( Reference Cross, Smith and Hoy 8 ) and of current migraine is more than 10 % in adults( Reference Stovner, Hagen and Jensen 4 ). Painful conditions can seriously influence quality of life, lead to work disability, and result in major economic burdens for both individuals and the health system( Reference Ehrlich 2 , Reference Stovner, Hagen and Jensen 4 , Reference Phillips 5 , Reference Cross, Smith and Hoy 8 – Reference Yelin 12 ).
Vitamin D comprises a group of fat-soluble secosteroids( Reference Holick 13 ) and the receptor of vitamin D has been identified in muscle tissue( Reference Simpson, Thomas and Arnold 14 ). Although the optimal level of serum 25-hydroxyvitamin D (25(OH)D) is a topic of ongoing research, vitamin D deficiency is typically defined as 25(OH)D<50 nmol/l( Reference Ross, Manson and Abrams 15 ). Vitamin D deficiency is very common in both developed( Reference Hilger, Friedel and Herr 16 ) and developing countries( Reference Arabi, El Rassi and El-Hajj Fuleihan 17 ). There is increasing evidence from observational studies that vitamin D deficiency is associated with a wide range of acute and chronic diseases( Reference Hossein-nezhad and Holick 18 ), including diseases with pain as a prominent symptom( Reference Thacher and Clarke 19 ). Previous research has provided inconsistent findings on the association between vitamin D status and pain. A meta-analysis of seven observational studies with 2420 statin-treated patients found that 25(OH)D levels were lower in those with myalgia than in those without( Reference Michalska-Kasiczak, Sahebkar and Mikhailidis 20 ). However, it used a fixed-effects model, not justified by the high heterogeneity of the results (I 2=94 %), which when repeated with a random-effects model was no longer significant (mean difference (MD): −3·52; 95 % CI −8·55, 1·51 ng/ml; P=0·17). Another meta-analysis of twelve observational studies with 1854 participants reported inconsistent results, with significantly increased odds of vitamin D deficiency associated with chronic widespread pain, but no difference in mean 25(OH)D levels between people with and without chronic widespread pain( Reference Hsiao, Hung and Chang 21 ). Moreover, these previous meta-analyses have not reported the association between 25(OH)D concentration and other pain-related conditions, such as arthritis and headache. In addition, recent reviews of randomized controlled trials have reported inconsistent conclusions about whether vitamin D supplementation improves chronic pain, with two being qualitative reviews( Reference Shipton and Shipton 22 , Reference Straube, Derry and Straube 23 ) and only one using quantitative methods( Reference Wu, Malihi and Stewart 24 ).
Given the limited evidence and inconsistent conclusions from previous reviews and meta-analyses, which for observational studies only searched up to September 2014( Reference Michalska-Kasiczak, Sahebkar and Mikhailidis 20 , Reference Hsiao, Hung and Chang 21 ), we conducted an updated meta-analysis of all observational studies reporting data on 25(OH)D levels and pain, including studies of non-statin users and participants with different pain conditions, to determine if there is an association between these two variables.
Methods
Search strategy
Two trained researchers (Z.W., Z.M.) independently searched MEDLINE, EMBASE and Cochrane Central Register of Controlled Trials (up to April 2017) using the following key words: Vitamin D, Vitamin D2, Vitamin D3, Cholecalciferol, Ergocalciferol, 25-hydroxyvitamin D, Pain, Myalgia, Myopathy, Myalgic, Headache, Migraine, Arthritis and Sciatica, for original publications pertinent to vitamin D levels and pain (search strategy listed in the online supplementary material, Supplement 1). In addition, we manually searched the reference lists of eligible articles and previous reviews for additional studies.
Aims
Two aims were predefined in the meta-analysis. The primary aim was the difference in mean circulating 25(OH)D concentration (nmol/l) between participants with and without pain-related conditions. The secondary aim was the difference in proportions of hypovitaminosis D in the participants with and without painful conditions. For the latter, we used the original definition of hypovitaminosis D from each paper (eight studies with a threshold of 75 nmol/l; thirty-four studies with 50 nmol/l; two studies with 25 nmol/l; six studies with other definitions, which were 20 nmol/l, 30 nmol/l, 37·5 nmol/l, 80 nmol/l and 100 nmol/l).
Eligibility criteria
We included observational studies in the current meta-analysis if the study: (i) was a cohort, case–control or cross-sectional study; (ii) enrolled adult participants (≥18 years old); (iii) described specific information on pain, such as a pain definition or category; and (iv) reported the 25(OH)D level and/or the proportion of hypovitaminosis D in participants with and without pain. There was no language or ethnicity restriction. In addition, the studies which selected controls with pain conditions were excluded.
Data extraction
Reviewers (Z.W., Z.M.) independently identified the included articles by screening title, abstract and full text (κ coefficient=0·73), and the main data were extracted based on a standardized data collection form developed for the study. Any inconsistencies were resolved by consensus and discussion.
Quality assessment of individual studies
The quality of each included study was assessed using the Newcastle–Ottawa scale( Reference Wells, Shea and O’Connell 25 ). Specifically, there were five items for cross-sectional studies, and eight items for cohort and case–control studies. We used the same score to categorize the quality of studies as reported previously( Reference Takahashi and Hashizume 26 ): 5 as very good, 4 as good, 3 as satisfactory and 0–2 as unsatisfactory in cross-sectional studies; similarly, 7–8 as very good, 5–6 as good, 4 as satisfactory and 0–3 as unsatisfactory in case–control or cohort studies.
Synthesis and analysis
Mean and sd of serum 25(OH)D levels, and number of participants with and without pain, were collected for the continuous exposure measurement. All 25(OH)D levels were transformed to nmol/l in the meta-analysis. Digitizer software (GetData Graph Digitizer version 2.26; www.getdata-graph-digitizer.com/) was used to extract the data from graphs, and Wan et al.’s( Reference Wan, Wang and Liu 27 ) methods were used to estimate the mean and sd by reported median and range, or median and interquartile range. Sample size and the proportion with hypovitaminosis D were collected for the dichotomous exposure measurement.
Weighted MD and 95 % CI were calculated for continuous exposure, and OR and 95 % CI were calculated for dichotomous exposure, to allow the combining of different study designs in the synthesis analysis. Heterogeneity was measured using Cochran’s Q test and the I 2 statistic (I 2>50 % denotes large or extreme heterogeneity). Random-effects models were used in the meta-analysis( Reference Borenstein, Hedges and Higgins 28 ). Predefined analyses were performed to detect the relationship of 25(OH)D with pain by different painful conditions (arthritis, muscle pain, chronic widespread pain, and headache or migraine), type of study design, statin user and different cut-off points of vitamin D deficiency (25 nmol/l, 50 nmol/l and 75 nmol/l). Interactions were tested between different subgroups using a standard method( Reference Altman and Bland 29 ). In addition, we also conducted meta-regression to examine other sources of heterogeneity (e.g. year, sample size, mean age, female proportion, type of study). Sensitivity analyses were also conducted by individually excluding each study in turn, and by collectively excluding low-quality studies or those that used other definitions of vitamin D deficiency (as listed above). We generated funnel plots for visual assessment of publication bias, as well as performed the Egger test( Reference Egger, Davey Smith and Schneider 30 ). All tests were two-tailed and P≤0·05 was considered statistically significant. We conducted the meta-analysis using the Stata statistical software package version 13.1 and Review Manager software (Revman version 5.2).
Results
Included studies
A total of 2340 unique articles were identified by searching the three electronic databases and by applying snowballing techniques. After reviewing titles and abstracts, 2155 publications were excluded. The full texts of the remaining 185 studies were assessed for their eligibility and a further 107 were excluded because they did not meet the eligibility criteria (see Supplement 1). The remaining seventy-eight publications, which reported eighty-one observational studies with data on vitamin D concentration and pain, were included in the current meta-analysis (Fig. 1).
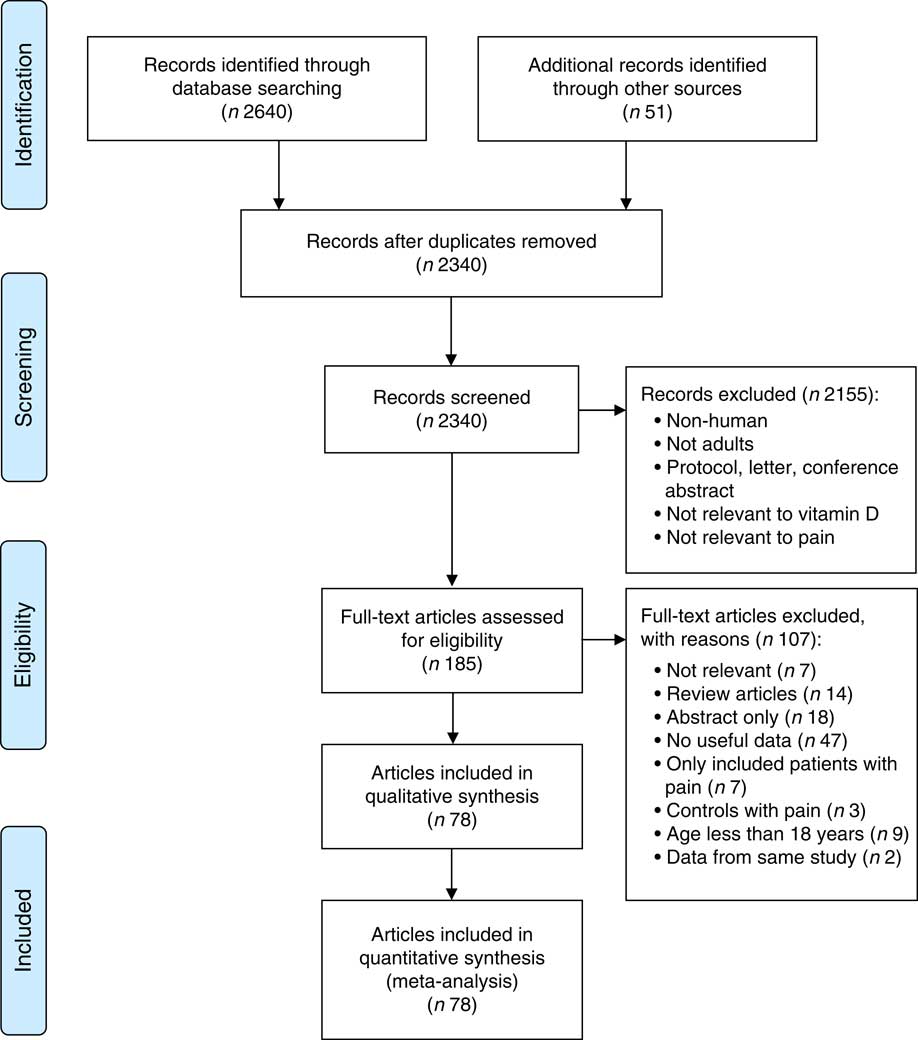
Fig. 1 (colour online) PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram of study selection
The eighty-one observational studies involved nineteen cross-sectional studies( Reference Macfarlane, Palmer and Roy 31 – Reference Virtanen, Giniatullin and Mantyselka 48 ), fifty-six case–control studies( Reference Pietschmann, Machold and Wolosczuk 49 – Reference Wong, Gupta and Radhakrishnan 102 ) and six cohort studies( Reference Laroche, Coste and Medkour 103 – Reference Calza, Magistrelli and Colangeli 108 ). Together, these studies included 50 834 participants, 21 723 of whom were reported as pain subjects, with a median mean age of 49·4 (median sd 10·3) years and median female proportion of 80·5 % (range: 0–100 %); and 29 111 participants who were community- or hospital-based controls without pain-related conditions, with a median mean age of 50·0 (median sd 10·3) years and median female proportion of 78·4 % (range: 0–100 %). The pain conditions or symptoms reported in these studies included arthritis, muscle pain, chronic widespread pain, and headache or migraine. Characteristics of included studies are shown in Table 1.
Table 1 Characteristics of studies included in the current meta-analysis on the association between vitamin D concentration and pain
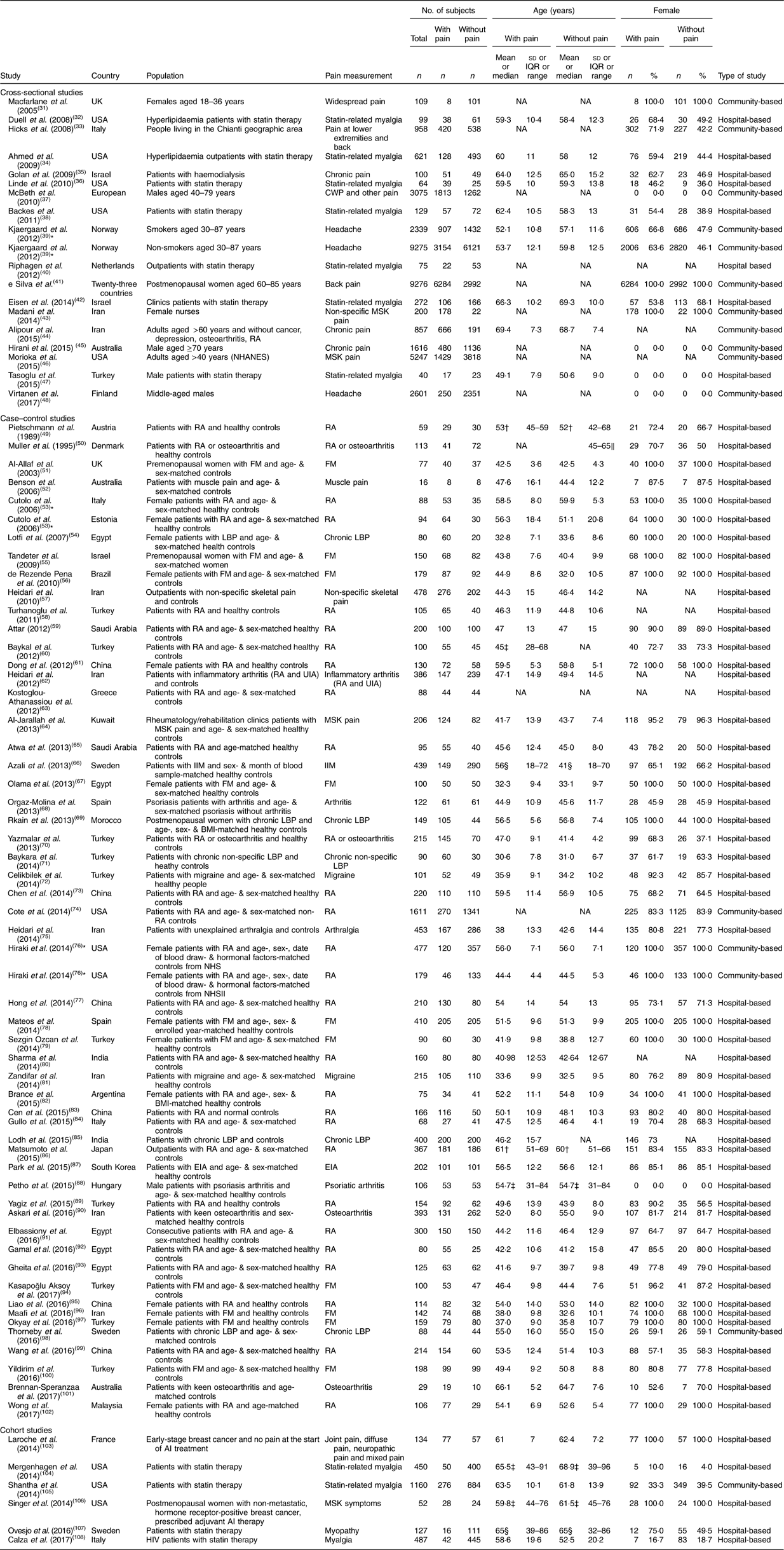
IQR, interquartile range; RA, rheumatoid arthritis; NHANES, National Health and Nutrition Examination Survey; FM, fibromyalgia; LBP, lower-back pain; UIA, undifferentiated inflammatory arthritis; MSK, musculoskeletal; IIM, idiopathic inflammatory myopathies; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; EIA, early inflammatory arthritis; AI, aromatase inhibitor; CWP, chronic widespread pain; NA, not available.
* One publication reported two studies.
† Median and IQR.
‡ Mean and range.
§ Median and range.
∥ Original article reported age range only.
Quality assessment in included studies
According to the Newcastle–Ottawa scale, sixty-two of the eighty-one observational studies were very good or good quality( Reference Hicks, Shardell and Miller 33 – Reference Golan, Haggiag and Os 35 , Reference McBeth, Pye and O’Neill 37 , Reference Kjaergaard, Eggen and Mathiesen 39 – Reference Madani, Alavi and Taghizadeh 43 , Reference Hirani, Blyth and Naganathan 45 , Reference Virtanen, Giniatullin and Mantyselka 48 , Reference Pietschmann, Machold and Wolosczuk 49 , Reference Al-Allaf, Mole and Paterson 51 , Reference Lotfi, Abdel-Nasser and Hamdy 54 – Reference Heidari, Shirvani and Firouzjahi 57 , Reference Attar 59 , Reference Baykal, Senel and Alp 60 , Reference Heidari, Hajian-Tilaki and Heidari 62 , Reference Al-Jarallah, Shehab and Abraham 64 – Reference Yazmalar, Ediz and Alpayci 70 , Reference Celikbilek, Gocmen and Zararsiz 72 – Reference Cote, Berger and Kirchner 74 , Reference Hiraki, Arkema and Cui 76 – Reference Brance, Brun and Lioi 82 , Reference Gullo, Mandraffino and Bagnato 84 , Reference Matsumoto, Sugioka and Tada 86 – Reference Yildirim, Solmaz and Akgol 100 , Reference Wong, Gupta and Radhakrishnan 102 – Reference Calza, Magistrelli and Colangeli 108 ), sixteen were satisfactory( Reference Macfarlane, Palmer and Roy 31 , Reference Alipour, Hosseini and Saadat 44 , Reference Morioka, Lee and Bertisch 46 , Reference Tasoglu, Kutsal and Tasoglu 47 , Reference Muller, Kriegbaum and Baslund 50 , Reference Benson, Wilson and Stocks 52 , Reference Cutolo, Otsa and Laas 53 , Reference Turhanoglu, Guler and Yonden 58 , Reference Dong, Xu and Bi 61 , Reference Kostoglou-Athanassiou, Athanassiou and Lyraki 63 , Reference Baykara, Dilek and Nas 71 , Reference Heidari, Heidari and Tilaki 75 , Reference Cen, Liu and Yin 83 , Reference Lodh, Goswami and Mahajan 85 , Reference Brennan-Speranza, Mor and Mason 101 ) and the remaining three were unsatisfactory( Reference Duell and Connor 32 , Reference Linde, Peng and Desai 36 , Reference Backes, Barnes and Ruisinger 38 ). Specifically, the quality for cross-sectional studies was good for twelve, satisfactory for four and unsatisfactory for three; for case–control studies, nine were very good, thirty-five were good and twelve were satisfactory; and for cohort studies, three were very good and three good. For the unsatisfactory studies, most of them did not have enough information to evaluate the representativeness for the target population. In addition, all the pain-related outcome measurements were based on questionnaire or self-report, and only a few of them validated the pain measurements. The quality assessment scores are shown in Supplement 2, Supplemental Table 1 (see online supplementary material).
Pooled results
Vitamin D concentration and pain
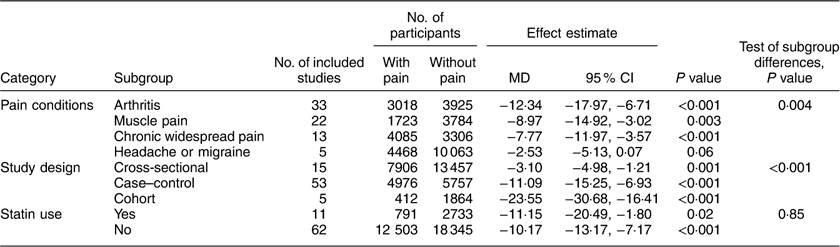
For the primary aim, seventy-three studies, containing 13 294 participants with pain and 21 078 without pain conditions, reported serum 25(OH)D levels( Reference Duell and Connor 32 , Reference Ahmed, Khan and Glueck 34 – Reference Riphagen, van der Veer and Muskiet 40 , Reference Eisen, Lev and Iakobishvilli 42 – Reference Hirani, Blyth and Naganathan 45 , Reference Tasoglu, Kutsal and Tasoglu 47 – Reference Muller, Kriegbaum and Baslund 50 , Reference Benson, Wilson and Stocks 52 – Reference Heidari, Heidari and Tilaki 75 , Reference Hong, Xu and Xu 77 – Reference Wong, Gupta and Radhakrishnan 102 , Reference Mergenhagen, Ott and Heckman 104 – Reference Calza, Magistrelli and Colangeli 108 ). Two publications reported the vitamin D concentration and pain conditions on different subgroups (smokers and non-smokers( Reference Kjaergaard, Eggen and Mathiesen 39 ) or Italian and Estonian( Reference Cutolo, Otsa and Laas 53 )), which are reported separately as four different studies in the current meta-analysis (Supplement 2, Supplemental Table 2).
Table 2 Association between 25-hydroxyvitamin D concentration and painful conditions
MD, mean difference.
Compared with controls, mean 25(OH)D concentration was significantly lower in patients with arthritis (MD=−12·34 nmol/l; P<0·001), muscle pain (MD=−8·97 nmol/l; P=0·003) and chronic widespread pain (MD=−7·77 nmol/l; P<0·001), but not in patients with headache or migraine (MD=−2·53 nmol/l; P=0·06; Table 2). These mean differences by disease condition were significantly different (P=0·004). Because of this interaction, forest plots are shown by pain condition rather than for all conditions combined (Fig. 2). Additionally, among age- and sex-matched case–control studies, similar lower vitamin D levels also were observed in patients with arthritis (MD=−12·03 nmol/l; P<0·001) and muscle pain (MD=−11·49 nmol/l, P<0·01).
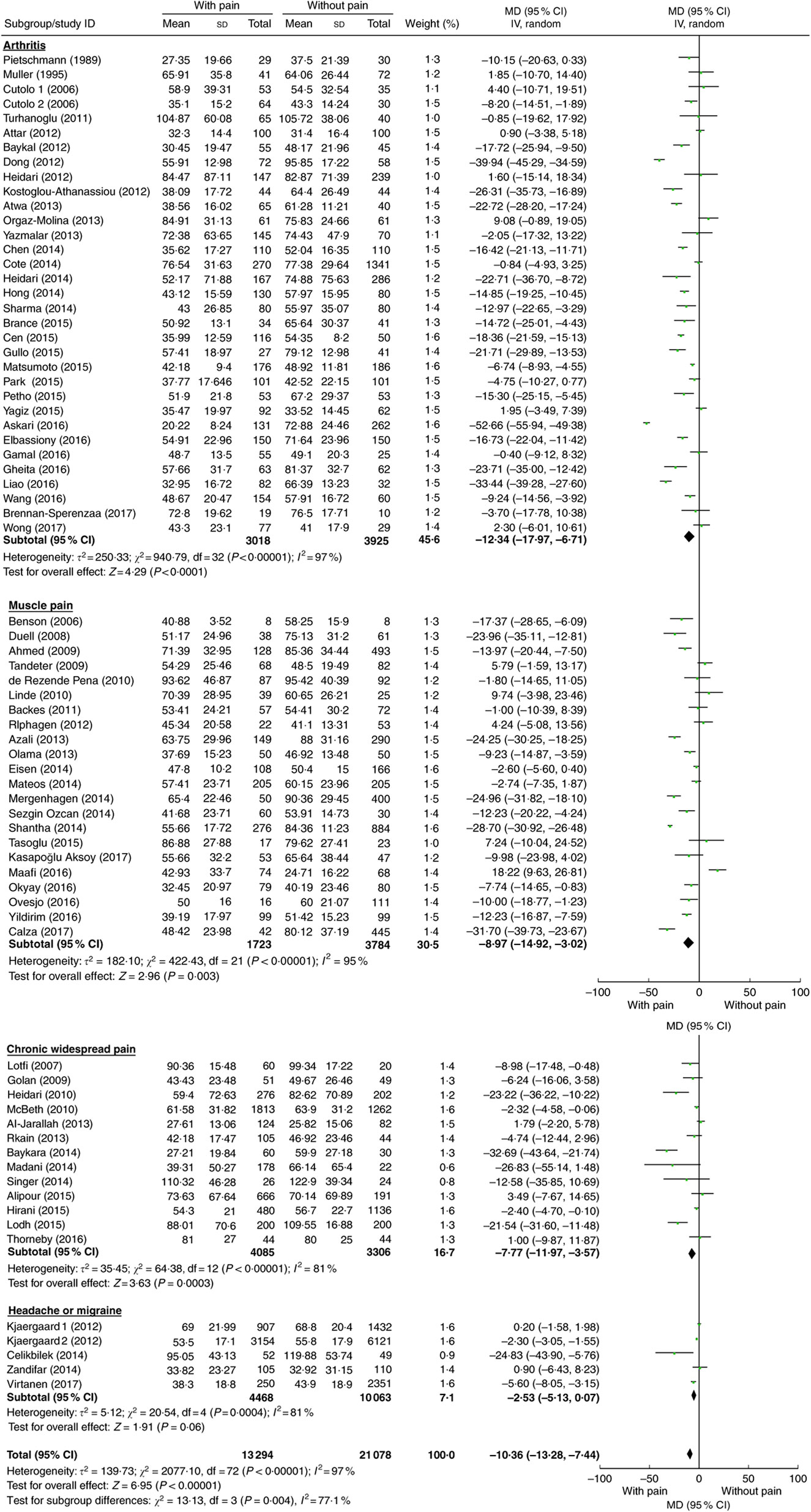
Fig. 2 (colour online) Meta-analysis of the difference in mean serum vitamin D concentration (nmol/l) between participants with and without pain-related conditions. The study-specific mean difference (MD) and 95 % CI are represented by the square and horizontal line, respectively; the centre of the diamond represents the pooled MD and its width represents the pooled 95 % CI. IV denotes inverse variance; random denotes random-effects model
In addition, interaction tests were conducted by type of study design and statin use (Table 2; Supplement 2, Supplemental Figs 1 and 2). There was a significant interaction between the three types of study design (P<0·001). For each study design, pain patients had significantly lower 25(OH)D concentration compared with those without pain, but the effect was strongest in cohort studies (MD=−23·55 nmol/l; P<0·001), moderate in case–control studies (MD=−11·09 nmol/l; P<0·001) and weakest in cross-sectional studies (MD=−3·10 nmol/l; P=0·001). However, there was no interaction between studies of statin users and non-statin users (interaction test P=0·85).
To investigate the impact of other covariables (year, sample size, mean age, female proportion, ratio of participants with and without pain, type of study) on the study-level estimate of the MD in 25(OH)D concentration, we performed random-effects meta-regression analyses. We did not observe any significant association for the above covariables and MD in 25(OH)D levels in both the univariate and multivariate meta-regression analyses (Supplement 2, Supplemental Table 3).
Table 3 Association between hypovitaminosis D and painful conditions
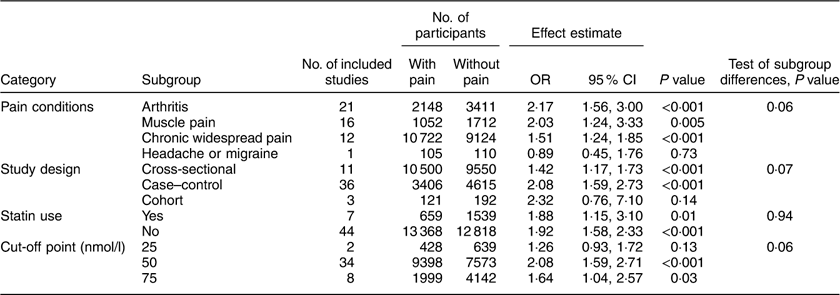
Vitamin D deficiency and pain
For the secondary aim, fifty studies reported the proportion of vitamin D deficiency among 14 027 patients with pain conditions and 14 357 without( Reference Macfarlane, Palmer and Roy 31 – Reference Ahmed, Khan and Glueck 34 , Reference Linde, Peng and Desai 36 – Reference Backes, Barnes and Ruisinger 38 , Reference e Silva, Lacativa and Russo 41 – Reference Madani, Alavi and Taghizadeh 43 , Reference Morioka, Lee and Bertisch 46 , Reference Al-Allaf, Mole and Paterson 51 , Reference Benson, Wilson and Stocks 52 , Reference Lotfi, Abdel-Nasser and Hamdy 54 – Reference Heidari, Shirvani and Firouzjahi 57 , Reference Attar 59 , Reference Dong, Xu and Bi 61 , Reference Heidari, Hajian-Tilaki and Heidari 62 , Reference Atwa, Balata and Hussein 65 – Reference Rkain, Bouaddi and Ibrahimi 69 , Reference Cote, Berger and Kirchner 74 – Reference Hong, Xu and Xu 77 , Reference Sezgin Ozcan, Oken and Aras 79 – Reference Cen, Liu and Yin 83 , Reference Park, Kim and Lee 87 – Reference Yagiz, Ustun and Paksoy 89 , Reference Elbassiony, Tawhid and Ahmad 91 – Reference Wang, Zhang and Wang 99 , Reference Laroche, Coste and Medkour 103 , Reference Singer, Cigler and Moore 106 , Reference Ovesjo, Skilving and Bergman 107 ). One publication( Reference Hiraki, Arkema and Cui 76 ) reported results from two studies which are included separately in the current meta-analysis (Supplement 2, Supplementary Table 4). To maintain consistency with the primary aim analyses (Table 2), the secondary aim of vitamin D deficiency was also analysed by pain condition, study design, statin use and cut-off point for vitamin D deficiency (Table 3 and Fig. 3). The odds of vitamin D deficiency was increased for arthritis, muscle pain and chronic widespread pain, but not for headache or migraine; and also increased for each of the study designs (cross-sectional, case–control and cohort) and for statin users and non-users separately (Supplement 2, Supplemental Figs 3 and 4). There was a significant interaction associated with the 25(OH)D cut-off point (P=0·06), with studies that used cut-offs below 50 or 75 nmol/l reporting significantly increased odds of vitamin D deficiency in patients with pain, but not at a very low cut-off of <25 nmol/l, although there were only two studies in the latter group (Table 3; Supplement 2, Supplemental Fig. 5). Meta-regression analyses did not find any other covariables that were significantly associated with the log(OR) of vitamin D deficiency (Supplement 2, Supplemental Table 5).

Fig. 3 (colour online) Meta-analysis of the difference in the proportion of vitamin D deficiency between participants with and without pain-related conditions. The study-specific OR and 95 % CI are represented by the square and horizontal line, respectively; the centre of the diamond represents the pooled OR and its width represents the pooled 95 % CI. M-H denotes Mantel–Haenszel; random denotes random-effects model
Sensitivity analysis and publication bias
For the primary and secondary aims, sensitivity analyses found similar summary measures to those shown in Figs 2 and 3 when studies were individually excluded (see Supplement 2, Supplemental Tables 6 and 7). In addition, after excluding studies with poor quality, we observed similarly lower 25(OH)D levels in arthritis, muscle pain and chronic widespread pain patients than in their controls (see Supplement 2, Supplemental Table 6). There was no convincing evidence of publication bias from funnel plots (Supplement 2, Supplemental Figs 6 and 7), nor from the Egger’s test for the primary and secondary aims (25(OH)D concentration: P values for publication bias were 0·49, 0·10, 0·17 and 0·66 for arthritis, muscle pain, chronic widespread pain, and headache or migraine conditions, respectively; while for the proportion of vitamin D deficiency: P values were 0·13, 0·64 and 0·06 for arthritis, muscle pain and chronic widespread pain conditions, respectively).
Discussion
Our results show lower mean 25(OH)D concentration among patients with widespread chronic pain, muscle pain and arthritis than among their controls (Fig. 2). This result was consistent with the increased odds of hypovitaminosis D associated with these three conditions (Fig. 3). In addition, our study found that the weighted MD in 25(OH)D concentration between patients with pain and control groups is large (arthritis: 12·34 nmol/l or 20 % difference; muscle pain: 8·97 nmol/l or 14 % difference; chronic widespread pain: 7·77 nmol/l or 11·7 % difference) compared with disease-related differences in previous studies, such as those which have reported a 3 nmol/l (5 %)( Reference Deleskog, Hilding and Brismar 109 ) and a 7 nmol/l (11 %) difference( Reference Gagnon, Lu and Magliano 110 ) between diabetes cases and controls. Overall, these results suggest that low vitamin D status may be associated with the development of painful conditions, with the overall quality of the evidence being rated as moderate according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria( Reference Guyatt, Oxman and Vist 111 ) because of the high heterogeneity, although there was no evidence of publication bias, the quality of studies was good and the association was strong (Supplement 2, Supplemental Tables 8 and 9).
Our findings are consistent with previous meta-analyses which found significantly lower 25(OH)D levels in patients on statin therapy with myalgia compared with those without( Reference Michalska-Kasiczak, Sahebkar and Mikhailidis 20 ) and a positive association between hypovitaminosis D and chronic widespread pain( Reference Hsiao, Hung and Chang 21 ). However, by including a further sixty-two studies in our meta-analysis (three studies that were included in the previous meta-analysis were excluded because of controls with pain conditions or no available data), we have extended previous meta-analyses to show that 25(OH)D levels are also lower in patients with pain not caused by statin therapy – for both the 25(OH)D concentration and hypovitaminosis D aims (Tables 2 and 3). The evidence for the primary aim from the five cohort studies( Reference Mergenhagen, Ott and Heckman 104 – Reference Calza, Magistrelli and Colangeli 108 ), which shows that low vitamin D levels at baseline predicted increased incidence of pain-related conditions (Table 2), supports a possible causal association. In addition, in analyses based on painful conditions, lower mean 25(OH)D levels were found in patients with arthritis, muscle pain and chronic widespread pain (compared with those without pain) for both primary and secondary aims, but not in patients with headache or migraine (Table 2). The latter finding could be due to chance because of the small number of studies (three cross-sectional comparisons from two publications, and two case–control studies) and further research is required to clarify this.
The strengths of the current meta-analysis include: (i) two aims that were predefined at the start of the study; (ii) a search of three electronic databases (MEDLINE, EMBASE and Cochrane Central Register of Controlled Trials), which reduces the possibility of missing relevant articles; (iii) assessment for publication bias by both funnel plots and Egger’s test; (iv) inclusion of broader pain conditions, particularly studies of patients with pain who were not on statins; and (v) evaluation of the quality of included studies by the Newcastle–Ottawa scale.
Nevertheless, there are several limitations of the current meta-analysis. Most of the included studies were case–control or cross-sectional in design, which could have resulted in reverse causation between pain and lower vitamin D levels. Some studies reported medians, and not means and sd, and information may have been lost in the transfer process. In addition, the included studies lack or have limited adjustment for potential confounders, so the unadjusted association must be interpreted with caution as the spurious associations can be result from potential confounders.
Of major importance is the high heterogeneity observed in the meta-analysis. We tried to identify the sources of this using subgroup, meta-regression and sensitivity analyses, but it remained even when analysing studies by type of pain condition or study design. The high heterogeneity could partly be due to the higher heterogeneity often seen in meta-analyses of observational studies where there is variable control of confounding, compared with randomized controlled trials where effects from standard interventions congregate more closely; and also due to the relatively large number of studies (up to thirty-three) included in the pooled analyses which increases the opportunity for heterogeneity. In our view, this does not lessen the validity of our findings as the results of individual studies almost all go in the same direction (Supplement 2, Supplemental Figs 1 and 2). Further, we used a random-effects model which allows for between-study variation of effect in its calculations.
In addition, the definition of pain varied in each individual publication, so that combining them may also have contributed to the heterogeneity of our results. Therefore, more objective outcome measurements, such as consumption of analgesics as reported in previous studies which have found increased opioid use in people with vitamin D deficiency( Reference Morioka, Lee and Bertisch 46 , Reference Bergman, Sperneder and Hoijer 112 ), or use of validated questionnaires to assess pain severity and function( Reference Hawker, Mian and Kendzerska 113 ), would help to clarify the association between vitamin D levels and pain in future studies.
Conclusion
In conclusion, our meta-analysis of eighty-one observational studies suggests that low vitamin D concentration is associated with arthritis, muscle pain and chronic widespread pain. Further well-designed randomized controlled trials should be conducted to confirm the relationship between vitamin D levels and painful conditions.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: The authors declare no conflicts of interest related to this study. Authorship: Z.W. conceived the idea, carried out the meta-analyses and drafted the text; Z.M. edited the manuscript and assisted with searching and extracting data; A.W.S. edited the manuscript and advised on statistical methods; C.M.M.L. edited the manuscript; R.S. contributed to the manuscript and provided critical revision to the study. Ethics of human subject participation: Not applicable.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980018000551








