Advances in combination antiretroviral therapy (cART) have improved morbidity and mortality for people living with HIV (PLHIV)(Reference Cheung, Ding and Sereda1–Reference Samji, Cescon and Hogg3). However, social and structural barriers, such as food insecurity (FI), homelessness, and poverty continue to prevent marginalized PLHIV from fully benefiting from cART(Reference Anema, Chan and Chen4–Reference Krüsi, Wood and Montaner6). Notably, FI, or self-reports of uncertain or inadequate food access due to limited financial resources, is associated with adverse HIV-related clinical outcomes(Reference Strike, Rudzinski and Patterson7–Reference Kalichman, Washington and Grebler9); FI has a known association with incomplete HIV viral load suppression(Reference Aibibula, Cox and Hamelin10,Reference Weiser, Yuan and Guzman11) , lower CD4 cell counts(Reference Aibibula, Cox and Hamelin12) and a heightened risk for mortality(Reference Anema, Chan and Chen4,Reference Lima, Anema and Bangsberg13) .
Research has suggested that the impact of FI on adverse HIV-related outcomes is due, in part, to its negative association with adherence to cART(Reference Kalichman, Washington and Grebler9,Reference de Pee, Grede and Mehra14,Reference Anema, Kerr and Milloy15) . A study based in San Francisco revealed that PLHIV who were food insecure were almost half as likely to be adherent to cART than their food-secure counterparts(Reference Weiser, Yuan and Guzman11). Additional research has illustrated mechanisms through which FI may impact cART adherence(Reference Young, Wheeler and McCoy16–Reference Hatcher, Tsai and Cohen19). For example, individuals may skip doses or discontinue treatment to mitigate the actual or anticipated side-effects of taking cART without food (e.g. nausea, stomach pain)(Reference Kalichman, Washington and Grebler9,Reference Young, Wheeler and McCoy16) .
While past studies have established a link between FI and suboptimal cART adherence(Reference Young, Wheeler and McCoy16,Reference Singer, Weiser and McCoy18,Reference Anema, Vogenthaler and Frongillo20) , this relationship has yet to be explored within the context of integrated care programmes that aim to attenuate the consequences of socio-structural inequities among PLHIV. For example, the Dr. Peter Centre (DPC) is an integrated care programme serving PLHIV in Vancouver, British Columbia (BC), Canada. PLHIV are eligible to access DPC programming if they are at risk of health deterioration and demonstrate a need (e.g. limited financial or social supports) for assistance to maintain independence(Reference Fernando, McNeil and Closson21). The DPC aims to reduce barriers to access and retention in HIV care by offering a wide array of harm reduction services(Reference Fernando, McNeil and Closson21). These services include counselling, therapies (e.g. art, music, recreational), nursing (e.g. wound care, foot clinic, cART support) and amenity access (e.g. nap room, showers)(Reference Fernando, McNeil and Closson21). DPC clients can also access two nutrient-rich meals per day, including balanced portions of meat/alternatives, dairy products, fruits and vegetables, and whole grains(22). While we acknowledge that food provision does not directly address the root cause of FI in resource-rich settings, which is inadequate financial resources(Reference Tarasuk23,Reference Tarasuk and Eakin24) , we hypothesize that this service, along with other supports that are offered in this setting, may help mitigate the relationship between FI and cART adherence. Therefore, we undertook a study to examine this relationship among clients of the DPC. Further understanding this relationship within an integrated care setting may have implications for optimizing HIV care among structurally vulnerable PLHIV.
Methods
The present study used data from a community-based observational study exploring the impact of the DPC’s services on health outcomes and HIV-related care for marginalized PLHIV. The quantitative study, described in detail elsewhere(Reference Fernando, McNeil and Closson21,Reference Collins, Parashar and Hogg25) , is comprised of a longitudinal cohort of DPC clients who participated in baseline (n 121) and follow-up (n 102) socio-behavioural surveys. Participant recruitment was conducted by peer research associates (i.e. individuals with common experiences to DPC clients) and DPC staff. Study invitations were placed at the DPC reception desk and included the study coordinator’s number, whom participants could call if interested in participating.
Individuals were eligible for the current analysis if they had been enrolled as a DPC client after 27 February 2011, had completed a baseline survey and were on cART at baseline. Baseline surveys that collected sociodemographic, behavioural and FI-related data were administered by the peer research associates to the DPC clients between February 2014 and March 2016. Follow-up surveys were conducted approximately 12 months after the baseline surveys. Participants received $CAN 30 honoraria as compensation for their involvement.
Survey data were supplemented with comprehensive clinical data from the HIV Drug Treatment Program (DTP) held at the BC Centre for Excellence in HIV/AIDS. The DTP provides cART free-of-charge to all PLHIV in the province of BC(Reference Hogg, Yip and Chan26). As described in detail elsewhere, individuals are enrolled in the DTP when they are first prescribed cART by any physician in BC and all subsequent measures of HIV-related clinical variables (e.g. CD4 count, HIV viral load, cART refill compliance) are stored in the DTP database(Reference Hogg, Yip and Chan26). Because our analysis required that DPC clients be on cART at baseline, all the participants in the present study were enrolled in the DTP.
Measures
The primary explanatory variable of interest was FI in the past 12 months, which was measured using the ten-item adult scale of Health Canada’s Household Food Security Survey Module (HFSSM)(Reference Kirkpatrick and Tarasuk27,28) . This tool classifies FI status based on the number of affirmative responses to the ten items. In accordance with Health Canada’s guidelines, zero or one affirmative response on the HFSSM indicates food security, while two or more affirmative responses denotes FI(28).
The outcome variable of interest for the current analysis was cART adherence, based on refill compliance, which is a previously validated method of estimating adherence when direct observation of medication consumption is not feasible(Reference Steiner and Prochazka29). Refill compliance is calculated as the number of days that cART was dispensed divided by the number of days of follow-up during the 12 months prior to the interview date(Reference Gross, Yip and Lo30,Reference Palepu, Tyndall and Joy31) . This measure was expressed as a percentage and dichotomized as optimal (adhering to ≥95 % of prescribed cART) or suboptimal (adhering to <95 % of prescribed cART) adherence; this cut-off has been validated as having clinical relevance for HIV viral load suppression(Reference Palepu, Tyndall and Joy31,Reference Low-Beer, Yip and O’Shaughnessy32) . Potential confounding variables for inclusion in the statistical models were selected a priori based on their hypothesized relationship with FI (exposure) and cART adherence (outcome).
Data analyses
Descriptive P values were calculated using Pearson χ 2 tests and Wilcoxon rank-sum tests for binary/categorical variables and continuous variables, respectively. Adjusted OR (aOR) were estimated by generalized estimating equations, quantifying the relationship between binary FI (food secure v. moderate/severely food insecure) and binary cART adherence (adhering to <95 % of prescribed cART v. adhering to ≥95 % of prescribed cART) with logistic regression(Reference Weiser, Leiter and Bangsberg33,Reference Shannon, Kerr and Milloy34) . Generalized estimating equations were used to account for the longitudinal nature of the baseline and follow-up measures taken from individual participants using an exchangeable correlation structure with robust se(Reference Ballinger35,Reference Zorn36) . To select the variables for the multivariable model, a change-in-estimate approach to confounder selection was used(Reference Maldonado and Greenland37,Reference Mickey and Greenland38) . Specifically, if the coefficient for FI changed by less than 5 % after the omission of a given confounder, the variable was not adjusted for in the final model(Reference Maldonado and Greenland37,Reference Lima, Geller and Bangsberg39) . All data were analysed using the statistical software package SAS version 9.4.
Results
Among the 121 DPC clients in the total cohort, 116 individuals and 215 total visits (observations) were included in the current analysis after excluding those who were not on cART at baseline or who were missing data on the FI or cART adherence measures. Table 1 reveals no significant differences in the proportions of responses to the HFSSM questions or overall FI status between baseline and follow-up. As shown in Table 2, at baseline, 74 % (n 86) of participants reported experiencing FI in the past 12 months and 67 % (n 78) of participants were adherent to cART in the past 6 months. The median (quartile 1–quartile 3) age of participants at baseline was 46 (39–52) years and 87 % (n 101) of participants were biologically male at birth. Notably, 35 % (n 41) identified as Indigenous, 70 % (n 81) had been diagnosed with hepatitis C and 53 % (n 62) had used illicit drugs (excluding marijuana) in the past 6 months.
Table 1 Baseline and follow-up responses to the ten-item adult scale of the Household Food Security Survey Module (HFSSM) of Dr. Peter Centre clients in Vancouver, Canada (February 2014–March 2016)
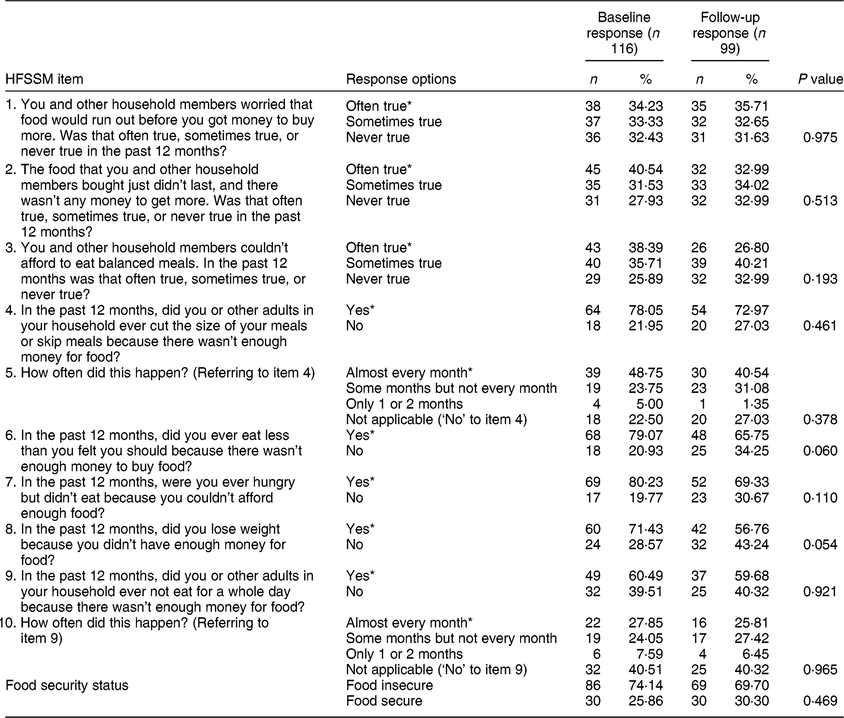
* An affirmative response on the HFSSM.
Table 2 Baseline descriptive characteristics of 116 Dr. Peter Centre (DPC) clients in Vancouver, Canada (February 2014–March 2016)
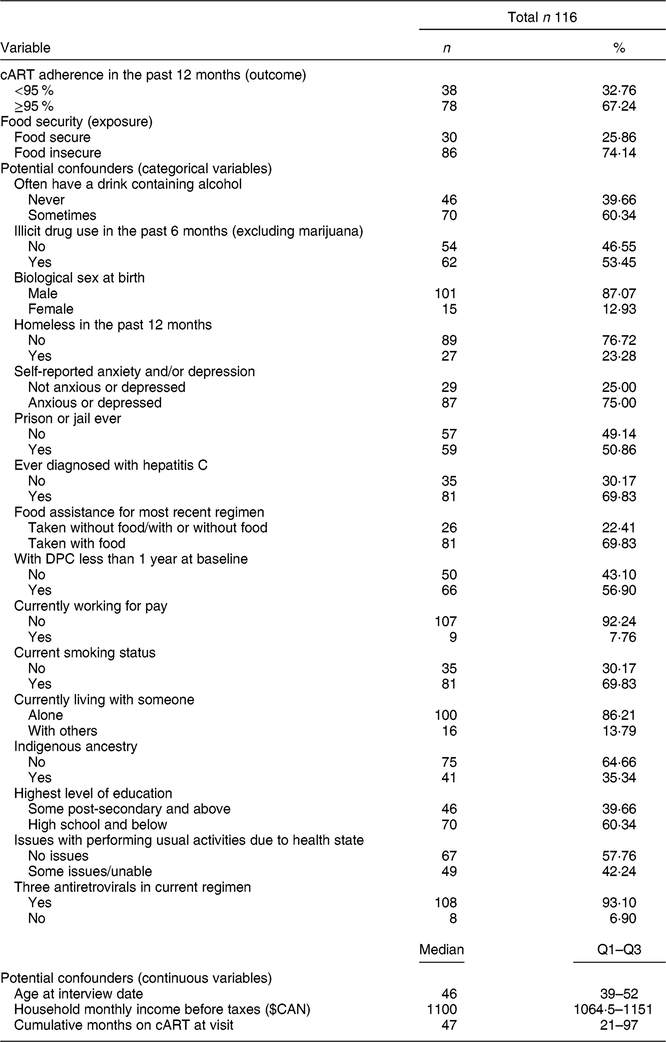
cART, combination antiretroviral therapy; Q1, quartile 1; Q3, quartile 3.
In the unadjusted analysis (Table 3), experiences of FI were associated with suboptimal cART adherence (unadjusted OR = 0·44, 95 % CI 0·24, 0·82). Furthermore, after adjustment for potential confounding factors, FI remained associated with suboptimal adherence (aOR = 0·47, 95 % CI 0·24, 0·93). In other words, those who experienced FI were approximately half as likely to be adherent to cART (≥95 %) compared with those who were food secure.
Table 3 Univariable and multivariable analyses of the relationship between food insecurity and ≥95 % combination antiretroviral therapy (cART) adherence among clients of the Dr. Peter Centre (DPC) in Vancouver, Canada (February 2014–March 2016)
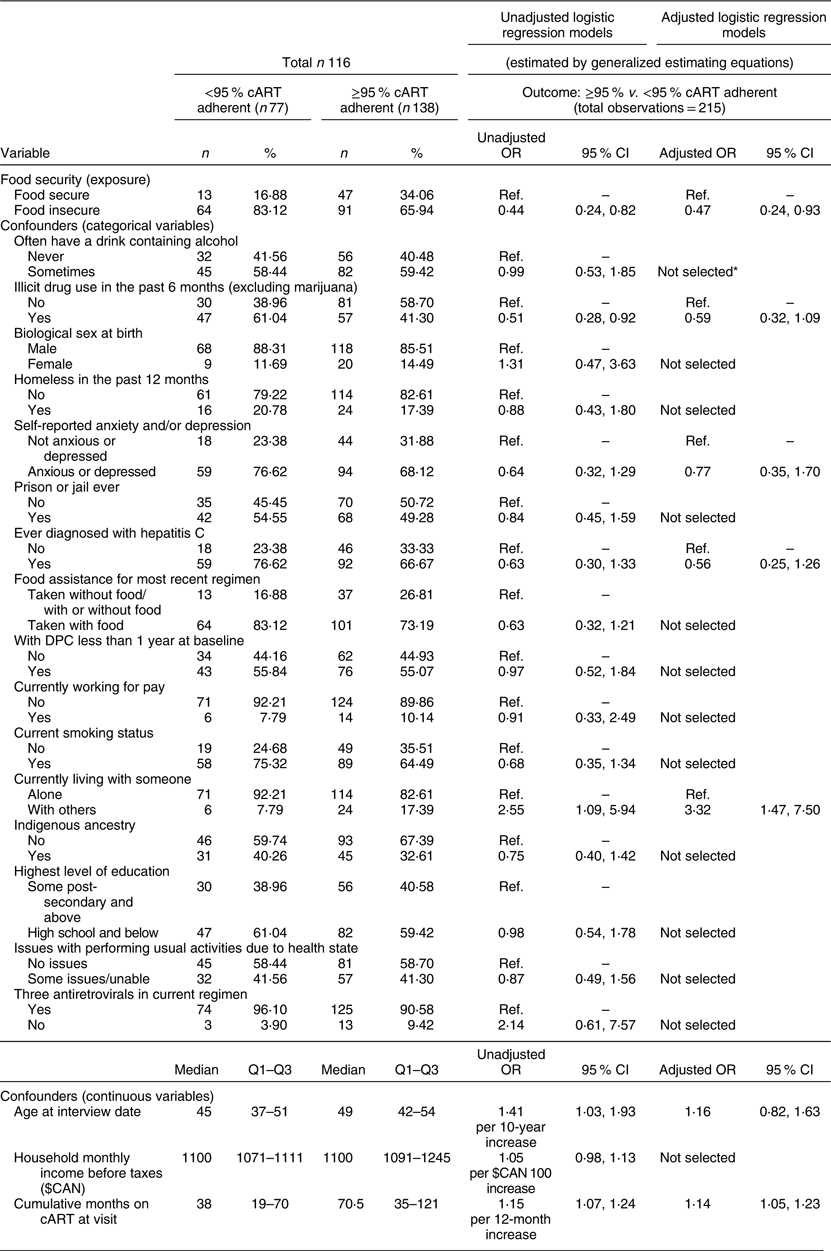
Ref., reference category; Q1, quartile 1; Q3, quartile 3.
* Not selected after change-in-estimate approach: if the coefficient for food insecurity changed by less than 5 % after the omission of a given confounder, the variable was not adjusted for in the final model.
Discussion
The present study examined the relationship between FI and cART adherence among a cohort of PLHIV who were clients of the DPC in Vancouver, Canada. Nearly three-quarters of DPC participants reported experiencing FI in the past 12 months. The high prevalence of FI among DPC clients was similar to that documented in other Canadian studies of PLHIV(Reference Strike, Rudzinski and Patterson7,Reference Anema, Fielden and Shurgold8,Reference Anema, Weiser and Fernandes40,Reference Miewald, Granger and Grieve41) . Two studies conducted in BC (2011 and 2016) found the percentage of food-insecure PLHIV to be almost identical to the 74 % of participants identified in the present study(Reference Anema, Fielden and Shurgold8,Reference Anema, Weiser and Fernandes40) . Also in line with other studies, individuals who were food insecure were approximately half as likely to be adherent to cART after adjusting for potential clinical, social and demographic confounders of the FI–cART adherence relationship(Reference Kalichman, Washington and Grebler9,Reference Kalichman, Hernandez and Cherry42) .
The present study’s results must be interpreted in the context of previous literature detailing how interventions that focus on food provision (e.g. food banks, community gardens) do not necessarily alleviate FI over an extended period of time, particularly in resource-rich settings(Reference Tarasuk23,Reference Kirkpatrick and Tarasuk43–Reference Miewald, McCann and Temenos45) . While food provision can provide other benefits (e.g. the mitigation of hunger(Reference Tarasuk and Eakin24) (a physical sensation experienced by those with severe FI)(Reference Tarasuk46), entry points to health-care services(Reference Collins, Parashar and Hogg25), promotion of social interactions(Reference Miewald, Granger and Grieve41,Reference Miewald, McCann and McIntosh47) and support for development of daily routines(Reference Collins, Parashar and Hogg25,Reference Miewald, McCann and Temenos45) ), the root driver of FI in resource-rich settings is inadequate financial resources(Reference Kirkpatrick and Tarasuk43,Reference Che and Chen48–Reference McIntyre, Connor and Warren50) . Our study further demonstrates this as FI remains prevalent among DPC clients despite the provision of food. In addition, there remains a relationship between this prevalent experience and suboptimal cART adherence in this integrated care programme.
While our study cannot evaluate any of the potential mechanisms by which FI leads to suboptimal cART adherence, our work provides impetus for additional research to better understand how to attenuate the relationship between these two factors in this setting and in other similar environments. For example, FI has a known association with depression(Reference Palar, Kushel and Frongillo51,Reference Vogenthaler, Hadley and Rodriguez52) and dependence on drugs and alcohol(Reference Kalichman, Grebler and Amaral53), all of which are linked with suboptimal cART adherence(Reference Lima, Geller and Bangsberg39,Reference Mugavero, Ostermann and Whetton54–Reference Anema, Wood and Weiser56) . FI, along with other needs (e.g. housing, transportation) that stem from limited financial resources, may also impact cART adherence when meeting these needs interferes with medication access or medical appointments(Reference Hatcher, Tsai and Cohen19,Reference Kushel, Gupta and Gee57,Reference Cunningham, Andersen and Katz58) . Analyses that explicate how these pathways may be leveraged to attenuate the impact of FI on cART adherence among structurally vulnerable PLHIV are necessary.
The findings of the present study also point to a need to consider the broader implications of food provision within integrated care models, beyond the scope of mitigating FI. In particular, the food programme at the DPC can be conceptualized within the organization’s broader harm reduction mandate, which aims to improve health and reduce health- and drug-related harms(Reference Fernando, McNeil and Closson21,Reference Miewald, Granger and Grieve41,Reference Miewald, McCann and Temenos45) . For example, the food programme at the DPC has been shown to be an integral element of the Centre and a primary access point for individuals interacting with the space(Reference Collins, Parashar and Hogg25,Reference Miewald, McCann and Temenos45) . Overall, the benefits of integrated care models that include food provision must consider how programming may positively impact clients through a harm reduction approach, even if experiences, such as FI, remain prevalent.
The DPC offers a unique environment in which to study FI and adherence to cART. However, our study warrants consideration of some potential limitations. Participants of the present study were not randomly selected and are thus not representative of the general population of PLHIV in BC. In fact, because the admission requirements for the DPC necessitate a deteriorating health status(Reference Fernando, McNeil and Closson21), the sample in the present study may over-represent individuals with complex health needs. In addition, while the HFSSM is a validated measurement tool for FI, fluctuations in FI within a 12-month period is an inherent limitation to the use of the HFSSM(Reference Tarasuk46). Another limitation of the study is that we are unable to stratify our analysis or adjust our regression models by whether a participant in fact received meals at the DPC. Therefore, we cannot directly attribute the impact of this particular service on the relationship between FI and adherence. However, previous work conducted among thirty DPC clients who used illicit drugs showed that 100 % (n 30) of clients surveyed accessed the DPC food programme for some of their meals, with 80 % (n 24) using the programme daily and the other 20 % (n 6) using the programme weekly (C Miewald, unpublished results). Our findings are contextualized based on this understanding, as well as other published literature including DPC clients(Reference Collins, Parashar and Hogg25,Reference Miewald, McCann and Temenos45) .
Conclusion
In conclusion, the present study documented a high prevalence of FI among DPC clients in Vancouver, Canada. As such, while food provision may have benefits related to harm reduction, there remains a relationship between this prevalent experience and cART adherence in this integrated care programme. Future studies that elucidate strategies to mitigate FI among PLHIV in this setting and in other similar environments are necessary.
Acknowledgements
Acknowledgements: The authors would like to thank the participants in the Dr. Peter Centre Study, the peer research associates, the DPC Community Advisory Committee, and study co-investigators: Rolando Barrios, Stuart Skinner, Silvia Guillemi, Susan Kirkland, M.-J. Milloy, Carol Strike, Bernadette Pauly, Hasina Samji, Kate Salters and Ciro Panessa. Financial support: The Dr. Peter Centre Study is funded by the Canadian Institutes of Health Research (CIHR; grant number CIHR R-PHE-122186); the DTP receives funding from the British Columbia provincial government through PharmaCare; T.M. is supported by a Canadian HIV Observational Cohort (CANOC) Centre Postdoctoral Award, a joint programme of CANOC and the CIHR Canadian HIV Trials Network (grant number CTN 242); and A.B.C. is supported by a Vanier Canada Graduate Scholarship. The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: R.S.H. and S.P. contributed to the study’s conception and implementation. K.K. and T.M. conceptualized the study design and analysis plan. L.W. and J.L. analysed the data. K.K. and A.B.C. prepared the original manuscript draft, with subsequent drafts led by K.K. and T.M. P.M., R.B.-T., C.M., K.A.S., R.S.H. and S.P. provided critical feedback on further revisions. All authors read and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Simon Fraser University and Providence Healthcare/University of British Columbia research ethics boards. Written informed consent was obtained from all subjects.





