According to the Joint UN Programme on HIV/AIDS( 1 ), 35·3 million people were living with HIV/AIDS at the end of 2012 and 260 000 children became newly infected in 2012.
Morbidity and mortality associated with HIV infection have been reduced dramatically in the paediatric population with the introduction of highly active antiretroviral therapy (HAART) into routine clinical use( Reference Rudin, Burri and Shen 2 – Reference Ramos, Matida and Hearst 4 ). Although beneficial to the prognosis of HIV infection, exposure to HAART has been associated with disturbances in the nutritional status of this population group( Reference Sarni, Souza and Battistini 5 – Reference Tremeschin, Sartorelli and Cervi 9 ), including body fat distribution abnormalities, which may represent an increased risk for premature CVD( Reference Sarni, Souza and Battistini 5 , Reference Jacobson, Patel and Siberry 8 ). HAART also does not seem to fully reverse the effects of HIV/AIDS on children’s growth( Reference Sutcliffe, Van Dijk and Munsanje 10 ). In Brazil, the free and universal access to HAART is relatively recent( Reference Matida, Ramos and Moncau 11 ), so there are very few studies discussing the side-effects of HAART in the paediatric population( Reference Reis, Rondó and Marques 12 ). Furthermore, associations between antiretroviral therapy and anthropometric disturbances in children and adolescents are poorly understood. Thus, the present study aimed to investigate the relationship between anthropometric parameters and body composition of perinatally HIV-infected children and adolescents under HAART, according to use and non-use of protease inhibitors.
Experimental methods
The present cross-sectional study involved 115 children and adolescents, aged 6–19 years, who acquired HIV infection perinatally and were under HAART. All participants were regularly followed at the Institute of Child Health, University of São Paulo, Brazil, one of the three largest reference centres for treatment of paediatric patients with HIV/AIDS in São Paulo city. The HIV/AIDS out-patient clinic of the Institute of Child Health receives patients not only from São Paulo, but also from other cities of the country. Thus, the sample can be considered representative of Brazilian children and adolescents living with HIV/AIDS.
Demographic, socio-economic, clinical and anthropometric data were obtained by a questionnaire, physical examination and medical records after the caregivers signed informed consent, between August and December 2007.
The recall period for medical history was limited to the past three years to ensure that the patients had access to the medicines that make up HAART.
CD4 values were characterized according to the WHO guidelines for the paediatric population( 13 ): no immunological suppression (≥500 cells/mm3), slight suppression (350–499 cells/mm3) and moderate/severe suppression (≤349 cells/mm3). Detectable viral load indicated the presence of HIV replication.
The patients were grouped according to the WHO( 14 ) definition for childhood (<10 years of age) and adolescence (10–19 years of age). Pubertal stage was self-assessed with drawings that showed the different Tanner stages for secondary sex characteristics( Reference Tanner 15 ).
The anthropometric assessment comprised determinations of weight, height, waist and neck circumferences, and triceps skinfold thickness. The measurements were taken in triplicate according to the standardized procedures of Frisancho( Reference Frisancho 16 ) and Lohman et al.( Reference Lohman, Roche and Martorell 17 ). Weight was measured using a portable electronic scale (Sohnle®, model 7500, Murrhardt, Germany), accurate to 100 g. Height was measured with a Leicester stadiometer (SECA® portable height measure model, Hamburg, Germany), accurate to 1 mm. BMI (kg/m2) was determined as weight divided by the square of the height. Z-scores were calculated for BMI-for-age and height-for-age using the WHO Anthro( 18 ) software. WHO( 19 ) cut-off points were used to categorize BMI into underweight, normal weight, overweight or obesity, and height into low or normal height. The mid-arm, waist and neck circumferences were obtained with an inextensible centimetre-graded measuring tape (Stanley®, model 34103, New Britain, CT, USA), accurate to 1 mm. Triceps skinfold thickness was taken with a calibrated Lange® adipometer (Beta-Technology, Santa Cruz, CA, USA) on the right side of the body; accurate to 1 mm. Triceps skinfold thickness was categorized into percentiles according to Frisancho( Reference Frisancho 20 ).
The mid-arm fat and muscle areas were calculated using the equations recommended by Frisancho( Reference Frisancho 20 ). The sex- and age-specific percentiles recommended by the Centers for Disease Control and Prevention( Reference McDowell, Fryar and Hirsch 21 ) were applied to categorize waist circumference and a percentile >75th was used to identify abdominal obesity as proposed by Savva et al.( Reference Savva, Tornaritis and Savva 22 ). Neck circumference was assessed at the level of the thyroid cartilage and classified according to age- and sex-specific cut-off values recommended by Nafiu et al.( Reference Nafiu, Burke and Lee 23 ).
Body fat percentage was obtained by bioelectrical impedance analysis using the Biodynamics® analyser (model 310, Seattle, WA, USA). The patient was positioned in horizontal decubitus with ornaments removed. Data on the patient’s age, gender, weight and height were entered into the analyser. Surface gel electrodes were dorsally placed on the right side of the body, according to the manufacturer’s instructions, at the wrist, hand, ankle and foot, after each site was cleaned with alcohol. The patient was instructed to slightly abduct his or her arms and legs.
The variables included in the analysis were: age, gender, per capita income, HAART exposure, antiretroviral therapy adopted in the last three years, CD4 count, viral load, pubertal stage, nutritional status (BMI-for-age, height-for-age, waist and neck circumferences, triceps skinfold thickness, body fat percentage, upper-arm fat area and upper-arm muscle area).
In order to evaluate the relationship among the variables investigated and the antiretroviral therapy adopted for the patients, the regimens were separated into protease inhibitor (PI)-containing and non PI-containing therapy.
Mean, median, standard deviation, minimum and maximum values were calculated to describe all the variables investigated. A t test was used to compare means of variables with normal distribution, according to variables with only two categories, and a non-parametric Wilcoxon (Mann–Whitney) compared the means of variables with non-normal distribution.
Correlations were assessed by the Spearman non-parametric test and associations were investigated by Pearson’s χ 2 test. The statistical significance was set at P<0·05. The statistical analysis was conducted with the Stata 9 statistical software package.
The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethical Committees of the School of Public Health and Institute of Child Health/School of Medicine, University of São Paulo, SP, Brazil. Written informed consent was obtained from all participants.
Results
At the time of enrolment, 120 children and adolescents seen in the HIV/AIDS out-patient clinic of the Institute of Child Health, São Paulo, were eligible. One patient refused to participate and four patients had not used any medication over the last three years. Therefore, the final sample consisted of 115 children and adolescents from 6 to 19 years of age who were vertically HIV-infected.
The demographic, socio-economic, clinical and nutritional characteristics of the patients are summarized in Table 1. The majority of the individuals were adolescents, female, from low-income background and in early pubertal stages. Most of the patients were taking PI, almost half (47·8 %) had CD4 ≥500 cells/mm3 and their mean viral load (copies/mm3) was 26 207·9. Approximately four-fifths (80·9 %) presented a normal weight (BMI-for-age Z-score ≥−2·0 and <+1·0), 15·6 % were overweight (BMI-for-age Z-score ≥+1·0) and 3·5 % underweight (BMI-for-age Z-score <−2·0). A high prevalence (20·9 %) of stunting (height-for-age Z-score <−2·0) and low prevalences of central adiposity, assessed by waist (2·6 %) and neck circumferences (5·2 %), were seen in this population. Depletion in adipose tissue (percentile <5th) was identified in 33·9 % children and adolescents according to the triceps skinfold thickness in percentiles.
Table 1 Characteristics of the vertically HIV-infected children and adolescents under HAART (n 115), São Paulo, Brazil, August–December 2007
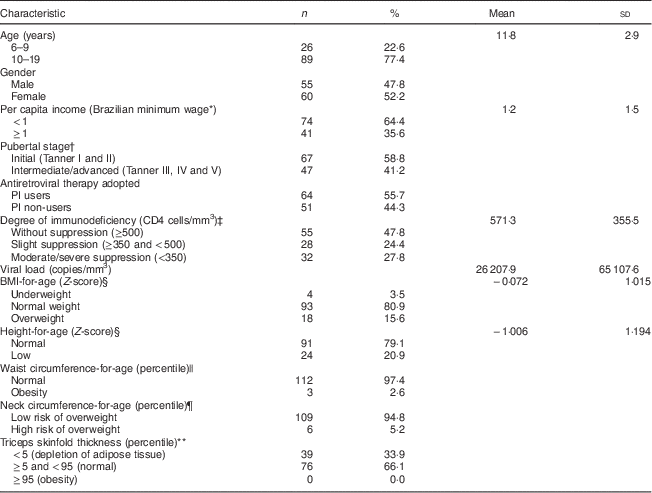
HAART, highly active antiretroviral therapy.
* 1 minimum wage (Brazilian Real)=US$ 172 at the time of the study.
† n 114; pubertal stage self-assessed with drawings( Reference Tanner 15 ).
‡ WHO guidelines for the paediatric population( 13 ).
§ WHO( 19 ) cut-off points.
|| Percentiles recommended by the Centers for Disease Control and Prevention( Reference McDowell, Fryar and Hirsch 21 ) categorized waist circumference; percentile>75th identified abdominal obesity( Reference Savva, Tornaritis and Savva 22 ).
¶ Age- and sex-specific cut-off values of Nafiu et al.( Reference Nafiu, Burke and Lee 23 ).
** Categorized into percentiles according to Frisancho( Reference Frisancho 20 ).
Table 2 compares the nutritional and clinical characteristics of the children and adolescents vertically HIV-infected under HAART, according to PI use. The results show that PI users had higher prevalence of stunting (P=0·03), lower BMI (P=0·03) and lower percentage of body fat (P=0·05) compared with PI non-users. There were no differences for the other variables investigated: age, gender, HAART exposure, CD4 count, viral load, pubertal stage, waist and neck circumferences, triceps skinfold thickness, upper-arm fat area and upper-arm muscle area.
Table 2 Nutritional and clinical characteristics of the vertically HIV-infected children and adolescents under HAART, according to pattern of PI use (n 115), São Paulo, Brazil, August–December 2007
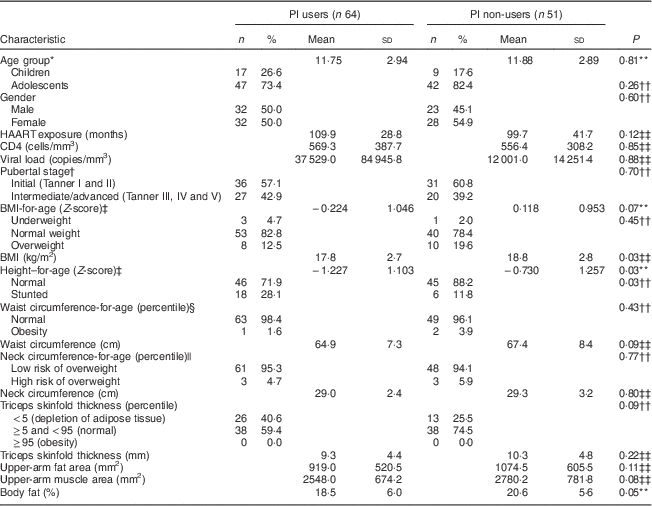
HAART, highly active antiretroviral therapy; PI, protease inhibitors.
* WHO definition of childhood and adolescence( 14 ).
† Pubertal stage self-assessed with drawings( Reference Tanner 15 ).
‡ WHO( 19 ) cut-off points.
§ Percentiles recommended by the Centers for Disease Control and Prevention( Reference McDowell, Fryar and Hirsch 21 ) categorized waist circumference; percentile>75th identified abdominal obesity( Reference Savva, Tornaritis and Savva 22 ).
|| Age- and sex-specific cut-off values of Nafiu et al.( Reference Nafiu, Burke and Lee 23 ).
¶ Categorized into percentiles according to Frisancho( Reference Frisancho 20 ).
** From t test.
†† From χ 2 text.
‡‡ From Wilcoxon rank sum test (Mann–Whitney).
Discussion
The patients included in the present study comprise a representative sample of the Brazilian HIV-infected paediatric population with long exposure to HAART. A high prevalence of stunting (20·9 %) was seen among those individuals, especially in PI users.
The latest Household Budget Survey, undertaken by the Brazilian Institute of Geography and Statistics( 24 ), showed that 6·8 % of children between 5 and 9 years of age were stunted and that the prevalence of this growth deviation decreased with age. Stunting has been observed in HIV-infected children( Reference Sarni, Souza and Battistini 5 , Reference Arpadi 25 – Reference Chiabi, Lebela and Kobela 28 ) and HAART is normally thought to improve growth( Reference Chhagan, Kauchali and Van den Broeck 27 ). However, HAART seems less effective in those under 1 year of age because of their poor clinical condition as they fulfilled the inclusion criteria for this therapy at a very young age, which suggests a more advanced disease( Reference Banerjee, Pensi and Banerjee 6 ). The mechanism by which HIV replication affects growth has not been totally elucidated, but suppression of HIV, through antiretroviral therapy, is thought to have a favourable effect on growth( Reference Arpadi 25 ).
The eligible children and adolescents of the present study received HAART regimens for prolonged periods. Thus, their clinical condition associated with the use of lifelong different antiretrovirals may have negatively influenced growth patterns.
Buonora et al.( Reference Buonora, Nogueira and Pone 29 ) assessed the impact of HIV infection on the growth parameters of vertically infected adolescents and demonstrated that, even on HAART, they tended to have lower growth parameters throughout childhood and adolescence compared with the healthy population. Chhagan et al.( Reference Chhagan, Kauchali and Van den Broeck 27 ) found that stunted children on HAART displayed inappropriate catch-up. These findings highlight the importance of adequate growth velocity assessment of these patients even after antiretroviral therapy is introduced.
Similar to our finding, Tremeschin et al.( Reference Tremeschin, Sartorelli and Cervi 9 ) referred that PI users presented lower mean BMI compared with PI non-users. Banerjee et al.( Reference Banerjee, Pensi and Banerjee 6 ) found that children under HAART had a significantly lower mean BMI Z-score compared with the no-HAART group. Weight and height are highly correlated and may be affected by the HIV infection itself, social factors, as well as medical therapy. It is well established that a poor trend of anthropometric measurements is likely to increase the risk of mortality of these children( Reference Chiabi, Lebela and Kobela 28 ). Chhagan et al.( Reference Chhagan, Kauchali and Van den Broeck 27 ) noticed that children under HAART who eventually died presented no improvements in anthropometry over time.
There is currently conflicting literature on whether lower mean or median BMI may represent the presence or absence of body fat redistribution. Aldrovandi et al.( Reference Aldrovandi, Lindsey and Jacobson 30 ) attributed the lower BMI in the HIV-infected group to differences in fat, primarily in the extremities. Alam et al.( Reference Alam, Cortina-Borja and Goetghebuer 31 ) observed that median BMI differed according to presence (19·4 kg/m2) or absence (17·8 kg/m2) of body fat abnormality in European HIV-infected children and adolescents.
Although many authors have documented an association between PI therapy and deviations in body fat redistribution( Reference Sarni, Souza and Battistini 5 , Reference Bockhorst, Ksseiry and Toye 32 , Reference Ramalho, Gonçalves and Carvalho 33 ), the present study did not observe such a relationship. However, metabolic disturbances (dyslipidaemia and insulin resistance) were observed among these patients and described in another publication( Reference Reis, Rondó and Marques 12 ). Jacobson et al.( Reference Jacobson, Patel and Siberry 8 ) suggested that body fat distribution in HIV-infected children follows a pattern associated with high risk of CVD and possibly related to antiretroviral therapy and disease severity. Other authors have also stated that ever having received PI therapy was not significantly associated with any fat distribution markers among HIV-infected children and adolescents( Reference Jacobson, Patel and Siberry 8 , Reference Arpadi, Bethel and Horlick 34 ).
In the current study PI users presented lower mean body fat percentage. The differences seen for stunting prevalence and body fat percentage according to HAART regimens might be correlated and derived from growth deviations. However, it is important to point out that some authors have not considered bioelectrical impedance analysis a good parameter for assessing body fat abnormalities in the paediatric population( Reference Contri, Berchielli and Tremeschin 7 , Reference Tremeschin, Sartorelli and Cervi 9 ).
Contri et al.( Reference Contri, Berchielli and Tremeschin 7 ) evaluated patients aged 3–17 years under HAART and found that both groups (receiving and not receiving PI therapy) had similar and unchanged body composition. According to these authors, bioelectrical impedance did not predict morphological changes in the children and adolescents evaluated. The authors suggested that other techniques should be applied to better characterize the fat redistribution of HIV-infected patients, including skinfold thickness measurements, segmental bioelectrical impedance technique, dual-energy X-ray absorptiometry and MRI. Despite the fact that bioelectrical impedance analysis has been used to assess body composition in HIV-infected children, it is difficult to analyse the results due to the lack of standards for children. Besides that, bioelectrical impedance analysis measures only whole-body fat and lean body mass, which does not allow the identification of body fat distribution abnormalities( Reference Tremeschin, Sartorelli and Cervi 9 ).
It is important to emphasize that alterations associated with antiretroviral therapy are likely a result of complex interactions between HIV infection, specific antiretroviral agents, age, gender, as well as host genetic and lifestyle factors( Reference Aldrovandi, Lindsey and Jacobson 30 ).
The underlying social determinants of malnutrition may contribute to poor nutrition among these patients( Reference Chhagan, Kauchali and Van den Broeck 27 ). Common causes for inadequate nutrition are chronic illness, poverty, food insecurity and side-effects of drugs( Reference Mothi, Karpagam and Swamy 35 ). Most of the children and adolescents evaluated in the present study were from low-income backgrounds. Although social support is provided by Brazilian government programmes, they might have been unable to overcome household food insecurity and poverty.
According to Contri et al.( Reference Contri, Berchielli and Tremeschin 7 ), long-term investigations with large samples are needed in order to ensure that the weight and height of HIV-infected paediatric patients can be maintained at normal rates. Considering that these parameters are highly correlated( Reference Buonora, Nogueira and Pone 29 ), HIV-infected children under HAART must be properly monitored in order to avoid deviations in their development and body composition, since other authors have suggested that stunting may have long-term effects on fat distribution( Reference Jacobson, Patel and Siberry 8 ).
Jacobson et al.( Reference Jacobson, Patel and Siberry 8 ) observed that stunted HIV-infected children had lower percentage of extremity fat and higher percentage of trunk fat. It is well established in the literature that stunted children are more likely to deposit fat centrally later in life( Reference Hoffman, Martins and Roberts 36 ). Stunted children must adapt to nutritional restriction that affects enzyme and hormone functions, as well as fat oxidation. These children seem to have impaired fat oxidation and are consequently at increased risk of obesity since fat that is not oxidized must be stored( Reference Hoffman, Sawaya and Verreschi 37 ). It is also well known that specific antiretrovirals and HIV disease severity are found to play a role in the acquisition or loss of body fat( Reference Jacobson, Patel and Siberry 8 ). Viral load, immune response and the production of inflammatory cytokines seem to adversely affect the growth parameters of HIV-infected patients( Reference Contri, Berchielli and Tremeschin 7 ).
Our findings suggest that HAART may be unable to reverse the effects of the disease on the children’s linear growth. The present study indicates the importance of monitoring the nutritional status of the paediatric population in routine care appointments so that these disturbances can be detected early and managed appropriately. These procedures could reduce the risk of mortality of HIV-infected children and adolescents.
A limitation of the present study is the lack of information regarding the use of antiretrovirals during the whole period of infection since the patients were born. It is also important to point out that this type of study (cross-sectional) does not allow for causality assertions.
Conclusions
The present study documented a high prevalence of stunting in HIV-infected children and adolescents, especially among PI users. The exposure to these antiretrovirals was also associated with lower mean for body fat percentage and lower BMI, which may indicate growth and development deviations. However, we did not see any association between PI exposure and body fat redistribution markers, as stated by many investigators.
Acknowledgements
Acknowledgements: The authors are grateful to the patients and their parents or caregivers who accepted to participate in the study. They would also like to thank Delanjathan Devakumar for the very useful comments and suggestions to improve the manuscript. Financial support: This research was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grant number 2007/50009-3). FAPESP had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: L.C.R. designed the study protocol, collected data, participated in the statistical analysis and interpretation of data, and undertook the main writing of the paper; P.H.C.R. secured funding, designed the study protocol, facilitated data collection, and participated in the interpretation of data and writing of the paper; H.H.S.M. assisted in locating the study areas, coordinated data collection, and participated in the interpretation of data and writing of the paper; N.J.S. performed statistical analysis and interpretation of data, and participated in the writing of the paper. All authors read and approved the final manuscript. L.C.R. is guarantor of the paper. Ethics of human subject participation: Informed written consent was obtained from all subjects. The protocol was approved by the Ethical Committees of the School of Public Health and Institute of Child Health/School of Medicine, University of São Paulo, SP, Brazil.




