Demographic transition is a challenging socio-economic aspect in the world, especially in low- and middle-income countries. Individuals aged 60 or over represent 12 % of the world’s population, which is estimated to reach 22 % in 2050(1). Populational aging is one factor responsible for the changes in the morbidity and mortality profile in the population, such as the increased prevalence of chronic non-communicable diseases, including mental and neurological disorders(Reference Alves, Leite and Machado2). Mental and neurological disorders affect more than 20 % of the older adult population worldwide, and dementia is among the most common disorders, affecting approximately 5 % of this age group(3). According to the World Alzheimer Report 2019, it is estimated that there are more than 50 million people in the world with some type of cognitive impairment, and the number is expected to exceed 152 million by 2050(4). In low- and middle-income countries, the number of people suffering from dementia is increasing rapidly with age(4). In addition to the impact on health, mental problems have significant economic repercussions. In 2019, it is estimated that the annual cost of mental disorders worldwide reached 1 trillion dollars(Reference Wimo, Guerchet and Ali5).
Understanding the risk factors for impaired cognitive function allows for the targeting of interventions to prolong autonomy or delay the onset of dementia(Reference Gauthier, Reisberg and Zaudig6). Evidence suggesting a relationship between vitamin D and brain development, neurotransmission, neuroprotection and immunomodulation has been growing(Reference Groves, McGrath and Burne7). In epidemiological studies, low levels of vitamin D in older adults have been associated with poorer cognitive performance(Reference Goodwill and Szoeke8,Reference Wilson, Houston and Kilpatrick9) . The maintenance of adequate levels of vitamin D could, therefore, represent an important protective factor in the prevention of neurological disorders related to aging. It is postulated that vitamin D deficiency represents a global health problem(Reference Holick10). In Brazil, despite high ultraviolet radiation availability to produce vitamin D in the skin throughout the year, studies have indicated a high prevalence of insufficiency and deficiency(Reference Eloi, Horvath and Szejnfeld11–Reference Pereira-Santos, Santos and Carvalho14), not supporting the common assumption that the level of radiation solar energy in the country guarantees adequate levels of vitamin D(Reference Mendes, Hart and Botelho15). As people age, the risk of vitamin D deficiency increases significantly, mainly due to the decreased capacity for synthesis in the skin(Reference Cominetti and Cozzolino16,Reference Meehan and Penckofer17) . In addition, older adults are among the risk groups for vitamin D deficiency which has been related to less sun exposure, decrease in food intake with Vitamin D and intestinal malabsorption(Reference Arabi, El Rassi and El-Hajj Fuleihan18,Reference Cesari, Incalzi and Zamboni19) .
In low- and middle-income countries, evidence on the association between vitamin D and cognitive impairment in the older population is scarce(Reference Chei, Raman and Yin20,Reference Lau, Mat Ludin and Rajab21) . Therefore, the objective of this study was to investigate the association between serum vitamin D (25-hydroxy-cholecalciferol) concentrations and cognitive impairment in the older population in the southern region of Brazil.
Materials and methods
Study design and population
This is a cross-sectional analysis of data collected in 2013–2014 from the database of household populational-based EpiFloripa Aging Cohort Study (www.epifloripa.ufsc.br). The EpiFloripa Aging Study design and methods have been previously published(Reference Confortin, Schneider and Antes22,Reference Schneider, Confortin and Bernardo23) . EpiFloripa’s baseline was established in 2009/2010 with a sample of 1702 older adults living in Florianópolis city, Santa Catarina State, Southern Brazil (Fig. 1). Older adults of both sexes, aged 60 years or more at the time of the interview, living in the sectors sampled by the survey were considered eligible at baseline. Older adults who were institutionalised (living in long-term care institutions, hospitals, prisons) were excluded. In the follow-up, carried out in 2013/2014, everyone who participated in the EpiFloripa baseline were included, resulting in 1197 interviews (response rate of 70·2 % in relation to the baseline)(Reference Schneider, Confortin and Bernardo23). All the older adults in the follow-up were invited to provide blood samples for analysis of biochemical markers, including vitamin D (n 604, response rate of 50·5 %)(Reference Confortin, Schneider and Danielewicz24). The average interval between interviews and blood collection was 107 d (with a median, 25th and 75th percentile of this difference of 100, 45 and 136 d, respectively). In this study, 572 older adults who participated in the second follow-up wave with complete data to assess cognitive impairment and vitamin D were included in the analysis. One outlier of serum vitamin D was excluded from the analysis (>96 ng/ml without supplementation), resulting in an analytical sample of 571 individuals. The interviews were conducted by trained interviewers at the older adult’s home, with the help of laptops. To ensure quality control, the use of validated instruments was prioritised for the composition of the questionnaire as well as the selection of interviewers with training in the health area and experience in research. Quality control was performed by telephone, using a short version of the questionnaire in 10 % of the sample.

Fig. 1 Flowchart of the populational-based EpiFloripa cohort study
Cognitive assessment
The cognition was assessed using the Mini-Mental State Examination (MMSE), translated and validated in Brazil by Bertolucci et al. (1994)(Reference Bertolucci, Brucki and Campacci25). The MMSE is one of the most widely used screening tools to identify cognitive impairment in clinical practice(Reference Aprahamian, Biella, Vanderlinde and Guanabara26,Reference Dias, Andrade and Duarte27) . The scores were categorised as probable cognitive impairment and absence of cognitive impairment, considering schooling of older adults, using the cutoff points 19/20 for illiterate and 23/24 for any level of education, as recommended by Almeida (1998)(Reference Almeida28).
Serum vitamin D (25-hydroxy-cholecalciferol)
For the measurement of 25-hydroxy-cholecalciferol (25(OH)D), individuals were invited to attend the Laboratory for Metabolism and Dietetics in the university between 7 and 10 a.m. Blood samples were collected after an 8-hour fast by venipuncture(Reference Confortin, Schneider and Danielewicz24). Blood samples were processed and analysed by the clinical analysis laboratory of the University Hospital of the Federal University of Santa Catarina. Serum 25(OH)D concentrations were measured using the Microparticle Chemiluminescence method/LIAISON, which is considered a rapid, accurate and accurate assay (Functional sensitivity: ≤ 2·0 ng/ml; inter-assay inaccuracy < 20 %)(Reference Bianchi, Maffei and Prontera29,Reference Ersfeld, Rao and Body30) . The LIAISON® 25OH vitamin D assay (Diasorin, São Paulo, Brazil) is certified of total 25-hydroxyvitamin D assays by CDC vitamin D Standardization-Certification Program (CDC VDSCP) since 2014(31). First, the blood samples were centrifuged (3500 rpm) for 10 min and the serum samples were immediately processed using the LIASON® according to the manufacturer(Reference Ersfeld, Rao and Body30). Briefly, an antibody specific to vitamin D was coated on magnetic particles, and 25(OH)D conjugated to an isoluminol derivative and diluted in phosphate buffer (pH 7·4). In the first incubation period, 25-OHD dissociated from the binding protein, and it interacts with the antibody. After the second incubation with the tracer reagent, microplate is washed with the buffer and starter reagents are added to generate the chemiluminescent signal, which is measured by a photomultiplier.
After that, serum concentration of vitamin D was divided into quartiles (Q1: 4·0–20·7 ng/ml; Q2: 20·8–26·6 ng/ml; Q3: 26·7–32·0 ng/ml; and Q4: 32·1–60·1 ng/ml) for statistical analysis.
Covariates
To characterise the sample and adjust the analysis, socioeconomic, demographic, behavioural and health aspects were categorised as follows: gender (male and female); age (63–69 years, 70–79 years or 80 years or more); schooling (0–4 years, 5–11 years or 12 years or more); per capita income in minimum wages (MW) according to the values in 2013 and 2014 (≤ 3 MW > 3, and ≤ 5 MW > 5); marital status (married, single/divorced, or widowed), smoking (no, past, or currently smoker); alcohol consumption (never, moderate (consumes up to one dose of alcohol on an average day and never consumes 5 or more doses on a single occasion), or high (consumes more than 2 doses in one a normal day or 5 or more doses on a single occasion)) according to the Alcohol Use Disorder Identification Test(Reference Babor, Higgins-Biddle and Saunders32); leisure-time physical activity (sufficiently active (≥150 min/week), and insufficiently active (<150 min/week)), according to the International Physical Activity Questionnaire(Reference Benedetti, Mazo and Barros33,Reference Benedetti, Antunes and Rodriguez-Añez34) ; number of morbidities diagnosed (none, 1, 2 or more (to include the following diseases: arthritis, cancer, diabetes, bronchitis, kidney disease, tuberculosis, cirrhosis, heart or CVD, stroke or cerebral ischemia, osteoporosis, hypertension/high blood pressure, depression))(35); BMI(Reference Garrow and Webster36) (underweight (<22 kg/m2), normal weight (22–27 kg/m2) and overweight (≥28 kg/m2))(37); season at time of blood collection (spring/summer, autumn/winter); vitamin D supplement use (yes/no). Supplementation of vitamin D was verified by medical prescription and verification of the presence of the supplement in the participant’s residence and self-reported use of vitamin D, registered in the EpiFloripa database according to the WHO Collaborating Center for Drug Statistic Methodology.
Statistical analysis
The sample was described according to socioeconomic, demographic, behavioural and health characteristics using absolute and relative frequencies, 95 % CI or mean and sd. The difference between probable and absent cognitive impairment was determined by Pearson’s Chi-square test and Fisher’s exact test. The normality of the vitamin D variable was verified by the Shapiro–Wilk test, and the outliers were identified by the boxplot. The difference in serum vitamin D concentrations between supplemented and not supplemented individuals and cognitive status was evaluated using Student’s t test and ANOVA followed by Bonferroni post hoc.
To explore the association between cognitive impairment (dependent variable) and vitamin D in quartiles (independent variable), logistic regression was used with crude and adjusted analysis. The adjusted analysis was initiated by socioeconomic and demographic variables (Model 1: gender, age group, income, season and vitamin D supplementation), followed by the inclusion of behavioural variables (Model 2: model 1 + physical activity, smoking and alcohol consumption) and health (Model 3: model 2 + number of morbidities and BMI). The analysis was performed considering Q4 (higher vitamin D levels) as the reference group to investigate the risk associated with vitamin D deficiency. The season during which the blood was collected was used to adjust for seasonal effects, considering that vitamin D levels are partially dependent on exposure to sunlight. Schooling was not included in the adjusted analysis, as it is already used in the classification criteria of the MMSE. The results were presented as OR with their respective 95 % CI. The value of P < 0·05 in the Wald test was used for making statistical decisions. To assess the adequacy of fit of the multiple logistic regression model, the Hosmer–Lemeshow test was used(Reference Hosmer and Lemesbow38). Statistical analysis was performed using the STATA® statistical program (Stata Corporation) version 14.0. The effect of the sample design by clusters was considered, incorporating the sample weight using the svy command. Statistical significance was set at P < 0·05.
Results
Of the 604 participants in the 2013–2014 wave, 571 older adults met the criteria and presented complete data to be included in the analysis (Fig. 1). Sixty-two percent were women (n = 371), and the mean age was 72·3 (sd ± 6·4) years. The prevalence of probable cognitive impairment in the total sample was 21·7 % (Table 1).
Table 1 Socioeconomic, demographic and behavioural characteristics of the elderly sample of the study according to the presence of cognitive impairment, EpiFloripa Aging cohort study, follow-up wave 2013–2014, Southern Brazil
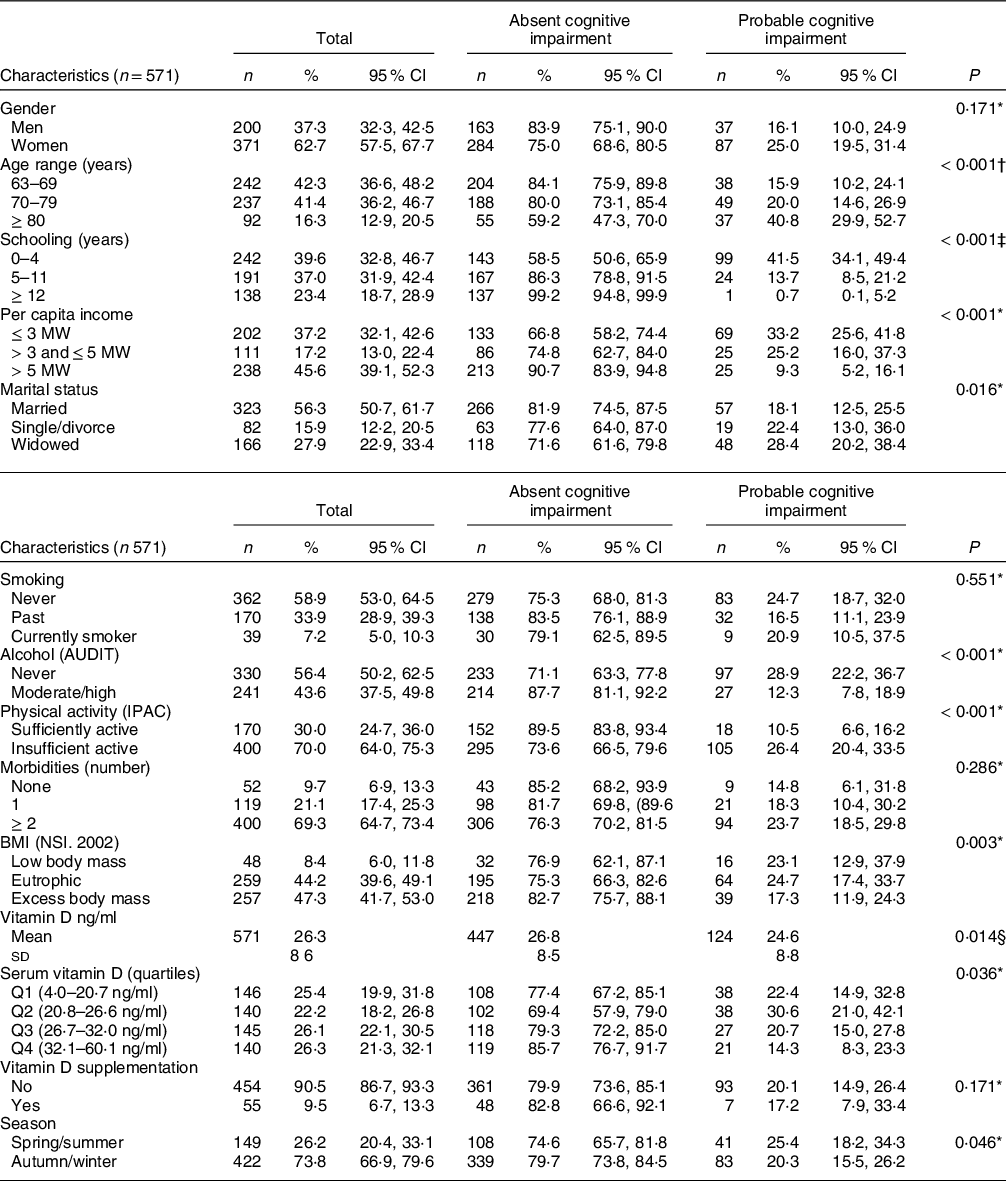
AUDIT, Alcohol Use Disorder Identification Test; IPAQ, International Physical Activity Questionnaire; MW, minimum wages; NSI, Nutrition Screening Initiative.
* Chi-square test.
† Trend chi-square.
‡ Fisher’s exact test.
§ Student t test.
Regarding the characteristics of the sample according to the presence and absence of probable cognitive impairment, there was a difference for almost all aspects, except for gender (P = 0·171), smoking (P = 0·551) and the number of morbidities (P = 0·286). The prevalence of cognitive impairment was higher among the older adults over 80 years of age (P < 0·001), with an income of up to 3 MW (P < 0·001), with less than 4 years of schooling (< 0·001), who are widowed (P = 0·0016), who never consumed alcohol (P < 0·001), who are physically inactive (P < 0·001) and with eutrophic BMI (P = 0·003). Regarding vitamin D, there was a lower prevalence of cognitive impairment in the highest vitamin D quartile (P = 0·036), and the mean vitamin D serum concentration was higher in those without cognitive impairment (26·8 v. 24·6, P = 0·014) (Table 1). The prevalence of vitamin D supplement use was low (n 55, 9·6 %) for the total sample, and among these, 48 (87·3 %) individuals did not present probable cognitive impairment. The prevalence of cognitive impairment was lower among the older adults supplementing vitamin D, but without a statistically significant difference between the groups (P = 0·171) (Table 1).
Regarding vitamin D serum concentrations, the results showed a high prevalence of inadequate levels, considering that 47·6 % of older adults were in Q1 and Q2, with levels between 4·0 and 26·6 ng/ml. The prevalence of vitamin D deficiency and insufficiency in the sample according to Endocrine Society cutoff points was 22·6 and 42·3 %, respectively, and according to Institute of Medicine (IOM) cutoff points was 4·7 % e 17·9 %, respectively (Supplementary Table).
Using Student’s t test, mean serum vitamin D concentrations were found to be higher in vitamin D-supplemented individuals than in those not supplemented (supplemented: mean 29·7, sd ± 7·3, 95 % CI 27·7, 31·7; not supplemented: mean 26·1 sd ± 8·7, 95 % CI 24·3, 26·9; P = 0·004). When vitamin D supplementation and cognitive impairment were evaluated together, using ANOVA followed by Bonferroni post hoc, statistical differences were observed for supplemented v. not supplemented groups (Fig. 2). Those individuals without cognitive impairment and taking vitamin D supplementation presented higher levels of vitamin D than those without cognitive impairment and without supplementation. There was a difference in the serum vitamin D concentrations between those with cognitive impairment without supplementation and those without cognitive impairment with supplementation.

Fig. 2 Serum vitamin D concentrations in elderly individuals in relation to supplementation of vitamin D (VitD) and probable presence of cognitive impairment. Data are presented as mean and SD. One-way ANOVA followed by Bonferroni post hoc was used (a P < 0·05 between the elderly without cognitive impairment, whether with supplementation or not; b P < 0·05 between older adults with cognitive impairment without supplementation and older adults without cognitive impairment with supplementation)
Logistic regression analysis investigating the association between serum vitamin D level and cognitive impairment are presented in Table 2. In the crude analysis, older adults in Q2 for vitamin D had a higher OR for probable cognitive impairment when compared with adults in Q4, the reference group (OR 2·65, 95 % CI 1·46, 4·81). This association remained statistically significant after the adjustment models. It was observed that individuals in Q1 for serum vitamin D presented an increased risk of probable cognitive impairment in models 1 and 2 (Model 1: OR 2·71, 95 % CI 1·22, 6·04; Model 2: OR 2·72, 95 % CI 1·19, 6·22). In model 3, all individuals below Q4 for serum vitamin D presented an increased risk of probable cognitive impairment (Q1: OR 3·03, 95 % CI 1·37, 6·73; Q2: OR 6·04, 95 % CI 2·78, 13·13; Q3: OR 2·68, 95 % CI 1·18, 6·08). The adjustment of the multivariate logistic regression model was considered adequate by the Hosmer–Lemeshow test (P = 0·9575)(Reference Hosmer and Lemesbow38).
Table 2 Crude and adjusted logistic regression to investigate the risk for cognitive impairment according to serum vitamin D concentrations in elderly, EpiFloripa Aging cohort study, follow-up wave 2013–2014, southern Brazil
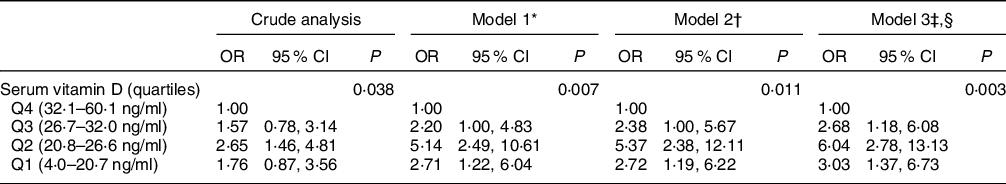
* Model 1: Gender, age, per capita income, marital status, season, vitamin D supplementation.
† Model 2: Model 1 + smoking, alcohol, physical activity.
‡ Model 3: Model 2 + morbidities, BMI.
§ Hosmer–Lemeshow fit test P = 0·9575.
Discussion
The main finding of this study is the association between lower concentrations of serum vitamin D and cognitive impairment independent of vitamin D supplementation and season, a statistically significant result even after adjusting for potential confounding factors. This is a truly relevant finding, especially because the EpiFloripa Aging study(Reference Schneider, Confortin and Bernardo23) design included a representative population from the city where data were collected in one of the most longevous cities in southern Brazil.
The prevalence of probable cognitive impairment in the total sample was 21·7 %, with 25·0 % for women and 16·1 % for men. Worldwide, there is a great variety in the prevalence of cognitive impairment, with 13·6 % in UK(Reference Llewellyn, Langa and Lang39), 18·7 % in Singapore(Reference Annweiler, Milea and Whitson40), 22·2 % in the USA(Reference Plassman, Langa and Fisher41) and 23·3 % in Spain(Reference Mateo-Pascual, Julián and Alarcón-Alarcón42). In middle-income countries, the prevalence ranged from 7·3 % to 24·1 %(Reference Vancampfort, Lara and Stubbs43). The prevalence of cognitive impairment could vary according to the rating scale used as well as the cutoff points for the MMSE applied in the study. Many articles found in the literature evaluating cognitive impairment in older adults used the MMSE as a rating scale(Reference Andrade, Corona and Lebrão44–Reference Holz, Nunes and Thumé46).
Our data have shown that the prevalence of probable cognitive impairment was different for women and men, but without statistical significance. Women accounted for the majority of the sample in our study. Regarding the difference between genders, studies have shown varying results in older adults(Reference Mateo-Pascual, Julián and Alarcón-Alarcón42,Reference Lobo, Marcos and Santabárbara47,Reference Petersen, Roberts and Knopman48) . However, the higher prevalence among women observed could be due to the education profile, in which men were more likely to have 12 years or more of schooling, and women with 1–4 years of schooling (data not shown). Years of schooling is one of the main factors associated with MMSE performance(Reference Valle, Castro-Costa and Firmo49) and cognitive impairment(Reference Langa and Levine50–Reference Meng and D’Arcy52). We also found differences in the prevalence of cognitive impairment according to marital status. Previous studies found that divorced and widowed elderly people are more vulnerable to cognitive impairment(Reference Liu, Zhang and Burgard53,Reference Hakansson, Rovio and Helkala54) and this association is stronger for men than women(Reference Feng, Ng and Yap55,Reference Kim56) . It is suggested that being married is associated with a reduced risk of dementia than lifelong single and widowed(Reference Sommerlad, Ruegger and Singh-Manoux57).
According to the Endocrine Society cutoff point, more than half of the elderly have inadequate levels of vitamin D, but this number is lower with the IOM definition. The 2nd International Conference on Controversies in Vitamin D discussed the cutoff points, recommending that 25(OH)D values below 12 ng/ml should be considered associated with an increased risk of rickets/osteomalacia, while 25(OH)D concentrations between 20 and 50 ng/ml seem to be safe and sufficient for bone health in the general population(Reference Giustina, Adler and Binkley58). The Brazilian Society of Endocrinology and Metabolism recommends values between 30 and 60 ng/ml for populations at risk, which includes older adults(Reference Maeda, Borba and Camargo59,Reference Ferreira, Maeda and Batista60) . Vitamin D deficiency below 20 ng/ml has been discussed as a risk factor for several health conditions and unfavorable skeletal outcomes, especially in older adults including fractures and bone loss, muscle function and risk of fall and the increased risk of mortality(Reference Bouillon, Marcocci and Carmeliet61–Reference Dudenkov, Mara and Petterson64).
In Brazil, due to its high solar radiation throughout the year, an adequate concentration of serum vitamin D in the population is expected. However, the results found in the literature(Reference Cabral, Borges and Maia65–Reference Saraiva, Cendoroglo and Ramos67) suggested that deficiency is common among older adults. It is important to note that only 26·2% of the blood collections in this study were performed in the spring and summer, the seasons in which the incidence of sunlight is highest(Reference Holick10), and 73·8% in the autumn and winter, when there is a higher prevalence of vitamin D deficiency(Reference Eloi, Horvath and Szejnfeld11,Reference Mateo-Pascual, Julián and Alarcón-Alarcón42,Reference Saraiva, Cendoroglo and Ramos67) , which may result in lower serum concentrations of this vitamin. Nevertheless, even when we controlled the analysis for the season, the association was sustained. In addition to the season, other factors also contribute to vitamin D deficiency/insufficiency in older adults, such as female sex, dark skin pigmentation, reduced intake, increased adiposity, shorter outdoor activities, decreased absorption, reduced renal function and medication use(Reference Meehan and Penckofer17). Our sample was composed mainly of women, people with insufficient physical activity, low per capita income and few years of schooling, which could influence food acquisition and information about vitamin D-rich foods and the acquisition of vitamin D supplements as well. In our sample, only 9·5 % of the elderly were receiving vitamin D supplements and had higher levels of vitamin D when compared with those who were not supplementing. With these data, we postulate that older individuals are a risk group for vitamin D deficiency and an effort of physicians, gerontologists and nutritionists to investigate this aspect and provide adequate supplementation earlier for the older adult population is needed.
The association between serum vitamin D concentrations and cognitive impairment found in our study is in agreement with previous evidence that analysed vitamin D in quartiles(Reference Chei, Raman and Yin20,Reference Llewellyn, Langa and Lang39,Reference Sakuma, Kitamura and Endo68) . In UK, a study with a representative sample of 1766 older people investigated the association of vitamin D with cognitive impairment, assessed by the Abbreviated Mental Test. The older adults in the first quartile of vitamin D (3·2–12·0 ng/ml) (OR 2·3; 95 % CI 1·4, 3·8) presented a greater chance of cognitive impairment when compared to those in the highest quartile (26·4 at 68·0 ng/ml). The results suggest that low serum vitamin D levels are associated with increased chances of cognitive impairment(Reference Llewellyn, Langa and Lang39). In a study with 644 older Japanese adults, using the highest quartile of vitamin D as a reference, those in the lowest quartile had almost 3 times more chance of cognitive impairment (OR 2·70; 95 % CI 1·38, 5·28), defined as a score less than or equal to 23 on the MMSE(Reference Sakuma, Kitamura and Endo68). In China, a study investigated this association in older population and found lower plasma vitamin D levels in individuals with cognitive impairment (score less than 18 in the MMSE) (12·76 ± 6·12 ng/ml) than in those without (18·24 ± 7·84 ng/ml). Older adults in the lowest quartile of vitamin D were twice as likely to have cognitive impairment when compared to those in the highest quartile (OR 2·15; 95 % CI 1·05, 4·41)(Reference Chei, Raman and Yin20). In 3325 elderly Americans, the adjusted logistic regression model showed that older adults who had severe vitamin D deficiency were more likely to have cognitive impairment (OR 3·68; 95 % CI 1·37, 9·90) compared to those with sufficient serum concentrations(Reference Llewellyn, Lang and Langa69).
The results of this study are in agreement with those observed in a systematic review with meta-analysis, in which lower concentrations of vitamin D were associated with worse cognitive performance in the older adult population (OR 1·24; 95 % CI 1·14, 1·35). However, the authors report important methodological limitations, such as the heterogeneity of the populations studied, the different forms of cognitive assessment, the different definitions of vitamin D deficiency and uncontrolled confounding factors(Reference Goodwill and Szoeke8), such as season, which we inserted in our models for the analysis.
Other studies that assessed global cognitive function by MMSE found conflicting findings. In 118 elderly Europeans, a significant association was found with vitamin D tertiles(Reference Brouwer-Brolsma, Feskens and Steegenga70). In Norway, a study of 2044 older adults also found no significant relationship between the vitamin D quartiles and the MMES score(Reference Jorde, Mathiesen and Rogne71). This association was also not found in a sample of 965 older American adults(Reference Laughlin, Kritz-Silverstein and Bergstrom72). In this way, studies investigating the association between serum vitamin D concentrations and cognition have found conflicting results. The variety of instruments used to assess the cognitive status and the different cutoff points to define vitamin D deficiency are limitations of these investigations(Reference Etgen, Sander and Bickel73).
The putative mechanisms by which vitamin D modulates cognitive processes in aging and the neurophysiopathology of dementia are complex and not well established. Its role in the brain is mediated by the presence of nuclear vitamin D receptors in neurons and glial cells(Reference Eyles, Smith and Kinobe74,Reference Kalueff and Tuohimaa75) . The location of these receptors in the hippocampus, hypothalamus, cortex and cerebral subcortex supports the hypothesis of their action in the regulation of neurocognitive functions(Reference Kalueff and Tuohimaa75–Reference Buell and Dawson-Hughes77). Although vitamin D cutoff points are well defined for the proper maintenance of bone metabolism(Reference Dawson-Hughes, Heaney and Holick78), the ideal concentrations to maintain cognitive function have not yet been identified(Reference Lau, Mat Ludin and Rajab21). To maximise its effects on body tissues other than bone mass, it has been suggested that vitamin D concentrations should be in the range of 28–40 ng/ml(Reference Sanders, Nicholson and Ebeling79). Thus, we verified the importance of more studies to elucidate new cutoff points for vitamin D for the maintenance of cognitive function in the older population.
This study has some limitations. The response rate to perform laboratory tests (response rate of 50·4 %) is the most important. This was due to the need to attend the exams, which can trigger a selection bias. The participation of older adults with better health conditions could result in underestimating the prevalence of probable cognitive impairment. Because they are walking and are subject to greater exposure to sunlight, the results of vitamin D may also have been overestimated in this sample, not being representative of the general population. A previous study in the EpiFloripa sample compared the refuses and losses with enrolled people and found differences in age (P < 0·001) (82·1 % in ≥ 80 years) and the probable presence of cognitive deficit (68·7 %). It was noticed that, with increasing age, there was a reduction in participation in the exams; this reduction was also noted in those with probable cognitive impairment(Reference Confortin, Schneider and Danielewicz24). In addition, potential confounding factors such as the use of sunscreen, time of sun exposure, air pollution, food intake, levels of parathormone and calcium were not controlled in the analysis.
Another possible limitation is the time of interval between the cognitive impairment screening and the blood sample collection to measure 25(OH)D. The screening of cognitive impairment is a result of an ongoing process over years. On the other hand, 25(OH)D corresponds to a specific moment. Data suggests that the world is experiencing vitamin D insufficiency in a pandemic manner, pointing some risk factor as skin colour (with increased skin melanin pigmentation), obesity, less sun exposure, especially in children, pregnant and elderly population(Reference Holick10). Also, decreased sun exposure associated with low consumption of foods containing vitamin D could be the main cause for this health issue(Reference Holick80). Furthermore, the low monitoring of vitamin D deficiency in some countries and the poor health coverage for the population, could make it difficult to treat it early, contributing to perpetuate this scenario in the world(Reference Holick10). In our study, only 55 older adults (∼10 % of the sample) reported the use of vitamin D supplements, despite the high prevalence of deficiency. In this way, if a high-risk population is not being monitored for 25(OH)D levels, it is possible to suggest that older adults in general could present vitamin D deficiency and are not receiving early treatment. This could partially explain the association between vitamin D deficiency and neuropsychiatric problems that we and other authors have been observed, considering that neuropsychiatric problems take some years to generate detectable symptoms.
Among the strengths, it is important to mention that the study has a probabilistic sampling, considering the distribution of the study population at the collection site. In addition, the use of validated and standardised instruments, training of the field team and quality control of the data performed in 10 % of the sample are factors that guaranteed the quality of the collected data. Furthermore, the adjustment for the season during which the blood sample was collected and for vitamin D supplementation are precautions that not all studies report.
Conclusion
Our results suggest that the lowest quartile of vitamin D is independently associated with the probable presence of cognitive impairment in a sample of older people. This finding contributes to the understanding of the involvement of vitamin D in cognitive function and emphasises the importance of this micronutrient for the older adult population, as it appears to be a risk group for vitamin D deficiency. Considering the possible relationship between vitamin D and the mental health of older adults, more research is needed to investigate whether the maintenance of adequate serum concentrations could represent a significant factor in the prevention of age-related neurological disorders as well as to verify the need for new cutoff points for older adults concerning mental health.
Acknowledgments
Acknowledgments:
We would like to thank EpiFloripa Aging research colleagues and professors for their efforts and dedication to the study and the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq for the scholarship of the coordinator. Finally, we would like to thank all older adults participating in the project.
Financial support:
The data in this study came from the 2013/2014 EpiFloripa Project. This project was made possible through partnerships with UFSC, other research projects and assistance from students and teachers involved in the research. All the infrastructure, equipment and instruments necessary to carry out research were made available by the UFSC. Laptops were provided by the Oswaldo Cruz Foundation (FIOCRUZ, Rio de Janeiro, Brazil). Serum biochemical analysis was financed by the CNPq (process number 475·904/2013–3) and developed within the scope of the Graduate Program in Collective Health at UFSC.
Conflict of interest:
There are no conflicts of interest.
Authorship:
L.H.M.: Conceptualisation, Methodology, Formal analysis, Writing - Original Draft. J.D.M.: Conceptualisation, Methodology, Writing - Review and Editing, Supervision. G.C.: Formal analysis, Writing - Review and Editing. S.C.C.: Formal analysis, Writing - Review & Editing. C.S.: Writing - Review and Editing. E.d’O.: Writing - Review and Editing. A.J.X.: Writing - Review and Editing. All authors read and approved the final version of the manuscript.
Ethics of human subject participation:
The EpiFloripa Aging Cohort Study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the Ethics Committee in Research with Human Beings of the Federal University of Santa Catarina, according to the protocols nº 329.650 and 526.126. Written informed consent was obtained from all subjects.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S1368980021004407







